A dual-synthon in pyridinium chloride: formation of ladder-like and columnar motifs through hydrogen bonds and cation–π interactions†
Shinji Yamada*, Nodoka Sako, Mai Okuda and Atsuko Hozumi
Department of Chemistry, Faculty of Science, Ochanomizu University, 2-1-1 Otsuka, Bunkyo-ku, Tokyo 112-8610, Japan. E-mail: yamada.shinji@ocha.ac.jp; Tel: +81 3 5978 5349
First published on 22nd October 2012
Abstract
A supramolecular dual-synthon with 2R4(10) ring and columnar motifs is observed in crystal structures of all 2-, 3- and 4-methylstyrylpyridinium chlorides and 3-bromo-, 3-nitro- and 4-trifluoromethylstyrylpyridinium chlorides. The dual-synthon assembled through N–H⋯Cl− and C–H⋯Cl− hydrogen bonds and cation–π interactions forms both ladder-like and head-to-head or head-to-tail columnar motifs. The position of the methyl group at the benzene ring affects the size and shape of the channel structure. Infinite hydrogen bond networks involving chloride anions, water and HCl molecules are found in the channels of 3- and 4-methylstyrylpyridinium chlorides. Variations in electron-withdrawing groups such as Br, NO2 and CF3 have little effect on the crystal structures.
Introduction
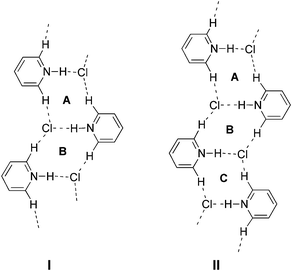
The exploitation of new supramolecular synthons1,2 in crystals is a key to the predetermination of the structural or functional properties of materials.3 A π–π stacking motif is widely observed as a synthon in crystals of various aromatic compounds.4 However, the crystals of pyridine derivatives, which structurally resemble benzene, consist of different types of homo- and heterosynthons as the pyridine nucleus has an N-acceptor and a C–H donor. Self-assembly of the molecules produces a weak C–H⋯N hydrogen bond synthon I,5 which produces a dimer or chain-like motif (Fig. 1a). Co-crystallization with carboxylic acids produces a robust heterosynthon II,6 in which O–H⋯N and C–H⋯O hydrogen bonds form a tape motif. Other synthons involving a pyridine moiety have also been reported.7 On the other hand, there have been few reports on the synthons of pyridinium salts, although they demonstrate a positive charge on the pyridine ring, a more acidic Cα–H and a counter anion.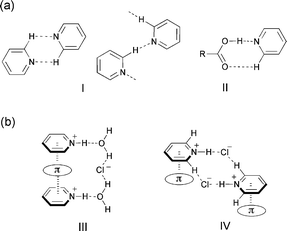 | ||
| Fig. 1 (a) Supramolecular synthons (I) and (II) observed in crystals and co-crystals of pyridine, respectively. (b) Reported supramolecular columnar motifs (III) for styrylpyridine hydrochloride hydrate and a supramolecular dual-synthon (IV) observed in substituted pyridinium chloride. | ||
Recently, we have reported the crystal structure of styrylpyridinium chlorides8,9 in which H-bond networks involving chloride anions and water molecules as well as cation–π interactions contribute to stabilize a head-to-tail columnar motif III.9 Continuing our research on the crystal engineering of pyridinium compounds through cation–π interactions,10 we found a different packing motif IV with a 2R4(10) ring system11 involving N–H⋯Cl− and C–H⋯Cl− hydrogen bonds as well as a columnar motif stabilized by pyridinium–π interactions as shown in Fig. 1b. In this paper, we report that a new supramolecular dual-synthon12 IV was commonly observed in the crystal structures of methylstyrylpyridinium chlorides (1a–1c)13 and 3-bromo- (2),14 3-nitro- (3)15 and 4-trifluorostyrylpyridinium chloride (4)16 (Fig. 2).
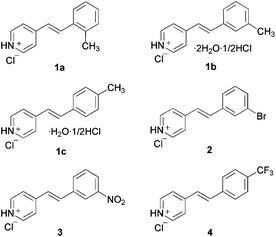 | ||
| Fig. 2 Substituted styrylpyridinium hydrochlorides. | ||
Experimental
Crystallization
The single crystals of 1a–1c, 2, 3 and 4 were grown from methanol solutions of corresponding (E)-styrylpyridines containing two equimolar amounts of conc. HCl. In each case, crystallization was performed at room temperature by slow evaporation of the solvent. Crystals 1a, 2, 3 and 4 were obtained as anhydrates, while 1b contained 0.5 equiv. of HCl and 2 equiv. of water molecules, and 1c contained 0.5 equiv. of HCl and 1 equiv. of water molecules (Fig. 2).Crystallographic details
All measurements were made on a Rigaku R-AXIS RAPID II diffractometer using graphite monochromated Cu Kα radiation. The data were collected at a temperature of −150 ± 1 °C to a maximum 2θ value of 136.5°. A total of 30 oscillation images were collected. A sweep of data was done using ω scans from 80.0 to 260.0° in 30.0° steps, at χ = 54.0° and ϕ = 0.0°. The exposure rate was 120.0 seconds per step. A second sweep was performed using ω scans from 80.0 to 260.0° in 30.0° steps, at χ = 54.0° and ϕ = 90.0°. The exposure rate was 120.0 seconds per step. Another sweep was performed using ω scans from 80.0 to 260.0° in 30.0° steps, at χ = 54.0° and ϕ = 180.0°. The exposure rate was 120.0 seconds per step. Another sweep was performed using ω scans from 80.0 to 260.0° in 30.0° step, at χ = 54.0° and ϕ = 270.0°. The exposure rate was 120.0 seconds per step. Another sweep was performed using ω scans from 80.0 to 260.0° in 30.0° step, at χ = 10.0° and ϕ = 60.0°. The exposure rate was 120.0 seconds per step. The crystal-to-detector distance was 127.40 mm. Readout was performed in the 0.100 mm pixel mode. The structures were solved by direct methods and expanded using the SHELXS-97 program17 and refined by full-matrix least-squares refinement on F2 using SHELXL-97.17 The non-hydrogen atoms were refined anisotropically. Hydrogen atoms were refined using the riding model. Figures were generated using the program Mercury.18Crystal data for 1a: C14H14NCl, M = 231.72, monoclinic, a = 4.84119(15), b = 31.5272(10), c = 7.9505(3) Å, β = 93.844(3)°, V = 1210.75(7) Å3, T = 123 K, space group P21/n, Z = 4, 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 451 reflections measured, 2173 unique (Rint 0.1540) reflections were used in all calculations. The final R1 and wR2 were 0.0929 and 0.2658(all data). CCDC 888277.
451 reflections measured, 2173 unique (Rint 0.1540) reflections were used in all calculations. The final R1 and wR2 were 0.0929 and 0.2658(all data). CCDC 888277.
Crystal data for 1b: (C14H14NCl)2·(H2O)4·(HCl), M = 580.03, monoclinic, a = 18.1754(4), b = 6.91256(13), c = 25.7534(5) Å, β = 110.5550(9)°, V = 3029.62(10) Å3, T = 123 K, space group P21/n, Z = 4, 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 040 reflections measured, 5380 unique (Rint 0.0608) reflections were used in all calculations. The final R1 and wR2 were 0.0741 and 0.2346(all data). CCDC 888278.
040 reflections measured, 5380 unique (Rint 0.0608) reflections were used in all calculations. The final R1 and wR2 were 0.0741 and 0.2346(all data). CCDC 888278.
Crystal data for 1c: (C14H14NCl)2·(H2O)2·(HCl), M = 535.94, triclinic, a = 4.8772(2), b = 16.2559(7), c = 18.5704(8) Å, α = 80.839(3), β = 87.297(2), γ = 84.649(3)°, V = 1446.41(11) Å3, T = 123 K, space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , Z = 2, 15
, Z = 2, 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 023 reflections measured, 5102 unique (Rint 0.1142) reflections were used in all calculations. The final R1 and wR2 were 0.0884 and 0.2236(all data). CCDC 888279.
023 reflections measured, 5102 unique (Rint 0.1142) reflections were used in all calculations. The final R1 and wR2 were 0.0884 and 0.2236(all data). CCDC 888279.
Crystal data for 2: C13H11NClBr, M = 296.59, monoclinic, a = 4.70335(13), b = 32.1116(9), c = 8.1926(3) Å, β = 98.0162(19)°, V = 1225.26(6) Å3, T = 123 K, space group P21/n, Z = 4, 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 515 reflections measured, 2230 unique (Rint 0.1167) reflections were used in all calculations. The final R1 and wR2 were 0.0658 and 0.1743(all data). CCDC 901989.
515 reflections measured, 2230 unique (Rint 0.1167) reflections were used in all calculations. The final R1 and wR2 were 0.0658 and 0.1743(all data). CCDC 901989.
Crystal data for 3: C13H11N2O2Cl, M = 262.70, triclinic, a = 4.79000(10), b = 8.28058(17), c = 16.2189(4) Å, α = 105.8270(14)° β = 91.5910(13)°, γ = 98.6340(14)°, V = 610.30(3) Å3, T = 123 K, space group P1, Z = 2, 6630 reflections measured, 3636 unique (Rint 0.0488) reflections were used in all calculations. The final R1 and wR2 were 0.0449 and 0.1981(all data). CCDC 901990.
Crystal data for 4: C14H11NF3Cl, M = 285.70, triclinic, a = 5.0232(3), b = 7.3643(3), c = 17.3038(8) Å, α = 92.657(3), β = 91.992(3), γ = 92.424(4)°, V = 638.42(5) Å3, T = 123 K, space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , Z = 2, 6734 reflections measured, 2267 unique (Rint 0.0899) reflections were used in all calculations. The final R1 and wR2 were 0.0631 and 0.1487(all data). CCDC 901991.
, Z = 2, 6734 reflections measured, 2267 unique (Rint 0.0899) reflections were used in all calculations. The final R1 and wR2 were 0.0631 and 0.1487(all data). CCDC 901991.
Thermal analysis
TG/DTA measurement of the solvated crystals of 1b and 1c was carried out using a Bruker TG-DTA 2000SA instrument to examine the thermal stability of the crystals and to determine the wt% of the solvent remaining in the crystals.Results and discussion
The X-ray crystallographic analyses of 1a–1c, 2, 3 and 4 were performed and their crystal structures were compared. Supramolecular dual-synthons were observed in all of the crystal structures. For compounds 1a–1c, the position of the methyl group at the benzene ring significantly affected the structure of the channels. The crystal structures of 2–4, having an electron withdrawing group, were also investigated to clarify the effect of the substituent on the cation–π interaction as well as to clarify the generality for the formation of the dual-synthon. A CSD search was also performed for styrylpyridinium chlorides, and the crystal structure of 3,5-dichlorostyrylpyridinium chloride (5) was found to contain the same synthon as those of 1–4.Crystal structures of 1a–1c and 2–4
Compound 1a crystallized in a monoclinic system with a P21/n space group. The crystal structure of 1a is shown in Fig. 3a. The molecules and chloride anions associated through the N–H⋯Cl− and C–H⋯Cl− hydrogen bonds to form a 2D sheet motif involving a new synthon with an 2R4(10) ring motif. This tape consists of two different centrosymmetric 10-membered rings A and B. The D⋯A distances of N1–H⋯Cl2, C1–H⋯Cl1 and C5–H⋯Cl3 are 3.025(4), 3.427(5) and 3.492(6) Å, respectively, showing that the hydrogen bond of N–H⋯Cl− is much stronger than those of C–H⋯Cl−.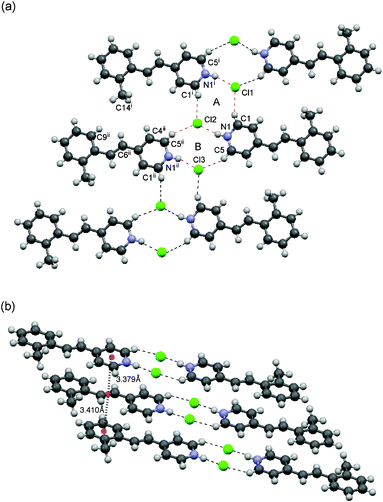 | ||
| Fig. 3 Crystal structure of 1a. (a) Top view of the packing structure. The 2D sheet motif contains dual-synthons, A and B. (b) Side view of the column with a head-to-head arrangement. | ||
The methyl group is directed toward the olefinic hydrogens of the neighboring molecule in the tape so as to avoid steric repulsion; the distances between the methyl carbon C14i and H4, H6 and H9 are 3.643, 3.568 and 3.690 Å, respectively. The molecules were arranged in a head-to-head and face-to-face fashion to form columns as shown in Fig. 3b. The pyridinium and benzene rings reside above and below the double bond moiety. The distances between the centroids of the double bond and those of the pyridinium and aryl moieties are 3.379 Å and 3.410 Å, respectively, suggesting that the columns are stabilized by cation–π10 and π–π interactions.
These structural features are speculated to result from the self-assembly of the dual-synthon motif IV (Fig. 1b). The H-bond networks contribute to the formation of a 2D sheet with a ladder-like motif and the cation–π interactions assist in the formation of the columnar motif perpendicular to the sheet, thus leading to an overall 3D network.
The crystals of 1b shown in Fig. 4 included an extra 0.5 equiv. of HCl and 2 equiv. of water molecules with a P21/n space group. The crystals contained two independent molecules in an asymmetric unit. Thermogravimetric analysis of 1b shows 20.4 wt% loss at 353 K (see the ESI† for the thermogravimetric analysis), which is in agreement with the involved 2H2O and 0.5HCl molecules as observed in the X-ray structure. The molecules were associated via the same dual-synthon as that of 1a to form a 2D sheet motif, which is very similar to that of 1a (Fig. 4a). This tape consists three ring systems A to C. A and C are centrosymmetric and B is acentrosymmetric, which form a ladder-like motif. The D⋯A distances of N1–H⋯Cl2 (3.032(4) Å) and N2–H⋯Cl1 (3.087(5) Å) are very close each other. Those of C1–H⋯Cl2ii, C5–H⋯Cl1, C15–H⋯Cl1 and C19–H⋯Cl2 are 3.488(6), 3.543(6), 3.673(5) and 3.547(6) Å, respectively (Fig. 4a). The orientation of molecules in a head-to-tail fashion, forming infinite columns perpendicular to the 2D sheet, is significantly different from that of 1a. The distances between the centroids of the benzene and pyridinium rings are 3.528 and 3.645 Å, suggesting the contribution of intermolecular cation–π interactions between the pyridinium ring and the aryl moiety. This arrangement allows the two double bonds to be close each other.
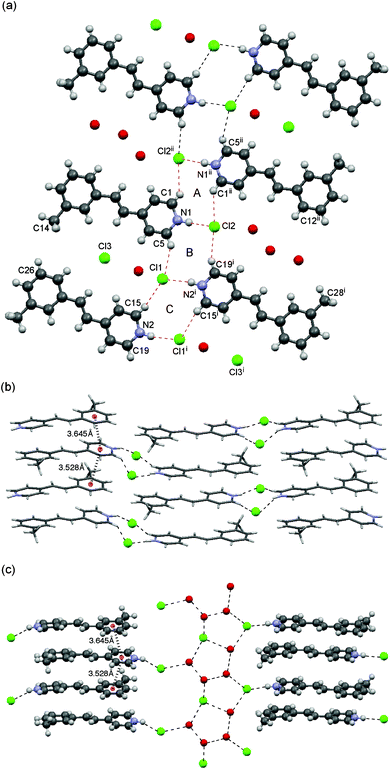 | ||
| Fig. 4 Crystal structure of 1b. (a) Packing structure from top view. The 2D sheet motif contains dual-synthons A–C. (b) Side view of the column with a head-to-tail arrangement. (c) Side view of the channel showing the incorporated chloride anion, HCl and water molecules. | ||
The methyl group at the 3-position is orientated toward the C26 of the neighboring molecule to form channels between the columns as shown in Fig. 4c. The channels are occupied by chloride anions as well as water and HCl molecules, which are linked to one another by a combination of O–H⋯Cl−, Cl–H⋯Cl− and O–H⋯O hydrogen bonds to form a tape motif containing a T5(2) ring system.19
The molecules in a column are alternatively linked to different hydrogen bond network systems; therefore, two hydrogen bond networks assist one head-to-tail column (Fig 4c). This is similar to the reported water-assisted assembly of styrylpyridinium chloride.9
A single crystal of 1c contains two independent molecules in an asymmetric unit with one equiv. of water and 0.5 equiv. of HCl molecules. 1c crystallized in a triclinic system with a P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group. Thermogravimetric measurement of 1c shows 12 wt% loss at 410 K (see the ESI† for the thermogravimetric analysis), which is almost in agreement with the involved H2O and 0.5HCl molecules as observed in the X-ray structure. The crystal structure is shown in Fig. 5. The dual-synthons A–C assemble to form a ladder-like motif similar to that in the crystals of 1b (Fig. 5a). The molecules are arranged in a head-to-head and face-to-face fashion to form columns as shown in Fig. 5b, which is very similar to the case of 1a. The pyridinium ring resides above the double bond moiety. The distances between the centroids of the double bond and the pyridinium and benzene rings are 3.403 Å and 3.464 Å, respectively.
space group. Thermogravimetric measurement of 1c shows 12 wt% loss at 410 K (see the ESI† for the thermogravimetric analysis), which is almost in agreement with the involved H2O and 0.5HCl molecules as observed in the X-ray structure. The crystal structure is shown in Fig. 5. The dual-synthons A–C assemble to form a ladder-like motif similar to that in the crystals of 1b (Fig. 5a). The molecules are arranged in a head-to-head and face-to-face fashion to form columns as shown in Fig. 5b, which is very similar to the case of 1a. The pyridinium ring resides above the double bond moiety. The distances between the centroids of the double bond and the pyridinium and benzene rings are 3.403 Å and 3.464 Å, respectively.
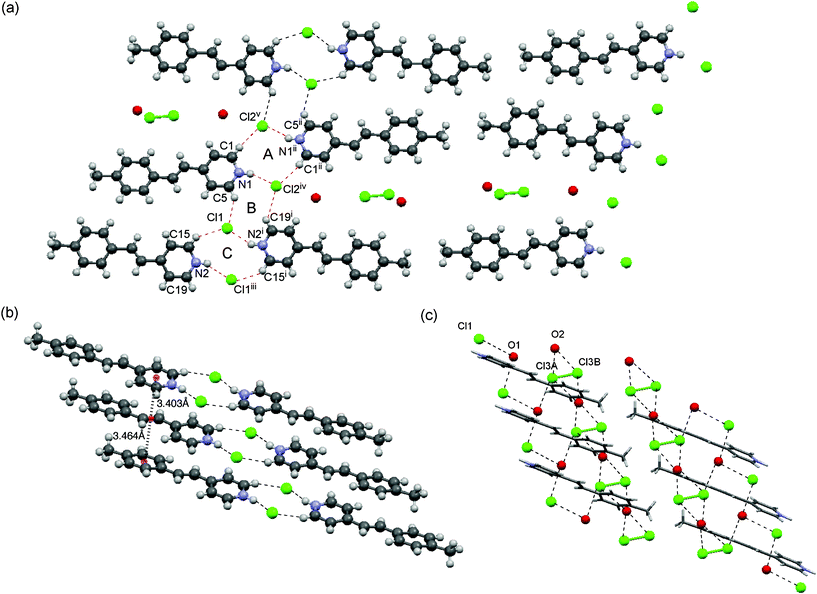 | ||
| Fig. 5 Crystal structure of 1c. (a) Top view of the packing structure. The 2D sheet motif contains dual-synthons A–C. (b) Side view of the column with a head-to-tail arrangement. (c) Side view of the channel showing the incorporated chloride anion, HCl and water molecules. | ||
The methyl group at the 4-position is located at the edge of the tape, thus facilitating the formation of a large channel in combination with the neighboring tape (Fig. 5a). This channel is much larger than that of 1b, and contains water and HCl molecules and chloride anions forming hydrogen bond networks. These form two 2D chain motifs (Fig. 5c). The HCl molecules occupy two different sites in the asymmetric unit with occupancies of 51.6 and 48.4%.
A comparison among these crystal structures of 1a–1c clearly shows that the tape motifs are grouped into types I and II as shown in Fig. 6. Type I consists of two centrosymmetric rings A and B. On the other hand, type II consists of three rings A–C, in which A and C are centrosymmetric and B is acentrosymmetric. These are repeated to form ladder-like motifs, A–B–A–B and A–B–C–A–B–C (Fig. 6). The ladder-like motif of 1a is classified into type I, whereas those of 1b and 1c are classified into type II, as the structures contain channels between columns.
 | ||
| Fig. 6 Two types of ladder-like motifs. | ||
The X-ray crystallographic analyses of 2–4 clarified that they have the same supramolecular dual-synthon IV as that observed in 1a. In all of the structures, a chloride anion is linked with three molecules through hydrogen bonds with C1–H, N1–H and C5–H, forming a type I ladder-like motif described above. The molecules were arranged in a head-to-head and face-to-face fashion to form columns through cation–π interactions between the pyridinium and the double bond. Table 1 summarized the distances and angles for the hydrogen bonds, C1–H⋯Cl1, N1–H⋯Cl2 and C5–H⋯Cl3, and the separation between the double bond with the pyridinium and aryl rings of 2–4 as well as 1a–1c for comparison.
| 1a | 1ba | 1ca | 2 | 3 | 4 | 5b | |
|---|---|---|---|---|---|---|---|
| a Data for synthons A and B.b Data obtained from the structure in ref. 23.c Distances of Py⋯Ar. | |||||||
| C1–H⋯Cl1 | |||||||
| C1⋯Cl1 (Å) | 3.427(5) | 3.488(6) | 3.587(8) | 3.495(7) | 3.509(10) | 3.331(4) | 3.518 |
| H⋯Cl1 (Å) | 2.633 | 2.789 | 2.673 | 2.654 | 2.678 | 2.834 | 2.874 |
| ∠C1–H–Cl1 (°) | 141.34 | 136.28 | 161.71 | 147.89 | 148.42 | 113.65 | 127.44 |
| N1–H⋯Cl2 | |||||||
| N1⋯Cl2 (Å) | 3.025(4) | 3.032(4) | 3.076(5) | 3.016(5) | 3.004(6) | 3.275(3) | 3.083 |
| H⋯Cl2 (Å) | 2.184 | 2.201 | 2.241 | 2.189 | 2.180 | 2.649 | 2.329 |
| ∠N1–H–Cl2 (°) | 159.66 | 157.04 | 158.37 | 156.58 | 156.08 | 129.06 | 146.58 |
| C5–H⋯Cl3 | |||||||
| C5⋯Cl3 (Å) | 3.492(6) | 3.547(6) | 3.416(7) | 3.400(6) | 3.495(8) | 3.575(3) | 3.400 |
| H⋯Cl3 (Å) | 2.639 | 2.726 | 2.778 | 2.742 | 2.859 | 2.987 | 2.694 |
| ∠C5–H–Cl3 (°) | 149.55 | 145.1 | 125.43 | 127.08 | 124.88 | 121.47 | 133.25 |
Py⋯C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (Å) C (Å) | 3.379 | 3.645c | 3.403 | 3.402 | 3.413 | 3.594 | 3.542 |
Ar⋯C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (Å) C (Å) | 3.410 | 3.528c | 3.464 | 3.436 | 3.467 | 3.614 | 3.485 |
Compound 2 crystallized in a monoclinic system with a P21/n space group. The D⋯A distances of N1–H⋯Cl2, C1–H⋯Cl1 and C5–H⋯Cl3 are listed in Table 1. These distances are shorter than those of 3-methyl derivative 1b. The distances between the centroids of the double bond and those of the pyridinium and aryl moieties are also shorter than those of 1b. These structural features may be responsible for the absence of channels in the crystal structure.
Compound 3 crystallized in a triclinic system, space group P1, the single crystal of which contains two independent molecules in an asymmetric unit. Although an electron-withdrawing group weakens the cation–π interaction,8,10a the separation between the molecules is close to those for 1a–1c and 2, suggesting that the interaction still exists between the molecules (Fig. 7). The nitro group at the 3-position plays an important role in the stabilization of the sheet motif. The nitro group at the 3-position links the neighboring tape through C9–H⋯O and C11–H⋯O hydrogen bonds (3.508 and 3.279, respectively)20 to form an infinite sheet structure. This may contribute to the stability of the packing structure and be responsible for the absence of channels in the crystal structure, which is contrary to the crystal structure of 1b.
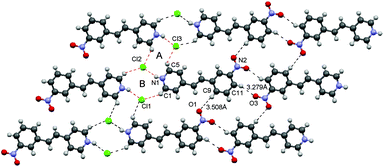 | ||
| Fig. 7 Crystal structure of 3. Top view of the packing structure. The 2D sheet motif contains dual-synthons A and B. | ||
Compound 4, having a CF3 group at the 4-position, crystallized in a triclinic system with a space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . The hydrogen bond networks were different from those of the others: a chloride ion links with the two N atoms and the four C atoms through N–H⋯Cl and C–H⋯Cl hydrogen bonds, respectively (see the ESI†). The N⋯Cl distances are longer and the bond angles for these hydrogen bonds are much smaller than those of the others as shown in Table 1. These structural features suggest that although 4 contains the same dual-synthon IV (Fig 1b), the additional hydrogen bonds led to distortion in the ladder-like motif.21 It should be noted that an F⋯F interaction was observed between the CF3 groups of the neighboring columns22 (Fig. 8). The 2.791 Å of the F⋯F distance is shorter than the sum of the van der Waals radii of the fluorine ions, thus contributing to the stability of the packing structure. This may be responsible for the absence of channels in the crystal structure, which is contrary to the crystal structure of 1c, which involves channels occupied by H2O and HCl molecules.
. The hydrogen bond networks were different from those of the others: a chloride ion links with the two N atoms and the four C atoms through N–H⋯Cl and C–H⋯Cl hydrogen bonds, respectively (see the ESI†). The N⋯Cl distances are longer and the bond angles for these hydrogen bonds are much smaller than those of the others as shown in Table 1. These structural features suggest that although 4 contains the same dual-synthon IV (Fig 1b), the additional hydrogen bonds led to distortion in the ladder-like motif.21 It should be noted that an F⋯F interaction was observed between the CF3 groups of the neighboring columns22 (Fig. 8). The 2.791 Å of the F⋯F distance is shorter than the sum of the van der Waals radii of the fluorine ions, thus contributing to the stability of the packing structure. This may be responsible for the absence of channels in the crystal structure, which is contrary to the crystal structure of 1c, which involves channels occupied by H2O and HCl molecules.
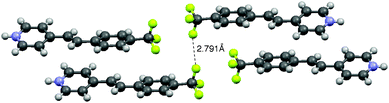 | ||
| Fig. 8 F⋯F interaction in the crystal structure of 4. | ||
CSD search for styrylpyridinium chlorides
A search of the Cambridge Structure Database (CSD Version 5.33, ConQuest 1.14, November 2011 update) gave 11 hits for styrylpyridinium chlorides. Among these crystals, 9 are hydrates and only 2 are anhydrates (see the ESI† for the refcodes). Similarly to he crystal structure of one of the anhydrates, 3,5-dichlorostyrylpyridinium chloride 5 (EQOTEK), 23 contained the same synthon IV and another anhydrate, perfluorostyrylpyridinium chloride (EQOTIO), contained a closely related motif to synthon IV. The crystal structure of 5 contains the 2R4(10) rings and columnar motifs with head-to-head and face-to-face arrangement similar to the others. On the other hand, the dual-synthon IV was not observed in the crystal structures of the 9 hydrates, although all of them involve columnar motifs with a head-to-tail orientation. Four of the 9 structures contained the same packing motif III indicated in Fig. 1b. The other 5 structures involved packing motifs that are very close to III. These structural features are summarized as follows: the supramolecular dual-synthon IV was preferentially formed in anhydrate crystals, whereas the packing motif III and related motifs were produced in hydrate crystals although exceptions were observed in the structures of 1b and 1c.Conclusion
In conclusion, we have found that a new supramolecular dual-synthon with an 2R4(10) ring and columnar motifs was commonly observed in all methylstyrylpyridinium chlorides 1a–1c, and in 3-bromo-, 3-nitro- and 4-trifluoromethylstyrylpyridinium chlorides 2–4. The dual-synthon assembled through N–H⋯Cl− and C–H⋯Cl− hydrogen bonds and cation–π interactions formed both ladder-like and head-to-head or head-to-tail columnar motifs. Although the position of the methyl group at the benzene ring affected the size and shape of the channel structures, the crystal structures were basically similar to each other. The crystals of 2–4 having an electron-withdrawing group also showed crystal structures similar to 1a and 1c, suggesting that the contribution of the cation–π interactions is important to the crystal packing structure even if the interaction is weakened by the substituent. The crystal structures of the 6 crystals described here and 11 styrylpyridinium chlorides crystals reported in the CSD were categorized into two supramolecular motifs III and IV (Fig. 1b). These features led to the conclusion that hydrate crystals tend to contain the supramolecular motif III, whereas anhydrate crystals, with the exception of 1b and 1c, contain the water-assisted supramolecular dual-synthon IV.Acknowledgements
This work was supported by JSPS KAKENHI Grant Number 21550097.References
- (a) G. R. Desiraju, Angew. Chem., Int. Ed., 2007, 46, 8342 CrossRef CAS; (b) B. Moulton and M. J. Zaworotko, Chem. Rev., 2001, 101, 1629 CrossRef CAS; (c) G. R. Desiraju, Chem. Commun., 1997, 1475 RSC; (d) G. R. Desiraju, Nature, 2001, 412, 397 CrossRef CAS; (e) G. R. Desiraju, Angew. Chem., Int. Ed. Engl., 1995, 34, 2311 CrossRef CAS.
- K. Biradha and L. Rajput, in Organic Crystal Engineering, ed. E. R. T. Tiekink, J. J. Vittal and M. J. Zaworotko, Wiley, 2010, p. 215 Search PubMed.
- G. R. Desiraju, J. Mol. Struct., 2003, 656, 5 CrossRef CAS.
- (a) R. T. Tiekink and J. Zukerman-Schpector, The Importance of Pi-Interaction in Crystal Engineering, Wiley, 2nd edn, 2012 Search PubMed; (b) K. Merz and V. Vasylyeva, CrystEngComm, 2010, 12, 3989 RSC.
- C. B. Aakeröy, A. M. Beatty, B. A. Helfrich and M. Nieuwenhuyzen, Cryst. Growth Des., 2003, 3, 159 Search PubMed.
- (a) T. Ueto, N. Takata, N. Muroyama, A. Nedu, A. Sasaki, S. Tanida and K. Terada, Cryst. Growth Des., 2012, 12, 485 CrossRef CAS; (b) S. L. Bekö, M. U. Schmidt and A. D. Bond, CrystEngComm, 2012, 14, 1967 RSC; (c) C. B. Aakeröy, S. Forbes and J. Desper, CrystEngComm, 2012, 14, 2435 RSC; (d) L. J. Thompson, R. S. Voguri, L. Male and M. Tremayne, CrystEngComm, 2011, 13, 4188 RSC; (e) A. Mukherjee and G. R. Desiraju, Chem. Commun., 2011, 47, 4090 RSC; (f) J. L. Wardell and E. R. T. Tiekink, J. Chem. Crystallogr., 2011, 41, 1418 CrossRef CAS; (g) M. Linares and A. Briceño, New J. Chem., 2010, 34, 587 RSC; (h) B. K. Saha and S. Bhattacharya, CrystEngComm, 2010, 12, 2369 RSC; (i) A. Lemmerer, J. Bernstein and V. Kahlenberg, CrystEngComm, 2010, 12, 2856 RSC; (j) S. Long and T. Li, Cryst. Growth Des., 2010, 10, 2465 CrossRef CAS; (k) R. Santra and K. Biradha, Cryst. Growth Des., 2009, 9, 4969 CrossRef CAS; (l) T. R. Shattock, K. K. Arora, P. Vishweshwar and M. J. Zaworotko, Cryst. Growth Des., 2008, 8, 4533 CrossRef CAS; (m) M. Du, Z.-H. Zhang, X.-G. Wang, H.-F. Wu and Q. Wang, Cryst. Growth Des., 2006, 6, 1867 CrossRef CAS; (n) N. J. Babu and A. Nangia, Cryst. Growth Des., 2006, 6, 1995 CrossRef CAS; (o) J. R. Bowers, G. W. Hopkins, G. P. A. Yap and K. A. Wheeler, Cryst. Growth Des., 2005, 5, 727–736 CrossRef CAS; (p) B. R. Bhogala, S. Basavoju and A. Nangia, CrystEngComm, 2005, 7, 551 RSC; (q) P. Vishweshwar, A. Nangia and V. M. Lynch, Cryst. Growth Des., 2003, 3, 783 CrossRef CAS; (r) R. D. B. Walsh, M. W. Bradner, S. Fleischman, L. A. Morales, B. Moulton, N. Rodriguez-Hornedo and M. J. Zaworotko, Chem. Commun., 2003, 186 RSC; (s) B. R. Bhogala, P. Vishweshwar and A. Nangia, Cryst. Growth Des., 2002, 2, 325 CrossRef CAS; (t) P. Vishweshwar, A. Nangia and V. M. Lynch, J. Org. Chem., 2002, 67, 556 CrossRef CAS; (u) V. R. Pedireddi, A. Ranganathan and S. Chatterjee, Tetrahedron Lett., 1998, 39, 9831 CrossRef CAS; (v) V. R. Pedireddi, S. Chatterjee, A. Ranganathan and C. N. R. Rao, Tetrahedron, 1998, 54, 9457–9474 CrossRef CAS; (w) C. V. K. Sharma and M. J. Zaworotko, Chem. Commun., 1996, 2655 RSC.
- (a) V. Stilinovic and B. Kaitner, Cryst. Growth Des., 2011, 11, 4110 CrossRef CAS; (b) E. Nauha, E. Kolehmainen and M. Nissinen, CrystEngComm, 2011, 13, 6531 RSC; (c) N. Lu, W.-H. Tu, J.-S. Shing, Y.-S. Wenb and L.-K. Liu, J. Mol. Struct., 2010, 980, 101 CrossRef CAS; (d) J. A. Bis, P. Vishweshwar, D. Weyna and M. J. Zaworotko, Mol. Pharmaceutics, 2007, 4, 401 CrossRef CAS; (e) R. Banerjee, B. K. Saha and G. R. Desiraju, CrystEngComm, 2006, 8, 680 RSC; (f) P. Vishweshwar, A. Nangia and V. M. Lynch, CrystEngComm, 2003, 5, 164 RSC.
- S. Yamada, N. Uematsu and K. Yamashita, J. Am. Chem. Soc., 2007, 129, 12100 CrossRef CAS.
- S. Yamada and Y. Nojiri, Chem. Commun., 2011, 47, 9143 RSC.
- (a) J. C. Ma and D. A. Dougherty, Chem. Rev., 1997, 97, 1303 CrossRef CAS; (b) S. Yamada and Y. Tokugawa, J. Am. Chem. Soc., 2009, 131, 2098 CrossRef CAS.
- J. Bernstein, R. E. Davis, L. Shimoni and N.-L. Chang, Angew. Chem., Int. Ed. Engl., 1995, 34, 1555 CrossRef CAS.
- (a) D. R. Turner, S. N. Pek and S. R. Batten, CrystEngComm, 2009, 11, 87 RSC; (b) D. R. Turner, S. N. Pek and S. R. Batten, Chem.–Asian J., 2007, 2, 1534 CrossRef CAS.
- A. Nakamura, H. Irie, S. Hara, M. Sugawara and S. Yamada, Photochem. Photobiol. Sci., 2011, 10, 1496 CAS.
- S. O. Sablin, M. J. Krueger, T. P. Singer, S. O. Bachurin and A. B. Khare, J. Med. Chem., 1994, 37, 151 CrossRef CAS.
- L. Horwitz, J. Org. Chem., 1956, 21, 1039 CrossRef CAS.
- B. Mondal, B. Captain and V. Ramamurthy, Photochem. Photobiol. Sci., 2011, 10, 891 CAS.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112 CrossRef.
- I. J. Bruno, J. C. Cole, P. R. Edgington, M. Kessler, C. F. Macrae, P. McCabe, J. Pearson and R. Taylor, Acta Crystallogr., Sect. B: Struct. Sci., 2002, 58, 389 CrossRef.
- L. Infantes, J. Chisholm and S. Motherwell, CrystEngComm, 2003, 5, 480 RSC.
- (a) C. V. K. Sharma and G. R. Desiraju, J. Chem. Soc., Perkin Trans. 2, 1994, 2345 RSC; (b) V. R. Pedireddi, J. A. R. P. Sharma and G. R. Desiraju, J. Chem. Soc., Perkin Trans. 2, 1992, 311 RSC.
- One of the referees suggested the reason of the distortion of the structure of 4.
- (a) K. Reichenbächer, H. I. Süss and J. Hulliger, Chem. Soc. Rev., 2005, 34, 22 RSC; (b) P. Panini and D. Chopra, CrystEngComm, 2012, 14, 1972 RSC.
- B. Mondal, B. Captain and V. Ramamurthy, Photochem. Photobiol. Sci., 2011, 10, 891 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Distances and angles of intermolecular hydrogen bonds in the crystals of 1b and 1c, TGA chart for 1b and 1c, crystal structures of 2–5, and refcode for styrylpyridinium chlorides. CCDC 888277–888279 and 901989–901991. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c2ce26547h |
| This journal is © The Royal Society of Chemistry 2013 |
