Oxydehydrogenation of C2–C4 hydrocarbons over Fe-ZSM-5 zeolites with N2O as an oxidant
Jolanta
Kowalska-Kuś
*,
Agnieszka
Held
and
Krystyna
Nowińska
Faculty of Chemistry, A. Mickiewicz University, Grunwaldzka 6, 60-780 Poznań, Poland. E-mail: jolakow@amu.edu.pl; Fax: +48 61 8291505; Tel: +48 61 8291472
First published on 24th October 2012
Abstract
Fe-ZSM-5 zeolites with a Si/Al ratio equal to 25 and 50, comprising 0.4 wt% of Fe and calcined at different temperatures, were used for oxidative dehydrogenation (ODH) of C2–C4 hydrocarbons. The calcination temperature (873 or 1173 K) slightly influenced hydrocarbon conversion, while it distinctly affected the selectivity towards respective olefins. It stems from the modification of acidic centers present in Fe-ZSM-5 samples. Calcination at 1173 K resulted in the decrease of total acidity, especially of strong Brønsted sites. UV-vis spectra evidenced the presence of very different iron(III) species, while the existence of Fe(II) ions was inferred from IR spectra of the adsorbed NO molecule. IR bands located at about 1840 and 1750 cm−1 indicate the presence of isolated iron(II) species, particularly in Fe-ZSM-5(1173) samples.
1. Introduction
Olefins belong to the most important intermediates employed in the organic industry. Currently they are produced mainly by means of high temperature (∼1000 K) paraffins cracking. Catalytic dehydrogenation of saturated hydrocarbons, another source of olefins, suffers from thermodynamic limitation. Oxidative dehydrogenation (ODH) of light paraffins may be considered as an alternative procedure for olefins production. It has been shown earlier that iron modified ZSM-5 zeolites, applied in the presence of N2O as an oxidant, demonstrate promising results in the ODH process.1–3The beneficial feature of the N2O–Fe-ZSM-5 system was discovered for the first time in the benzene to phenol hydroxylation process (BTOP),4 which was commercialized by Solutia in 2001. This success encouraged the search for new applications of the N2O–Fe-ZSM-5 system in oxidation reactions and it was successively used for the ODH reaction1–3 as well as for propene epoxidation.5 Despite the fact that a lot of research was involved in characteristics of iron complexes accommodated in high silica zeolite channels, the structure of iron species, responsible for oxidative activity in ODH and BTOP processes, as well as their generation and localization, has still not been fully explained. It has been suggested that high temperature treatment releases iron species accommodated in the zeolite structure6 or induces the penetration of exchanged ions into zeolite channels7 to generate active iron complexes of specific structure, containing Fe(II) species, called α-complexes. According to Dubkov et al.6 and Pirngruber et al.8 Fe(II) ions formation occurs as a result of high temperature treatment, by means of autoreduction of Fe(III) according to the reaction:8
| 4Fe3+ + 2O2− → 4Fe2+ + O2 |
Fe(II) ions interact with N2O to form active oxygen species, called α-oxygen. The nature of α-oxygen has been studied in detail by Panov and co-workers.6,9–11 The quoted authors indicated that α-oxygen may be formed as a result of N2O decomposition on the iron species accommodated in the zeolite matrix of the MFI structure. α-Oxygen was defined as a negatively charged oxygen radical O−, very reactive to CO and hydrocarbons. However, it was also underlined that α-oxygen cannot be identified with O− anion-radicals, commonly formed on transition metal oxides, as a result of oxygen adsorption. According to Panov et al.11 presence of α-oxygen may be demonstrated by IR spectra in a range of hydroxyl groups. The related band is recorded at 3670 cm−1. This specific OH group is formed as a result of interaction of α-oxygen with water molecule. It was demonstrated that α-oxygen formed on binuclear iron(II) complexes is indispensable for selective benzene to phenol hydroxylation. The activity for this reaction increases as a result of high temperature treatment of Fe-ZSM-5 catalysts,9,12 what is consistent with autoreduction of Fe(III), present in Fe-ZSM-5 channels, to Fe(II) after high temperature calcinations in air or in vacuum.6
It has been shown earlier that N2O decomposition, performed in the presence of transition metal oxides, results in the formation of different oxygen species. Aika and Lundsford13,14 opined that the predominant oxygen species, formed as a result of the contact of N2O with transition metal oxides, is an O− radical (not identical to α-oxygen). It has also been indicated that this radical participates in the oxydehydrogenation reaction, binding hydrogen released from hydrocarbons, following water formation. Accordingly, one can believe that different oxygen species, formed as a result of N2O decomposition, are utilized for the oxidation of different hydrocarbons when N2O is used as an oxidant.
In the presented paper, the effect of high thermal treatment of Fe-ZSM-5 catalysts on their activity in the oxidative dehydrogenation of short alkanes (C2–C4) was estimated. Considering the role of acidic centers for hydrocarbons activation, the concentration and the nature of acidic centers present on the catalysts calcined at 873 and 1173 K were estimated by means of TPD of ammonia and FT-IR spectra of adsorbed pyridine. To obtain some insight into the structure of different iron species in the catalysts under study, FT-IR spectra of adsorbed NO as well as UV-vis spectra were recorded and analyzed.
2. Experimental
2.1 Catalysts preparation
ZSM-5 zeolites with a Si/Al ratio equal to 25 and 50, kindly delivered from Süd Chemie, were modified with Fe(III) cations by means of an ionic exchange procedure from aqueous solution of 0.1 mol of Fe(NO3)3·9H2O at 333 K for 6 h. The exchange was performed as a single (for Si/Al = 25) or double (for Si/Al = 50) procedure, to obtain similar iron content. In the case of double exchange, the sample after the first process was centrifuged and the second exchange procedure was carried out. In the preliminary experiment the amount of iron introduced during the first exchange process was estimated after washing, drying and calcination. The exchanged samples were washed, dried and calcined at 873 and 1173 K for 2 h and labeled as Fe-ZSM-5(Si/Al ratio)LT and Fe-ZSM-5(Si/Al ratio)HT, respectively. The number of iron species introduced into zeolites and estimated from ICP measurements was about 0.4 wt% of Fe.2.2 Oxidative dehydrogenation (ODH) of light paraffins
The oxydehydrogenation reaction of C2–C4 hydrocarbons, in the presence of Fe-ZSM-5 zeolites as catalysts, was carried out in a continuous flow reactor in the range of temperature from 623 to 723 K, under atmospheric pressure, with WHSV = 52 h−1 and a contact time of 0.8 s. Nitrous oxide was used as an oxidant. Prior to oxidative experiments, the catalysts were pretreated in helium flow at 723 K. Substrates hydrocarbon and N2O were diluted with helium (molar ratio of hydrocarbon![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nitrous oxide
nitrous oxide![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) helium = 1
helium = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12.5). The products were analyzed using on-line GC, equipped with a TCD detector and in the case of C4 (n-butane and isobutane) ODH, also with a FID detector.
12.5). The products were analyzed using on-line GC, equipped with a TCD detector and in the case of C4 (n-butane and isobutane) ODH, also with a FID detector.
2.3 Catalysts acidity
2.4 Characterization of catalysts
The iron concentration in the studied catalysts was calculated from ICP measurements performed on a VARIAN ICP-OES VISTA-MPX spectrometer. The structure of active iron forms was characterized by UV-vis spectra and by FT-IR spectra of adsorbed NO as a probe molecule. UV-vis diffuse reflectance spectra were recorded on a VARIAN CARY 100 spectrometer. FT-IR spectra of NO adsorbed on Fe-ZSM-5 zeolites were recorded on a BRUKER FT-IR TENSOR 27 spectrometer. Prior to measurements, the catalyst wafer was placed in a vacuum cell and evacuated at 673 K for 2 h. FT-IR spectra were recorded at RT, after NO adsorption (directly after contact with catalyst and after 30 min) and after evacuation at RT for 1 h. FT-IR spectra are presented after subtraction of the spectrum of the untreated catalyst, recorded before contact with NO.3. Results and discussion
3.1 Catalysts acidity
The acidity of the studied samples was estimated on the grounds of TPD of ammonia. Fe-ZSM-5(25 and 50)LT showed well-defined peaks of desorption at about 460 and 680 K, while Fe-ZSM-5(25 and 50)HT samples displayed the peaks desorption of much weaker intensity, especially in the range of the higher temperature (Table 1). It indicates significant decrease of the acidity of iron modified ZSM-5 zeolites, calcined at high temperature. However, the Fe-ZSM-5(HT) samples still comprise some amount of strong acidic centers. IR spectra of adsorbed pyridine, employed for the estimation of the nature of acidic centers present on applied catalysts, delivered the information indicating a significant contribution of Brønsted acidic sites in the total acidity of Fe-ZSM-5(LT) and a dramatic decrease in Brønsted acidity after high thermal treatment (Fig. 1). Nevertheless, the very weak bands, originating from B-Py species, were still recorded for Fe-ZSM-5(HT) samples even after evacuation of pyridine at 673 K. Presence of strong and medium Brønsted acid sites was also confirmed by the results of a catalytic test in cumene cracking at 593 K, where Fe-ZSM-5(HT) zeolite still showed measurable activity (Table 1). It has been shown earlier that the acidity of Fe-ZSM-5 samples is affected by the Si/Al ratio15,16 and the temperature of calcinations.9 The number of acidic sites decreases along with an increase in Si/Al ratio as well as with an increase in the temperature of calcination. A comprehensive characteristic of iron modified zeolites ZSM-5 including acidic properties was presented by Schwidder et al.17 and also by Pérez-Ramírez et al.18 The quoted authors have shown that the acidity of Fe-ZSM-5 as well as the L/B ratio depends on the method of iron introduction, zeolite matrix, as well as the following treatment. Accordingly, high temperature calcination (HT) applied in our study resulted in a dramatic decrease in B acidity, while the intensity of the band of adsorbed pyridine related to Lewis acid sites changed slightly.| Symbol of catalyst | Si/Al ratio | Temperature of calcination [K] | Aciditya [μmol g−1] | Cumene conversion [%] |
|---|---|---|---|---|
| a Calculated on the basis of the area of the high-temperature peak. | ||||
| Fe-ZSM-5(25)LT | 25 | 873 | 562 | 90.7 |
| Fe-ZSM-5(25)HT | 25 | 1173 | 169 | 49.6 |
| Fe-ZSM-5(50)LT | 50 | 873 | 341 | 86.5 |
| Fe-ZSM-5(50)HT | 50 | 1173 | 118 | 24.1 |
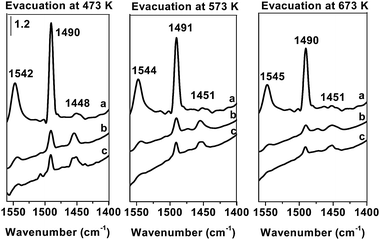 | ||
| Fig. 1 FT-IR spectra of pyridine adsorbed on (a) Fe-ZSM-5(25)LT; (b) Fe-ZSM-5(25)HT; (c) Fe-ZSM-5(50)HT. | ||
3.2 UV-vis spectra
UV-vis reflectance spectra recorded for Fe-ZSM-5(LT and HT) zeolites showed well separated strong bands at about 215 and 270 nm with a distinct shoulder at 245 nm, a band at 350 nm and broad bands in the range of 370–440 nm (Fig. 2). Metal ions with d electrons show spectroscopic bands in the range of visible-NIR vibrations, characterizing coordination and strength of metal–ligand bonds. Profound analysis of the bands recorded for Fe-silicalite in the range of UV-vis spectroscopy has been carried out by Zecchina et al.19 and also by Mihaylov et al.20 In the extended discussion, the quoted authors indicate that considering the low value (<1) of the extinction coefficient characterizing the d–d bands of Fe3+, mainly LMCT transitions, recorded in the range of 200–300 nm, have been observed for isolated Fe3+ complexes of tetrahedral coordination. Considering that Fe3+ complexes of octahedral coordination are recorded for similar wavelengths, the bands observed in this range do not allow the differentiation of tetrahedral and octahedral complexes. Also Schwidder et al.17,21 and Kumar et al.22 attributed the bands recorded up to 300 nm to the presence of isolated Fe(III) ions in tetrahedral or octahedral coordination. Clustering the iron species results in the shift of UV-vis bands to the lower energy region.19,20 Considering that, the band at about 350 nm should be assigned to oligomeric iron clusters, the band above 400 nm indicates the presence of oxide particles. The bands presented in Fig. 2 are relatively well separated, while UV-vis bands related to LMTC transitions are usually broad and overlapped. However, it has been shown earlier that intensity and separation of UV-vis bands were affected by different parameters such as method of introduction of iron ions as well as by iron concentration and the thermal treatment.23 Schwidder et al. in the quoted paper23 present spectra of Fe-ZSM-5 catalysts prepared in a different way and containing different iron concentrations (from 0.2–3.2 wt% of Fe). The mentioned spectra showed well separated bands below 300 nm and also a well-defined band at 350 nm and a distinct shoulder above 400 nm. Considering the above results, it seems that low iron concentration (0.4 wt% of Fe) and presence of both isolated, oligonuclear and oxide particles bring about small amount of specific iron species, which result in better separation of related bands. According to Pirngruber et al.24 both monomeric and dimeric iron species may be observed in UV-vis spectra in the region up to 300 nm and only large clusters are characterized by the bands at about 350 nm (Fig. 2). On the grounds of the quoted papers we can assume that both monomeric and dimeric iron complexes with iron ions in tetrahedral or octahedral coordination, as well as larger clusters and Fe2O3 particles, are present in the catalysts under study. According to XPS measurements presented in the paper,7 calcination of iron exchanged zeolite ZSM-5, in the range of temperature from 773–1173 K, results in penetration of iron ions into zeolite channels bringing about a decrease in the surface concentration and an increase of the number of iron species inside the channels. This may explain the alteration of contribution of different iron species in the samples after calcination at 1173 K indicated by UV-vis spectra. The bands characteristic for monomeric and dimeric iron species (described by the bands up to 300 nm) predominate in the spectra of Fe-ZSM-5(HT) (Table 2). High thermal treatment influences not only iron ions location but concurrently facilitates the reduction of Fe(III) ions present in Fe-ZSM-5 to Fe(II).6,8 A singular iron ion penetrating into the channel may undergo reduction with the formation of isolated Fe(II) species. However, UV-vis spectra deliver the information exclusively about coordination of Fe(III), not Fe(II) species. Considering the lower metal charge of Fe(II) ions, the related d–d transitions are shifted to lower wave numbers, when compared to Fe(III) ions and bands characteristic of Fe(II) complexes are observed in the NIR not in the UV-vis region.19 Partial autoreduction may be an explanation of lower intensities of the bands recorded for Fe-ZSM-5(HT) samples (Fig. 2). Fe(II) species generated in Fe-ZSM-5 zeolites may be characterized on the grounds of IR spectra of adsorbed NO molecules.19,20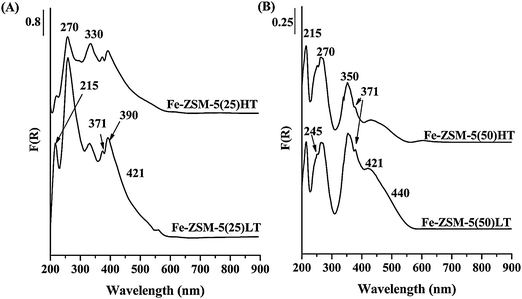 | ||
| Fig. 2 UV-vis spectra of (A) Fe-ZSM-5(25) and (B) Fe-ZSM-5(50) calcined at different temperatures (873 and 1173 K). | ||
| Symbol of catalyst | Si/Al ratio | A 1 [%] | A 2 [%] | A 3 [%] |
|---|---|---|---|---|
| a Isolated Fe(III) ions in tetrahedral and higher coordination. b Oligomeric Fe(III)xOy clusters. c Aggregated Fe2O3 particles. | ||||
| Fe-ZSM-5(25)LT | 25 | 30.5 | 29.5 | 40.0 |
| Fe-ZSM-5(25)HT | 25 | 54.3 | 35.5 | 10.2 |
| Fe-ZSM-5(50)LT | 50 | 28.6 | 26.8 | 44.6 |
| Fe-ZSM-5(50)HT | 50 | 47.2 | 24.3 | 28.5 |
3.3 FT-IR spectra of adsorbed NO
IR spectra of NO adsorbed on iron modified zeolite ZSM-5 were studied in a number of papers19,20,22,25–31 and demonstrated that both wavelength and the intensity of specific bands depend on different parameters influencing an iron species location and concentration.19,28,29,31 From the analysis of recorded bands and on the grounds of the other techniques, the quoted authors indicated the mechanism of NO–catalyst interaction, as well as pointed out different forms of iron active species operating in the catalyst. The comprehensive study on the interaction of NO molecules with the iron species of Fe-ZSM-5 zeolites was presented by Zecchina et al.19 and by Rivallan et al.25 The quoted authors have shown that depending on the method of introduction of iron ions, the iron concentration as well as the presence of framework aluminum ions, the location of the bands related to NO-Fe-ZSM-5 interaction is shifted or their intensity changes. Considering these studies we have tried to assess the nature of iron species generated on the studied catalysts looking for the species active for the ODH reaction.NO adsorption on Fe-ZSM-5 zeolites, performed at room temperature and monitored by IR spectroscopy, indicated the presence of the bands in the range of 1600–2200 cm−1. Considering that the spectra were presented after earlier subtraction of the untreated catalyst, the bands may be considered as characteristic for complexes between iron ions and NO molecules.
The strongest band recorded in the spectra of NO adsorbed on Fe-ZSM-5(LT) was located at 2133 cm−1 (Fig. 3). According to earlier papers,25–27 the band at 2133 cm−1 should be attributed to NO+ groups occupying the Brønsted acid sites. This suggestion was confirmed by disappearance of the band at 3610 cm−1, corresponding to strong Brønsted acid sites, after contact of NO with Fe-ZSM-5(LT) (Fig. 4). The band at 3745 cm−1, related to silanol groups, was still present. Short evacuation resulted in removal of the band at 2133 cm−1 and in restoration of the band of OH groups at 3610 cm−1. Contact of NO with Fe-ZSM-5(HT) resulted in the formation of only a very weak band at 2133 cm−1 which is consistent with low Brønsted acidity of that sample (Fig. 5). Interestingly, the very weak band at about 3670 cm−1 has also been recorded on the fresh Fe-ZSM-5(50)LT catalyst indicating the presence of α-oxygen.11 The following contact with NO removes this band indicating the interaction of NO with the Fe3+–OH group. According to Mihaylov et al.20 and Blain-Aube et al.31 an interaction of NO with Fe3+–OH results in reduction of Fe3+ and formation of Fe2+ ions resulting in the formation of a Fe2+–NO complex characterized with the IR band at about 1890 cm−1. Indeed, the weak band at about 1890 cm−1, formed as a result of contact with NO, has been noted (Fig. 5). According to quoted papers20,31 the initially formed mononitrosyl complex may be transformed to polynitrosyl. This transformation, which is an activated process, may occur considering the location of α-sites in the straight channels of the zeolite.
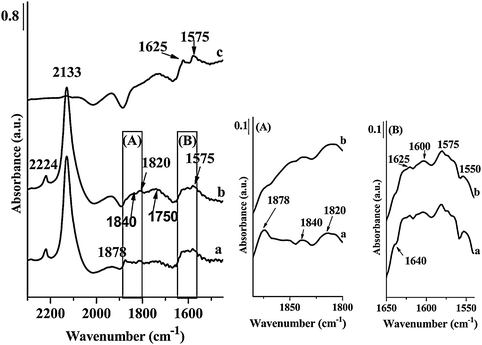 | ||
| Fig. 3 FT-IR spectra of Fe-ZSM-5(50)LT; (a) spectrum registered immediately after NO adsorption at RT, (b) after 30 min, (c) after evacuation at RT. | ||
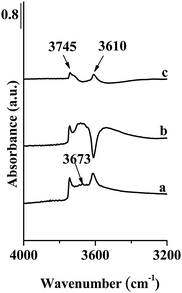 | ||
| Fig. 4 FT-IR spectra of Fe-ZSM-5(50)LT in the range of OH vibrations; (a) initial zeolites matrix, before NO adsorption, (b) after adsorption of NO at RT, (c) after evacuation at RT. | ||
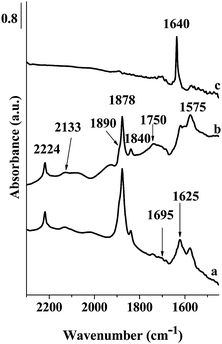 | ||
| Fig. 5 FT-IR spectra of Fe-ZSM-5(50)HT; (a) spectrum registered immediately after NO adsorption at RT, (b) after 30 min, (c) after evacuation at RT. | ||
The bands indicating the interaction of NO with iron ions become much stronger and distinct when recorded for the sample calcined previously at 1173 K (Fig. 5). The origin of the strongest band, located at 1878 cm−1, was discussed in a number of papers.19–22,26–30 and their assignment is still ambiguous. Nechita et al.30 and also Zecchina et al.19 suggest the attribution of this band to the nitrosyl group bonded to Fe(III) ions. On the other hand, the analysis of DFT calculation32 as well as Mössbauer spectra31 delivered the evidence pointing at the attribution of this band to the nitrosyl group adsorbed rather to Fe(II) ions. Blain-Aube et al.31 and Mihaylov et al.20 assumed that FT-IR bands of adsorbed NO, recorded in the range of 1870–1880 cm−1, should be attributed to mononitrosyl groups of Fe(II)(NO), however, the location of relevant iron ions in zeolite channels is still under discussion. Analyzing results presented in Fig. 3 and 5 one can conclude that the band at 1878 cm−1 is much stronger in the spectra recorded after NO contact with Fe-ZSM-5 calcined at 1173 K, when compared to the bands of NO-Fe-ZSM-5(873). Considering that high temperature calcination of Fe-ZSM-5 catalysts brings about iron autoreduction with the formation of additional Fe(II) ions,6,8 the simultaneous increase in the IR band (1878 cm−1) intensity confirms the attribution of that band to nitrosyl groups connected to Fe(II) ions. The assignment of the band at about 1880 cm−1 to Fe(NO) complexes was also suggested by Mul et al.33 and by Pirngruber and Pieterse.29 However, the contribution of the nitrosyl group bonded to Fe(III) ions cannot be ruled out completely.19,30,34 The IR band at 1878 cm−1, recorded for the sample Fe-ZSM-5(HT), is accompanied by a weakly marked shoulder at 1890 cm−1. The weak shoulder at 1890 cm−1 is attributed to the mononitrosyl group located on Fe(II) ions generated as a result of reduction of Fe3+–OH groups accommodated on α-sites. On the other hand, it was suggested that this center is located in the neighborhood of Al Lewis sites, possibly formed as a result of framework dealumination.35 Some removal of Al(III) ions may be expected as a result of high temperature calcination.
The attribution of two weak bands at about 1840 and 1820 cm−1 was discussed in the literature.27,31,33,36 The weak band at 1820 cm−1 recorded as a result of NO contact with Fe-ZSM-5(LT) samples was noted when NO was adsorbed on the Fe/SiO2 catalyst and it was attributed to NO relative to Fe(II) ions formed on Fe2O3 particles.27 If NO was adsorbed on Fe-ZSM-5(HT) (Fig. 5), the band at 1820 cm−1 was not recorded. The band at 1840 cm−1, according to Mul et al.,33 as well as Joyner and Stockenhuber,36 has been assigned to nitrosyl groups located on isolated Fe(II) ions. According to Hadjiivanov et al.20,26 the weak bands at about 1840 and 1750 cm−1 should be attributed to Fe(II)(NO) species formed on isolated Fe(II) sites. Both bands were recorded on Fe-ZSM-5 calcined at 873 and 1173 K, which may indicate the presence of some amount of isolated Fe(II) species.
Interaction of NO with Fe-ZSM-5 at RT resulted also in the appearance of the bands at 1575 and 1625 cm−1. Prolongation of the contact time with NO at RT does not change the location of these bands, however, influences their intensities. Evacuation of Fe-ZSM-5 samples at RT results in a weak band at 1575 cm−1 and a distinct, strong band at 1640 cm−1 (Fig. 3 and 5). Hensen et al.37 analyzing the effects of NO adsorption on Fe-ZSM-5 zeolites suggest that Fe–O–Al iron species formed, thanks to partial dealumination (by thermal treatment or by steaming), are responsible for the formation of the bands at about 1640 cm−1 as a result of contact with NO. The higher intensity of the bands on Fe-ZSM-5(HT) samples confirms this interpretation, considering the more probable dealumination as a result of high thermal treatment. According to Pirngruber and Pieterse29 and also Lobree et al.28 the presence of the bands in the range of 1550–1650 cm−1 indicates the formation of bridging nitrate species strongly adsorbed on Fe(II) ions. This kind of bands was observed as a result of catalyst contact with a NO + O2 mixture.37 Considering that only oxygen free NO was introduced into a vacuum cuvette, oxygen may stem from NO to N2O transformation. The band of low intensity at 2224 cm−1 (after gas phase subtraction) indicates the presence of N2O in the system and may confirm the occurrence of the following reactions:
| 2NO → N2O + 1/2O2 |
| 3NO ↔ N2O + NO2 |
Interestingly, evacuation of the samples indicates a different stability of recorded species. Majority of the bands, originating from NO adsorption, disappears after short evacuation at RT, while the band at 1625 cm−1 is shifted to1640 cm−1 and becomes even stronger. High stability of nitrate species characterized with the band at about 1640 cm−1 was indicated in a number of papers.20,29,33,38 An increase in the intensity of the band attributed to NO2 species after mild evacuation or as a result of purging with helium has also been observed in the quoted papers. However, one should remember that traces of water may also influence the band at about 1640 cm−1. According to Pirngruber and Pieterse,29 two different forms of NO2, characterized with the bands in the region of 1500–1640 cm−1, may be indicated. The band at 1575 cm−1, recorded after contact of Fe-ZSM-5 with NO, may be treated as intermediate in the formation of gas-phase NO2. This band almost disappears after short evacuation. The following adsorption of gas NO2 on the sites released by desorption of nitrosyl groups cannot be ruled out. According to Mul et al.33 increase in the intensity of the bands assigned to nitrate species with simultaneous decrease of the intensity of the bands of adsorbed NO indicates that adsorption of NO2 species may occur on the same centers.
The analysis of IR spectra of adsorbed NO and UV-vis spectra recorded for Fe-ZSM-5 samples calcined at 873 and 1173 K indicates the presence of both isolated and dimeric38 iron complexes, larger oligomeric iron clusters and also small amount of Fe2O3 particles.
3.4 Catalysts activity
Fe-ZSM-5(LT) and Fe-ZSM-5(HT) catalyze the oxidative dehydrogenation of light paraffins in the presence of nitrous oxide as an oxidant in a wide range of temperature. Already at 623 K, about 10% of ethane was transformed to ethene with a selectivity of almost 90% and with CO2 as the only byproduct (Fig. 6A). Ethane conversion and CO2 contribution in products increase along with the increase in reaction temperature, while the selectivity to ethene diminishes gradually. Propane oxidative dehydrogenation is a more complicated process (Fig. 6B). Propene being a main product of the propane ODH reaction interacts with acidic centers and as a consequence the cracking reaction occurs with the formation of C2 and C1 hydrocarbons. Along with the increase in reaction temperature, propane conversion also increases, while the selectivity to propene diminishes. Contribution of the cracking product and selectivity to CO2 are increased. If ODH of butanes was performed over Fe-ZSM-5(LT), olefins made up less than 50% of reaction products, while cracking products predominated (Fig. 7).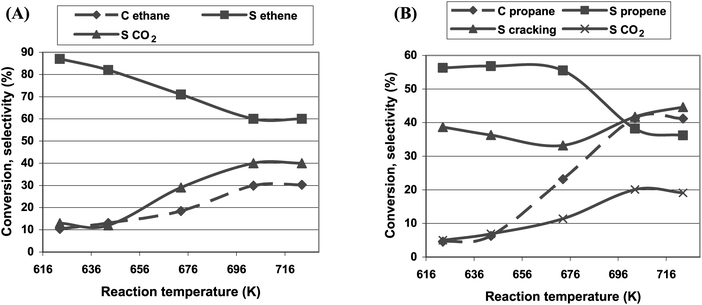 | ||
| Fig. 6 The effect of the reaction temperature on the initial activity of the Fe-ZSM-5(50)HT catalyst in ethane (A) and propane (B) ODH reaction. | ||
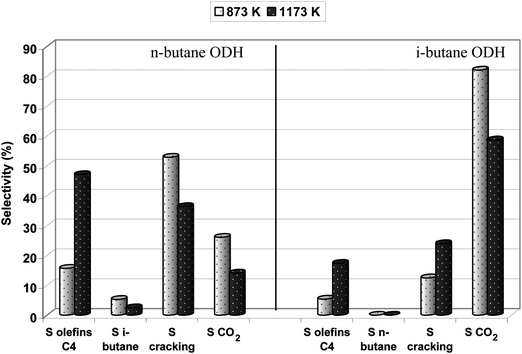 | ||
| Fig. 7 Effect of thermal treatment (873 K, 1173 K) of the Fe-ZSM-5(50) catalyst on its selectivity in the ODH reaction of n-butane and i-butane (reaction temperature: 673 K). | ||
Prolongation of reaction time resulted in some, but not significant, deactivation of catalysts (Fig. 8). If ethane was oxidized, the ethane conversion was almost stable for more than 3 h on stream (Fig. 8A). Selectivity to ethene showed a small, gradual increase at the expense of CO2 formation (Fig. 8B). Along with time on stream some increase in selectivity towards olefins was also recorded for propane and butanes (Fig. 8B) oxidation reactions, however, the selectivity towards cracking products was still high. The initial activity was recorded after 15 min on stream, when the temperature of the reaction was fully stabilized. It has been reported earlier39 that fast deactivation of iron modified zeolites ZSM-5, applied as catalysts for the propane ODH reaction, occurs during the very first minutes of time on stream (in the quoted paper the initial activity was measured after 2 min on stream) and after about 20 min., propane conversion was almost stable, which is consistent with our observation. Deactivation results from coke formation over acidic sites present in catalysts. The regeneration procedure of spent catalysts restores the initial activity utterly and the catalysts could be applied for a few times with full activity restoration (Fig. 9).
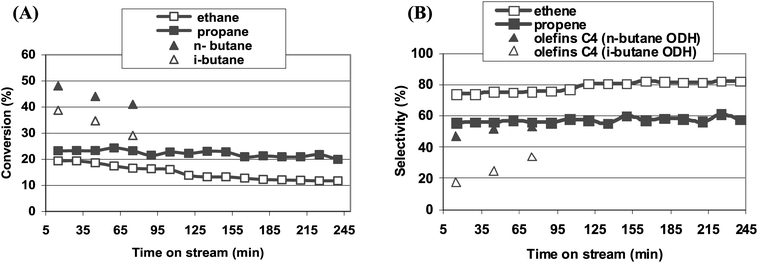 | ||
| Fig. 8 Change of the conversion of hydrocarbons (A) and selectivity towards olefins (B) on the Fe-ZSM-5(50)HT catalyst with time on stream (reaction temperature: 673 K). | ||
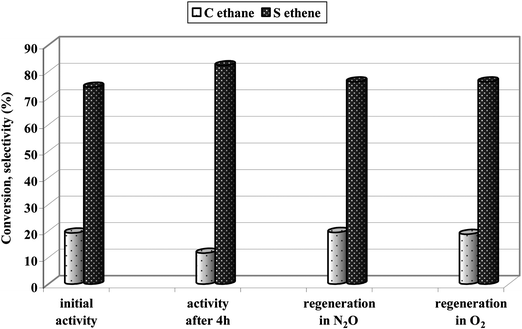 | ||
| Fig. 9 Comparison of the initial activity of Fe-ZSM-5(50)HT and activity after regeneration in N2O or O2 (4 h, 823 K). | ||
It is commonly accepted that acidic centers are responsible for coke formation. On the other hand, according to Derouane et al.40 and also to other authors,41,42 Brønsted acidic centers of a middle strength play an important role in hydrocarbons activation. One could expect that modification of surface acidity and elimination of strong Brønsted acidic sites should limit both selectivity towards cracking products and coke deposit formation and simultaneously enhance the olefin productivity. Modification of zeolite acidity could be performed by introduction of alkaline cations16,43 or by high thermal treatment of iron modified zeolite. It has been shown that introduction of alkaline cations into Fe-ZSM-5 catalysts, on one hand, enhances selectivity towards olefins but, on the other hand, reduces considerable hydrocarbons conversion.44 The beneficial effect of high thermal treatment of Fe-ZSM-5 on its catalytic activity for the BTOP reaction was reported by Sobolev et al.9 and also was indicated in our earlier paper.12 It has been shown that high thermal treatment (at 1173 K) of the Fe-ZSM-5 catalyst modifies iron ions distribution,29 influences the iron oxidation step8 and also diminishes the catalyst acidity.9 These results stimulated the application of high thermal treated Fe-ZSM-5 catalysts in the light paraffins ODH reaction. However, in contrast to results reported for BTOP, the effect of high thermal treatment of Fe-ZSM-5 catalysts on light paraffins susceptibility to oxidation appeared insignificant and hydrocarbons oxidative conversion over Fe-ZSM-5 calcined at 873 and 1173 K differed slightly (Fig. 10). These results suggest that iron complexes involved in BTOP and ODH reactions are not the same.
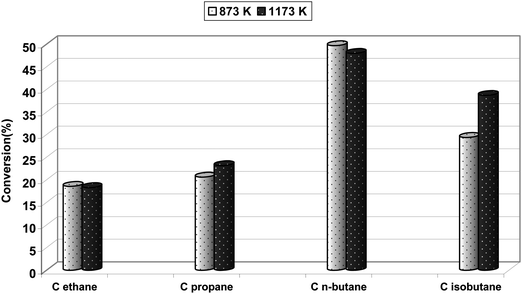 | ||
| Fig. 10 Effect of thermal treatment (873 K, 1173 K) of the Fe-ZSM-5(50) catalyst on its activity in the light paraffins ODH reaction (reaction temperature: 673 K). | ||
Even though thermal treatment of Fe-ZSM-5 catalysts does not affect hydrocarbons oxidative conversion, it influences significantly the selectivity to different oxidation products, which can be an effect of modification of zeolite acidity by high temperature calcination (Fig. 7 and 11). Increase in the temperature of calcinations (from 873 to 1173 K) resulted in a certain rise of selectivity to ethene and some decrease in COx formation in the ethane ODH reaction (Fig. 11). On the other hand, high temperature treatment influences significantly the selectivity to different products formed from propane and butanes oxidation. Both propane and butanes ODH reaction resulted in a complicated mixture of products being an effect of the presence of iron species of different structure showing an oxidative character and also acidic centers resulting in acid catalyzed transformation of hydrocarbons (Fig. 7 and 11). Fe-ZSM-5(LT) catalyzed the cracking process to a considerable extent (60 and 50% in the case of propane and n-butane respectively). Calcination at 1173 K resulted in lowering of Brønsted acidity (Table 1, Fig. 1) and as a consequence in a reduction of selectivity towards cracking products. Simultaneously, an increase in selectivity, as well as yield to respective olefins, has been noted. Considering the conversion of light paraffins, performed over Fe-ZSM-5 calcined at 873 and 1173 K (Fig. 10), and related selectivity to olefins (Fig. 7 and 11) one can conclude that the modification of the samples acidity by thermal treatment, on one hand, still provides sufficient number of middle acidic centers, indispensable for alkanes activation, while, on the other hand, reduces the cracking direction in the ODH reaction and increases selectivity to olefins.
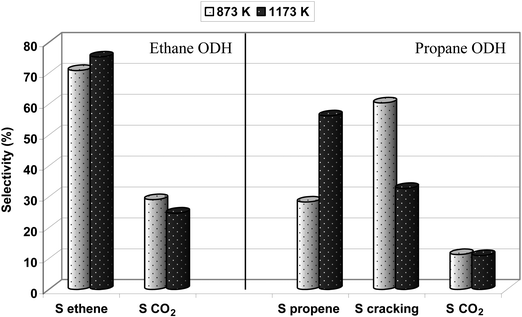 | ||
| Fig. 11 Effect of thermal treatment (873 K, 1173 K) of the Fe-ZSM-5(50) catalyst on its selectivity in the ODH reaction of ethane and propane (reaction temperature: 673 K). | ||
The oxidative dehydrogenation of hydrocarbons is influenced by chain length, which results from significant diversity of susceptibility to the oxidation reaction depending on the chemical structure. The highest oxidation activity was noted for n-butane (Fig. 10). High susceptibility to the oxidation reaction of n-butane, when compared to C2 and C3, results from the lower energy of the C–H bond of C4 hydrocarbons.45,46 According to quoted authors, the C–H bond in n-butane is characterized with energy equal to 401 kJ mol−1, while for the C–H bond in ethane, the energy of this bond was calculated to be 420 kJ mol−1. Elongation of the hydrocarbons chain length results in lowering of the dissociation energy of the C–H bond and as a consequence in lower energy of hydrogen removal. The activity for isobutane ODH was, however, clearly lower, which probably stems from geometric limitation, considering the size of the isobutane molecule. The diameter of isobutane is about 0.50 nm,47 while the diameter of ZSM-5 channels is about 0.55 nm.48 Penetration of isobutane inside the channels occurs very slowly and the reaction proceeds mainly on the outside wall of the zeolite catalyst and results mainly in CO2 formation. When the V/MCM-41 catalyst, with a pore diameter of about 2.7 nm, was used for alkanes C2–C4 oxidative dehydrogenation,49 no diffusion limitation was noted and alkanes oxidative conversion follows the order C2 < C3 < n-C4 ≤ iso-C4.
HT treatment of iron modified ZSM-5 catalysts results in partial reduction of iron(III), as well as influences the iron species distribution. The transformation of iron species can be concluded from UV-vis spectra and from IR spectra of adsorbed NO. According to earlier papers,21,22 UV-vis spectra allow the estimation of the presence of isolated and oligonuclear iron(III) species as well as the formation of oxide like clusters. On the grounds of discussion presented by Pérez-Ramírez et al.18,50 and also by Schwidder et al.21 one can accept that extinction coefficients for UV-vis bands describing the different iron species show the same order of magnitude. Therefore, the values of related areas may be treated as related to the contribution of different specific iron(III) species noted in the catalyst. Analyzing the results presented in quoted papers,18,50 one can deduce that the method of introduction of iron ions influences significantly the distribution of different iron species. When iron ions are introduced into the structure during the hydrothermal synthesis following their release by calcination or steaming, mainly isolated iron species are formed.50 Ionic exchange from aqueous solution, as well as sublimation method, results mainly in oligomeric species formation, while contribution of isolated iron species is lower. The exchange procedure brings about the formation of some amount of bigger oxide clusters.50 Estimation of contribution of specific iron species (Table 2), carried out on the grounds of areas related to specific UV-vis bands, indicates that HT treatment increases the contribution of isolated iron species, while the contribution of oligomeric complexes changes insignificantly. The relatively high attendance of bigger iron clusters in Fe-ZSM(LT) decreases with high temperature calcination. On the basis of an earlier XPS study7 we can believe that clusters generated as a result of ionic exchange from aqueous solution are located at least partly on the external surface of the zeolite matrix. High temperature calcination stimulates penetration of iron ions inside the channels. It results in the formation of isolated iron species and simultaneously reduced participation of large clusters. Considering that contribution of oligomeric iron species remains practically stable for both temperature of calcinations and simultaneously, HT treatment influences hydrocarbons conversion in the ODH reaction only slightly it seems highly probable that oligomeric iron complexes play an essential role in ODH of light alkanes. Kondratenko and Pérez-Ramírez51 analyzing the activity of Fe-silicalite comprising mainly isolated iron species and Fe-ZSM-5 with oligonuclear iron complexes have also suggested the oligonuclear iron species as favorable for ODH of propane.
It has been shown earlier6,8 that HT treatment of the Fe-ZSM-5 system results not only in redistribution of iron ions but also brings about partial reduction of iron(III) to iron(II), which was confirmed by IR spectra of adsorbed NO which could also be inferred from the lower intensity of UV-vis spectra of Fe-ZSM-5(HT) samples when compared to related Fe-ZSM-5(LT). A study on BTOP performed over the Fe-ZSM-5 system with N2O as an oxidant indicated the important role of iron(II) species generated in the applied catalyst. The autoreduction process of Fe(III) ions as a result of HT treatment has been reported by Dubkov et al.6 and was related to reduction of binuclear iron species operating as isolated iron α-sites. The susceptibility of isolated iron species to reduction was also suggested by Pirngruber et al.8 Considering the insignificant effect of HT treatment on alkanes conversion in the ODH reaction (Fig. 10) one can believe that the presence of iron(II) species stabilized in the Fe-ZSM-5 zeolite structure influences slightly the activity of this system for the studied reaction.
4. Conclusions
Iron exchanged ZSM-5 zeolites calcined at 873 and 1173 K were applied for hydrocarbons (C2–C4) oxidative dehydrogenation in the presence of N2O as an oxidant. Hydrocarbons conversion increased along with carbon chain elongation, while the selectivity towards olefins came down due to increasing amounts of side products formation. The temperature of thermal treatment (873 or 1173 K) of Fe-ZSM-5 catalysts influenced slightly the hydrocarbon conversion whereas the selectivity to olefins increased along with the increase in temperature of calcination. The increase in olefin selectivity can be explained with substantial modification of the sample acidity. Both the number and strength of Brønsted acidic centers diminished after high temperature treatment (1173 K), but they were not removed completely. Modification of surface acidity and reduction of strong acidic Brønsted sites substantially increases the selectivity towards olefins in the ODH reaction of C2–C4 alkanes.High temperature treatment of Fe-ZSM-5 samples stimulates partial reduction of Fe(III) to Fe(II), as well as influencing the distribution of iron species. Alteration of the iron species structure was confirmed by decrease in the UV-vis band intensity and the change in the relationship between bands characterizing the iron species in specific arrangement (isolated and dimeric, oligomeric species and oxide aggregates). The correlation of contribution of different iron species generated in Fe-ZSM-5 with oxidative activity in the ODH reaction indicates the important role of oligonuclear iron species in the reaction under study.
Generation of iron(II) complexes in Fe-ZSM-5(HT) samples was confirmed both by lowering in the intensity of UV-vis bands and from IR spectra of adsorbed NO. It seems, however, that the presence of previously reduced iron species does not affect the oxidative activity for the ODH reaction significantly.
References
- J. Pérez-Ramírez and A. Gallardo-Llamas, J. Catal., 2004, 223, 382 CrossRef.
- K. Nowińska, A. Wącław and A. Izbińska, Appl. Catal., A, 2003, 243, 225 CrossRef.
- J. Pérez-Ramírez and E. V. Kondratenko, Chem. Commun., 2003, 2152 RSC.
- G. I. Panov, CATTECH, 2000, 4, 18 CrossRef CAS.
- Q. Zhang, Q. Guoa, X. Wang, T. Shishido and Y. Wanga, J. Catal., 2006, 239, 105 CrossRef CAS.
- K. A. Dubkov, N. S. Ovanesyan, A. A. Shteinman, E. V. Starokon and G. I. Panov, J. Catal., 2002, 207, 341 CrossRef CAS.
- S. Kowalak, K. Nowińska, M. Święcicka, M. Sopa, A. Jankowska, G. Emig, E. Klemm and A. Reitzmann, in Proceedings of the 12th International Zeolite Conference, ed. M. M. J. Treacy, B. K. Marcus, M. E. Bisher and J. B. Higgins, 1999, p. 2847 Search PubMed.
- G. D. Pirngruber, P. K. Roy and R. Prins, J. Catal., 2007, 246, 147 CrossRef CAS.
- V. I. Sobolev, A. Dubkov, E. A. Paukshtis, L. V. Pirutko, M. A. Rodkin, A. S. Kharitonov and G. I. Panov, Appl. Catal., A, 1996, 141, 185 CrossRef CAS.
- E. V. Starokon, K. A. Dubkov, L. V. Pirutko and G. I. Panov, Top. Catal., 2003, 23, 137 CrossRef CAS.
- G. I. Panov, E. V. Starokon, L. V. Pirutko, E. A. Paukshtis and V. N. Parmon, J. Catal., 2008, 254, 110 CrossRef CAS.
- A. Wącław, K. Nowińska and W. Schwieger, Appl. Catal., A, 2004, 270, 151 CrossRef.
- K.-I. Aika and J. H. Lundsford, J. Phys. Chem., 1977, 81, 1393 CrossRef CAS.
- K.-I. Aika and J. H. Lundsford, J. Phys. Chem., 1978, 82, 1794 CrossRef CAS.
- Z. Song, A. Takahashi, N. Mitura and T. Fujitani, Catal. Lett., 2009, 131, 364 CrossRef CAS.
- N. Rahimi and R. Karimzadeh, Appl. Catal., A, 2011, 398, 1 CrossRef CAS.
- M. Schwidder, S. M. Kumar, U. Bentrup, J. Pérez-Ramírez, A. Brückner and W. Grünert, Microporous Mesoporous Mater., 2008, 111, 124 CrossRef CAS.
- J. Pérez-Ramírez, J. C. Groen, A. Brückner, M. S. Kumar, U. Bentrup, M. N. Debbagh and L. A. Villaescusa, J. Catal., 2005, 232, 318 CrossRef.
- A. Zecchina, M. Rivallan, G. Berlier, C. Lamberti and G. Ricchiardi, Phys. Chem. Chem. Phys., 2007, 9, 3483 RSC.
- M. Mihaylov, E. Ivanova, N. Drenchev and K. Hadjiivanov, J. Phys. Chem. C, 2010, 114, 1004 CAS.
- M. Schwidder, S. M. Kumar, K. Klementiev, M. M. Pohl, A. Brückner and W. Grünert, J. Catal., 2005, 231, 314 CrossRef CAS.
- S. M. Kumar, M. Schwidder, W. Grünert, U. Bentrup and A. Brückner, J. Catal., 2006, 239, 173 CrossRef.
- M. Schwidder, S. Heikensa, A. De Toni, S. Geisler, M. Berndt, A. Brückner and W. Grünert, J. Catal., 2008, 259, 96 CrossRef CAS.
- G. D. Pirngruber, P. K. Roy and R. Prins, Phys. Chem. Chem. Phys., 2006, 8, 3939 RSC.
- M. Rivallan, G. Ricchiardi, S. Bordiga and A. Zecchina, J. Catal., 2009, 264, 104 CrossRef CAS.
- K. Hadjiivanov, J. Saussey, J. L. Freysz and J. C. Lavalley, Catal. Lett., 1998, 52, 103 CrossRef CAS.
- M. Lezcano, V. I. Kovalchuk and J. L. d'Itri, Kinet. Catal., 2001, 42, 104 CrossRef CAS.
- L. J. Lobree, I.-C. Hwang, J. A. Reimer and A. T. Bell, J. Catal., 1999, 186, 242 CrossRef CAS.
- G. D. Pirngruber and J. A. Z. Pieterse, J. Catal., 2006, 237, 237 CrossRef CAS.
- M.-T. Nechita, G. Berlier, G. Ricchiardi, S. Bordiga and A. Zecchina, Catal. Lett., 2005, 103, 33 CrossRef CAS.
- V. Blain-Aube, O. Marie, J. Saussey, A. Plesniar, M. Daturi, N. Nguyen, C. Hamon, M. Mihaylov, E. Ivanova and K. Hadjiivanov, J. Phys. Chem. C, 2009, 113, 8387 Search PubMed.
- M. F. Fellach, J. Phys. Chem. C, 2011, 115, 1940 Search PubMed.
- G. Mul, J. Pérez-Ramírez, F. Kapteijn and J. A. Moulijn, Catal. Lett., 2002, 80, 129 CrossRef CAS.
- A. R. Beltramone and O. A. Anunziata, Catal. Lett., 2004, 92, 131 CrossRef CAS.
- P. K. Roy, R. Prins and G. D. Pirngruber, Appl. Catal., B, 2008, 80, 226 CrossRef CAS.
- R. Joyner and M. Stockenhuber, J. Phys. Chem. B, 1999, 103, 5963 CrossRef CAS.
- E. J. M. Hensen, Q. Zhu, R. J. Janssen, P. C. M. M. Magusin, P. J. Kooyman and R. A. van Santen, J. Catal., 2005, 233, 123 CrossRef CAS.
- H.-Y. Chen, El-M. El-Malki, X. Wang, R. A. van Santen and W. M. H. Sachtler, J. Mol. Catal. A: Chem., 2000, 162, 159 CrossRef CAS.
- J. Pérez-Ramírez and A. Gallardo-Llamas, Appl. Catal., A, 2005, 279, 117 CrossRef.
- E. G. Derouane, H. He, S. B. Derouane-Abd Hamid and I. Ivanova, Catal. Lett., 1999, 58, 1 CrossRef CAS.
- A. A. Teixeira-Neto, L. Marchese, G. Landi, L. Lisi and H. O. Pastore, Catal. Today, 2008, 133, 1 CrossRef.
- J. Bandiera, M. Dufaux and Y. B. Taârit, Appl. Catal., A, 1997, 148, 283 CrossRef CAS.
- J. S. Jung, J. W. Park and G. Seo, Appl. Catal., A, 2005, 288, 149 CrossRef CAS.
- J. Kowalska-Kuś, A. Held and K. Nowińska, Catal. Lett., 2010, 136, 199 CrossRef.
- E. Heracleous, M. Machli, A. A. Lemonidou and I. A. Vasalos, J. Mol. Catal. A: Chem., 2005, 232, 29 CrossRef CAS.
- M. A. Bañares, Catal. Today, 1999, 51, 319 CrossRef.
- K. S. W. Sing and R. T. Williams, Part. Part. Syst. Charact., 2004, 21, 71 CrossRef CAS.
- M. Park, S. C. Sin, C. L. Choi, D. H. Lee, W. T. Lim, S. Komarneni, M. C. Kim, J. Choi and N. H. Hoe, Microporous Mesoporous Mater., 2001, 50, 91 CrossRef CAS.
- O. Ovsister and E. V. Kondratenko, Catal. Today, 2009, 142, 138 CrossRef.
- J. Pérez-Ramírez, M. S. Kumar and A. Brückner, J. Catal., 2004, 223, 13 CrossRef.
- E. V. Kondratenko and J. Pérez-Ramírez, Appl. Catal., A, 2004, 267, 181 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2013 |
