Colonic catabolism of dietary phenolic and polyphenolic compounds from Concord grape juice
Angelique
Stalmach
a,
Christine A.
Edwards
a,
JoLynne D.
Wightman
b and
Alan
Crozier
*a
aSchool of Medicine, College of Medical, Veterinary and Life Sciences, University of Glasgow, Glasgow G12 888, UK. E-mail: alan.crozier@glasgow.ac.uk; Tel: +44-(0)141-330-4613
bWelch Foods Inc., 749 Middlesex Turnpike, Billerica, MA 01821, USA
First published on 16th August 2012
Abstract
After acute ingestion of 350 ml of Concord grape juice, containing 528 μmol of (poly)phenolic compounds, by healthy volunteers, a wide array of phase I and II metabolites were detected in the circulation and excreted in urine. Ingestion of the juice by ileostomists resulted in 40% of compounds being recovered intact in ileal effluent. The current study investigated the fate of these undigested (poly)phenolic compounds on reaching the colon. This was achieved through incubation of the juice using an in vitro model of colonic fermentation and through quantification of catabolites produced after colonic degradation and their subsequent absorption prior to urinary excretion by healthy subjects and ileostomy volunteers. A total of 16 aromatic and phenolic compounds derived from colonic metabolism of Concord grape juice (poly)phenolic compounds were identified by GC-MS in the faecal incubation samples. Thirteen urinary phenolic acids and aromatic compounds were excreted in significantly increased amounts after intake of the juice by healthy volunteers, whereas only two of these compounds were excreted in elevated amounts by ileostomists. The production of phenolic acids and aromatic compounds by colonic catabolism contributed to the bioavailability of Concord grape (poly)phenolic compounds to a much greater extent than phase I and II metabolites originating from absorption in the upper gastrointestinal tract. Catabolic pathways are proposed, highlighting the impact of colonic microbiota and subsequent phase II metabolism prior to excretion of phenolic compounds derived from (poly)phenolic compounds in Concord grape juice, which pass from the small to the large intestine.
1. Introduction
The role of the colonic microbiota in degrading dietary (poly)phenolic compounds is a topic of increasing interest, due to the potential health benefits occurring in situ or associated with catabolites absorbed into the circulatory system.1–8In vitro and animals studies have highlighted beneficial effects associated with intact undigested dietary (poly)phenolic compounds and degradation products of colonic catabolism. For instance, rats fed a high-fat diet supplemented with 0.5% polyphenols for 3 weeks exhibited a reduction of faecal secondary bile acids, a known risk factor of colon cancer.9 Similarly, 3′,4′-dihydroxyphenylacetic acid, a phenolic acid produced by colonic degradation of a number of (poly)phenolic compounds, has antiproliferative properties in vitro,10 while urolithins and pyrogallol have antiglycative properties and dihydrocaffeic acid, dihydroferulic acid and feruloylglycine are potentially protective against neurodegeneration.7A previous investigation showed that after acute intake of 350 ml of Concord grape juice containing 528 μmol of (poly)phenolic compounds by human volunteers, various quantities of the ingested phenolics were detected as metabolites in plasma and urine. Peak plasma concentrations ranged from 1 nM to 355 nM and urinary excretion varied from 0.3% to 24% of intake. When the same juice was consumed by ileostomists, 40% of the compounds initially ingested was recovered intact in the 0–24 h ileal effluent.11 This indicates that in healthy subjects these components would pass from the small to the large intestine where they would be subject to the action of the microbiota, which would result in their degradation to an array of simpler phenolic acids before absorption in the circulatory system via portal vein and ultimately excretion in urine.
The aim of the present study was to identify and quantify the phenolic acids and aromatic compounds resulting from colonic catabolism of Concord grape juice (poly)phenolics using an in vitro model of colonic fermentation with faecal samples. The in vivo fate of these catabolites following absorption in the colon was also investigated by GC-MS analysis of 24-urine collected after acute intake of 350 ml of the juice by both healthy subjects and ileostomy volunteers.
2. Results
2.1. Phenolic and polyphenolic compounds in Concord grape juice
Previous analysis of the Concord grape juice used in this study resulted in the identification and quantification of 60 flavonoids and related phenolic compounds which were present at an overall concentration of 1508 ± 31 μM.12 A total of 25 anthocyanins were detected, which were mono- and di-O-glucosides, O-acetylglucosides, O-p-coumaroyl-O-diglucosides and O-p-coumaroylglucosides of delphinidin, cyanidin, petunidin, peonidin and malvidin. The anthocyanins represented 46% of the total phenolic content of the juice (680 μM). Tartaric esters of hydroxycinnamic acids, namely trans-caftaric and trans-coutaric acids, and to a lesser extend trans-fertaric acid, accounted for 29% of the phenolic content, with a total concentration of 444 μM, of which 85% comprised of trans-caftaric acid. Free hydroxycinnamic acids at 8.4 μM contributed less than 1% of the total phenolic content. The other groups of (poly)phenolic compounds present in the juice, accounting for 24% of the total, comprised of monomeric and oligomeric units of (epi)catechin and (epi)gallocatechin (248 μM), flavonols (76 μM), gallic acid (51 μM) and trans-resveratrol (1.5 μM).122.2. In vitro faecal incubation with Concord grape juice
A total of 34 phenolic acids and aromatic compounds were identified either in the 0–24 h urine samples of healthy volunteers following a single intake of Concord grape juice, and/or in the faecal slurries following incubation of the juice with simulated colonic fermentation medium. The compounds were identified by GC-MS based on their co-chromatography with authentic standards, matching retention times, target and qualifier ions (m/z), the NIST library, or previous identifications (Table 1).| Compounds | R t (min) | Target ion (m/z) | Qualifier ions (m/z) | Identification | Location |
|---|---|---|---|---|---|
| a Based on the retention time and mass spectra of commercially available standards, from identification using the built-in NIST library or from previous published work. b FS, faecal slurry; U, urine; IS, internal standard. | |||||
| Benzoic acid | 5.85 | 105 | 179, 135, 77 | Standard | FS |
| Phenylacetic acid | 6.22 | 164 | 193, 91, 75, 73 | Standard | FS |
| Pyrocatechol | 6.37 | 254 | 239, 166, 151, 136, 73 | Standard | FS |
| Resorcinol | 6.92 | 239 | 254, 147, 133, 112, 73 | Standard | FS |
| 3-(Phenyl)propionic acid | 7.35 | 104 | 222, 207, 91, 75, 73 | Standard | FS |
| Phenoxyacetic acid | 7.49 | 224 | 165, 147, 135, 73 | Standard | U |
| Mandelic acid | 7.98 | 179 | 253, 147, 73 | Standard | U |
| Pyrogallol | 8.83 | 239 | 342, 211, 133, 73 | Standard | FS, U |
| 3-Hydroxybenzoic acid | 9.33 | 267 | 282, 223, 193, 73 | Standard | U |
| 3-(Phenyl)lactic acid | 9.54 | 193 | 147, 73 | Standard | FS |
| 3′-Hydroxyphenylacetic acid | 10.09 | 164 | 296, 281, 252, 147, 73 | Standard | U |
| 4-Hydroxybenzoic acid | 10.48 | 267 | 223, 193, 126, 73 | Standard | FS, U |
| Tartaric acid | 10.54 | 292 | 219, 147, 73 | Standard | FS, U |
| 4′-Hydroxyphenylacetic acid | 10.62 | 179 | 296, 281, 252, 164, 73 | Standard | FS, U |
| Phloroglucinol | 10.67 | 342 | 327, 268, 147, 73 | Standard | FS |
| 3-(3′-Hydroxyphenyl)propionic acid | 12.71 | 205 | 310, 192, 177, 73 | Standard | FS, U |
| 3-(4′-Hydroxyphenyl)propionic acid | 13.58 | 179 | 310, 192, 73 | Standard | FS |
| 3-Methoxy-4-hydroxybenzoic acid | 13.78 | 297 | 312, 282, 267, 253, 223, 126 | Standard | U |
| 3′-Methoxy-4′-hydroxyphenylacetic acid | 13.87 | 209 | 326, 311, 267, 179, 73 | Standard | U |
| 3-(4′-Hydroxyphenyl)mandelic acid | 14.17 | 267 | 341, 207, 147, 73 | Standard | U |
| 3,4-Dihydrobenzoic acid | 15.39 | 193 | 370, 367, 355, 311, 281, 73 | Standard | FS |
| 3′,4′-Dihydroxyphenylacetic acid | 15.63 | 179 | 384, 267, 73 | Standard | FS, U |
| Hippuric acid | 16.21 | 206 | 236, 105, 73 | Standard | U |
| 3-(3′-Hydroxyphenyl)hydracrylic acid | 16.54 | 267 | 398, 147, 73 | NIST | U |
| 3′-Methoxy-4′-hydroxymandelic acid | 17.50 | 297 | 371, 267, 194, 147, 73 | Standard | U |
| 3-(4′-Hydroxyphenyl)lactic acid | 18.16 | 179 | 308, 293, 267, 147, 73 | Standard | FS, U |
| p-Coumaric acid | 19.68 | 219 | 308, 293, 281 | Standard | FS |
| 3-(3′,4′-Dihydroxyphenyl)propionic acid | 19.89 | 179 | 398, 266, 73 | Standard | FS, U |
| Gallic acid | 20.60 | 281 | 443, 179, 73 | Standard | FS |
| Ferulic acid | 26.31 | 338 | 323, 307, 293, 249 | Standard | FS |
| Caffeic acid | 28.61 | 219 | 396, 306, 191, 73 | Standard | FS |
| 3′-Hydroxyhippuric acid | 29.03 | 294 | 281, 207, 193, 73 | Standard | U |
| 5-(3′,4′-Dihydroxyphenyl)-γ-valerolactone | 29.67 | 352 | 267, 179 | Roowi et al.20 | FS, U |
| 2′,4′,5′-Trimethoxycinnamic acid | 31.69 | 279 | 310, 295, 236, 221, 163, 73 | Standard | IS |
| 4′-Hydroxyhippuric acid | 32.58 | 193 | 294, 73 | Standard | U |
Following incubation of Concord grape juice with faecal slurries obtained from three healthy donors, a total of 22 phenolic acids and aromatic compounds were detected (Table 2). Certain phenolic compounds, such as benzoic acid, phenylacetic acid, 3-(phenyl)propionic acid and 4′-hydroxyphenylacetic acid, were present in incubates both with and without the grape juice, in concentrations that were not significantly different, suggesting that they were not derived principally from the catabolism of grape juice (poly)phenolics. Other compounds, such as resorcinol and 3-(phenyl)lactic acid, were present in greater concentrations in the incubated juice samples than the control samples, but this difference was not statistically significant across all time points due to high inter-individual variations (% CV 60–70%) over the 48 h incubation period (see Table 2).
| Compounds | Treatment | 0 h | 2 h | 6 h | 24 h | 48 h |
|---|---|---|---|---|---|---|
| a Expressed as mean value in μM ± SE (n = 3), quantified using the available standard, unless otherwise stated. b Values followed by an asterisk denote a statistically significant difference in the levels produced between the control and the juice (paired t-test, p < 0.1). c Expressed as 3-(3′,4′-dihydroxyphenyl)propionic acid equivalents; nd, not detected. | ||||||
| 5-(3′,4′-Dihydroxyphenyl)-γ-valerolactonec | — | nd | nd | nd | nd | nd |
| Juice | nd | 0.6 ± 0.2 | 2.7 ± 0.4 | 2.8 ± 0.2 | 2.6 ± 0.3 | |
| 3-(Phenyl)propionic acid | — | 11 ± 5 | 10 ± 5 | 11 ± 5 | 37 ± 20 | 71 ± 39 |
| Juice | 8.9 ± 3.7 | 15 ± 5 | 46 ± 18 | 46 ± 20* | 48 ± 19 | |
| 3-(3′-Hydroxyphenyl)propionic acid | — | nd | 0.1 ± 0.1 | 0.4 ± 0.3 | 1.0 ± 0.1 | 0.9 ± 0.0 |
| Juice | nd | 16 ± 8 | 61 ± 7* | 62 ± 7* | 60 ± 2* | |
| 3-(4′-Hydroxyphenyl)propionic acid | — | nd | nd | 1.0 ± 0.1 | 3.2 ± 1.1 | 0.9 ± 0.2 |
| Juice | 1.4 ± 0.2 | 30 ± 14 | 59 ± 17* | 53 ± 17* | 55 ± 18* | |
| 3-(3′,4′-Dihydroxyphenyl)propionic acid | — | nd | nd | nd | nd | nd |
| Juice | 0.5 ± 0.3 | 11 ± 3 | 1.7 ± 0.4 | 1.7 ± 0.9 | 2.1 ± 1.3 | |
| 3-(Phenyl)lactic acid | — | nd | 4.1 ± 0.7 | 28 ± 4 | 18 ± 7 | 10 ± 6 |
| Juice | nd | 11 ± 2 | 46 ± 9* | 78 ± 29 | 94 ± 37 | |
| 3-(4′-Hydroxyphenyl)lactic acid | — | 0.0 ± 0.0 | 0.7 ± 0.2 | 3.9 ± 0.5 | 3.7 ± 1.8 | 1.7 ± 1.3 |
| Juice | 0.2 ± 0.1 | 2.4 ± 0.1* | 9.1 ± 1.8* | 15 ± 3* | 20 ± 6* | |
| Phenylacetic acid | — | 38 ± 21 | 45 ± 23 | 59 ± 28 | 140 ± 65 | 303 ± 183 |
| Juice | 32 ± 14 | 49 ± 25 | 96 ± 41 | 86 ± 38 | 108 ± 44 | |
| 4′-Hydroxyphenylacetic acid | — | 0.8 ± 0.5 | 2.2 ± 0.2 | 3.6 ± 0.6 | 11 ± 6 | 21 ± 15 |
| Juice | 0.7 ± 0.4 | 2.8 ± 0.3 | 4.9 ± 0.9 | 4.4 ± 1.5 | 4.5 ± 0.9 | |
| 3′,4′-Dihydroxyphenylacetic acid | — | nd | nd | nd | nd | nd |
| Juice | nd | 3.3 ± 0.2 | 4.8 ± 0.9 | 3.7 ± 0.3 | 4.0 ± 0.6 | |
| Benzoic acid | — | 2.2 ± 0.8 | 1.9 ± 0.3 | 2.3 ± 0.4 | 3.0 ± 0.7 | 3.3 ± 0.7 |
| Juice | 1.5 ± 0.3 | 1.9 ± 0.4 | 4.4 ± 1.4 | 4.2 ± 0.8 | 4.6 ± 0.9* | |
| 4-Hydroxybenzoic acid | — | 0.5 ± 0.3 | 1.0 ± 0.1 | 0.9 ± 0.2 | 0.7 ± 0.1 | 1.0 ± 0.1 |
| Juice | 1.2 ± 0.1 | 1.7 ± 0.1* | 2.4 ± 0.4* | 2.1 ± 0.2* | 2.0 ± 0.3* | |
| 3,4-Dihydrobenzoic acid | — | nd | nd | nd | nd | nd |
| Juice | 0.9 ± 0.9 | 2.8 ± 0.2 | 3.7 ± 0.5 | 2.4 ± 0.2 | 2.9 ± 0.8 | |
| Gallic acid | — | nd | nd | nd | nd | nd |
| Juice | 8.4 ± 1.1 | 14 ± 3 | 14 ± 9 | 11 ± 4 | 7.2 ± 4.7 | |
| Resorcinol | — | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.1 |
| Juice | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.1 | 0.2 ± 0.0* | 0.2 ± 0.0 | |
| Catechol | — | nd | nd | nd | 0.2 ± 0.2 | 0.4 ± 0.4 |
| Juice | nd | nd | 3.8 ± 1.6 | 3.9 ± 0.9* | 4.0 ± 0.8* | |
| Pyrogallol | — | nd | nd | nd | nd | nd |
| Juice | 0.2 ± 0.1 | 0.9 ± 0.5 | 4.3 ± 0.8 | 6.0 ± 2.9 | 5.5 ± 2.4 | |
| Phloroglucinol | — | nd | nd | nd | nd | nd |
| Juice | nd | 1.1 ± 0.5 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.3 ± 0.2 | |
| p-Coumaric acid | — | nd | nd | nd | nd | nd |
| Juice | 9.4 ± 2.1 | 7.6 ± 3.9 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.2 ± 0.2 | |
| Caffeic acid | — | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| Juice | 5.7 ± 1.0* | 6.3 ± 3.5 | 0.1 ± 0.1 | 0.0 ± 0.0 | 0.1 ± 0.1 | |
| Ferulic acid | — | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.0 |
| Juice | 1.4 ± 0.1* | 0.6 ± 0.4 | 0.0 ± 0.0 | 0.1 ± 0.1 | 0.1 ± 0.1 | |
| Tartaric acid | — | nd | nd | nd | nd | nd |
| Juice | 35 ± 31 | 35 ± 25 | 5.2 ± 5.2 | 4.4 ± 4.4 | 7.2 ± 7.2 | |
| Total | — | 53 ± 26 | 66 ± 29 | 114 ± 36 | 222 ± 83 | 415 ± 229 |
| Juice | 107 ± 37 | 215 ± 36* | 382 ± 76* | 404 ± 76* | 454 ± 86 | |
The majority of the phenolic acids and aromatic compounds produced from the in vitro catabolism of the (poly)phenolic compounds in the juice by the colonic microbiota were in the 6–48 h incubations. The presence of tartaric acid (35 μM), p-coumaric acid (9.4 μM), caffeic acid (5.7 μM) and ferulic acid (1.4 μM) was at highest concentrations in the baseline samples, which were collected less than 5 min after incubation of the juice with the faecal slurries, suggest that the colonic microbiota are capable of very rapidly hydrolysing the tartaric acid moiety from trans-caftaric, trans-coutaric and trans-fertaric acids, thus releasing the three free hydroxycinnamates (Fig. 1, Table 2). The released hydroxycinnamates remained in the medium for up to 2 h after incubation, and their concentrations gradually declined to reach subsequent concentrations of less than 0.1 μM after 6 h.
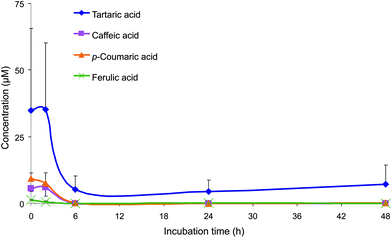 | ||
| Fig. 1 Mean concentrations (corrected by subtracting concentrations from the control samples) of tartaric acid, p-coumaric acid, ferulic acid and caffeic acid detected in the faecal incubations of Concord grape juice with colonic microbiota from three healthy donors. Error bars represent the standard error of the mean (n = 3). | ||
4-Hydroxybenzoic acid was detected in both control and treated samples, but a significant increase in the 2 h-incubation and subsequent periods was observed, indicating the hydroxybenzoic acid was a catabolite formed from the degradation of the juice (poly)phenolic compounds (Fig. 2 and Table 2). 3,4-Dihydroxybenzoic acid was detected only in the slurries incubated with the juice, and reached a peak concentration in the medium of 3.7 μM after 6 h (Table 2). Similarly, gallic acid (3,4,5-trihydroxybenzoic acid) was present only in the samples incubated with the juice, and the concentration increased slowly from baseline (8.4 μM) to 14 μM after 2–6 h and decreased slowly to 7.2 μM after 48 h (Fig. 2 and Table 2). Gallic acid was present in the juice at a concentration of 51 μM,12 equivalent to an initial 0 h concentration of 10.2 μM when the juice was incubated with faecal slurries. The 14 μM maximum concentration suggests that gallic acid was also produced from the degradation of other (poly)phenolic compounds present in the juice, most probably from the delphinidin-based anthocyanins.13,14
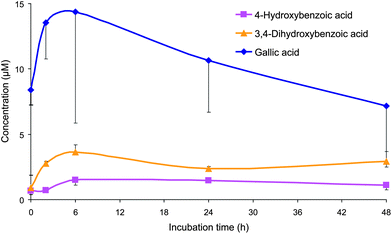 | ||
| Fig. 2 Mean concentrations (corrected by subtracting concentrations from the control samples) of 4-hydroxybenzoic acid, 3,4-dihydroxybenzoic acid and gallic acid (2,3,4-dihydroxybenzoic acid) detected in the faecal incubations of Concord grape juice with colonic microbiota from three healthy donors. Error bars represent the standard error of the mean (n = 3). | ||
3-(3′,4′-Dihydroxyphenyl)propionic acid (aka dihydrocaffeic acid) was detected only in the samples incubated with the juice, with a peak concentration of 11 μM, observed after 2 h, followed by a decrease to reach a constant concentration of ∼2 μM from 6–48 h. In contrast, concentrations of 3-(3′-hydroxyphenyl)propionic acid and 3-(4′-hydroxyphenyl)propionic acid increased rapidly in the medium over 6 h, and reached a plateau of ∼60 and 50 μM, respectively, over the remaining 24–48 h period (Fig. 3 and Table 2).
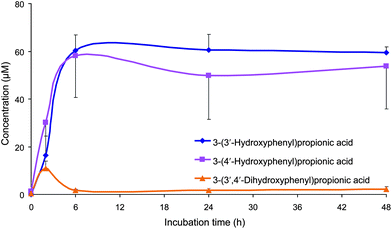 | ||
| Fig. 3 Mean concentrations (corrected by subtracting concentrations from the control samples) of 3-(3′-hydroxyphenyl)propionic acid 3-(4′-hydroxyphenyl)propionic acid and 3-(3′,4′-dihydroxyphenyl)propionic acid detected in the faecal incubations of Concord grape juice with colonic microbiota from three healthy donors. Error bars represent the standard error of the mean (n = 3). | ||
Other low molecular weight dihydroxy- and trihydroxybenzene derivatives, namely catechol (1,2-dihydroxybenzene), phloroglucinol (1,3,5-trihydroxybenzoic acid) and pyrogallol (1,2,3-trihydroxybenzene), were also produced when the Concord grape juice was incubated with colonic microbiota. The peak concentration of phloroglucinol (1.1 μM) was reached after a 2 h incubation and decreased afterwards and remained constant at 0.3–0.4 μM until the end of the 48 h incubation period (Fig. 4 and Table 2). Pyrogallol and catechol reached maximum concentrations of 6.0 and 4.0 μM, respectively, after 24 h and 48 h, and in both instances the levels changed relatively little after 6 h (Fig. 4 and Table 2).
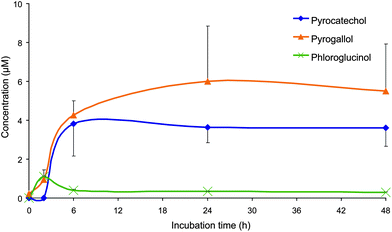 | ||
| Fig. 4 Mean concentrations (corrected by subtracting concentrations from the control samples of pyrogallol (1,2,3-trihydroxybenzene), catechol (1,2-dihydroxybenzene) and phloroglucinol (1,3,5-trihydroxybenzene) detected in the faecal incubations of Concord grape juice with colonic microbiota from three healthy donors. Error bars represent the standard error of the mean (n = 3). | ||
3′,4′-Dihydroxyphenylacetic acid (3.3–4.8 μM), 3-(4′-hydroxyphenyl)lactic acid (2.4–20 μM) and 5-(3′,4′-dihydroxyphenyl)-γ-valerolactone (0.6–2.8 μM) were also detected in the faecal suspensions following incubation with the juice (Table 2).
From the 22 phenolic acids and aromatic compounds with increasing levels, in some cases transient, detected using the in vitro model of colonic fermentation, 16 were highlighted as potential catabolites derived from microbial degradation of Concord grape juice (poly)phenolic compounds.
2.3. Urinary excretion of phenolic acids and aromatic compounds after acute intake of Concord grape juice
Urine samples were collected from groups of healthy volunteers (n = 8) and ileostomists (n = 4) who were without a functional colon but otherwise healthy. Urine was collected for 24 h periods both before and after acute intake of 350 ml of Concord grape juice containing 528 μmol of a mixture of (poly)phenolic compounds. The phenolic acids and aromatic compounds of the urine samples were analysed quantitatively by GC-MS, representing urinary excretion of these compounds in their unconjugated form.A total of 21 phenolic acids and aromatic compounds were identified in the urine of the healthy volunteers, 15 of which were also detected in the urine of the ileostomists (Tables 3 and 4). The baseline level of urinary phenolic compounds, that is urine collected over a 24 h period prior to supplementation, was 4-fold lower with ileostomists (69 μmol) than the healthy volunteers (298 μmol). After consuming the juice the production of phenolic acids increased to 161 μmol in the ileostomy group and to 541 μmol in the individuals with a functional colon. The majority of the compounds detected in the urine of the ileostomists did not increase significantly after intake of the juice. Six compounds, namely 3-hydroxybenzoic acid, 3′-hydroxyhippuric acid, 3-(3′-hydroxyphenyl)propionic acid, 3-(3′,4′-dihydroxyphenyl)propionic acid, 3-(3′-hydroxyphenyl)hydracrylic acid and 5-(3′,4′-dihydroxyphenyl)-γ-valerolactone, were detected in urine from healthy volunteers but not in the ileostomists urine (Table 4). The only compounds excreted in significantly greater amounts by the ileostomy group after juice intake were 4′-hydroxyhippuric acid and tartaric acid, accounting for 1.8% and 12% of the total (poly)phenolics ingested (Table 4). The total increase of phenolic acids excreted accounted for 17% of the amount initially ingested by the ileostomists. In contrast, the levels of 13 phenolic acids and aromatic compounds increased significantly from baseline levels after intake of the juice by the healthy subjects (Table 3), with amounts excreted (corrected for baseline excretion) ranging from 0.1 ± 0.0 μmol (mandelic acid) to 109 ± 31 μmol (hippuric acid). The total amount excreted in 24 h following intake of 528 μmol of (poly)phenolic compounds in the grape juice was 243 ± 36 μmol (corrected for baseline excretion), corresponding to 46 ± 7% of intake. The only compound excreted in similar amounts by both groups of volunteers after consumption of the juice was tartaric acid, resulting from the hydrolysis of trans-caftaric, trans-coutaric and trans-fertaric acids (58 ± 14 and 65 ± 14 μmol excreted in the healthy and ileostomy groups, respectively).
| Compounds | Baseline | Juice | % Intakec |
|---|---|---|---|
| a Data expressed as mean values in μmol ± SE (n = 8), quantified using the available standard, unless otherwise stated. b Values followed by an asterisk denote a statistically significant increase in the amount excreted 0–24 h after juice consumption compared to excretion over the 24 h period before ingestion (paired t-test, p < 0.05). c Significant increases expressed as a percentage of (poly)phenolic compounds ingested in 350 ml of Concord grape juice (528 μmol), corrected for baseline excretion. d Expressed as 3-(3′,4′-dihydroxyphenyl)propionic acid equivalents. e Expressed as mandelic acid equivalents; nd, not detected. | |||
| 5-(3′,4′-Dihydroxyphenyl)-γ-valerolactoned | nd | 0.2 ± 0.1* | 0.04 ± 0.01 |
| 3-(3′-Hydroxyphenyl)propionic acid | 0.03 ± 0.02 | 0.5 ± 0.3* | 0.09 ± 0.05 |
| 3-(3′,4′-Dihydroxyphenyl)propionic acid | 0.09 ± 0.07 | 0.9 ± 0.2* | 0.2 ± 0.0 |
| 3-(4′-Hydroxyphenyl)lactic acid | 0.8 ± 0.3 | 0.8 ± 0.2 | — |
| 3-(3′-Hydroxyphenyl)hydracrylic acide | 1.4 ± 0.4 | 5.7 ± 0.8* | 0.8 ± 02 |
| Mandelic acid | 0.2 ± 0.0 | 0.3 ± 0.0* | 0.01 ± 0.00 |
| 4′-Hydroxymandelic acid | 2.9 ± 0.4 | 2.8 ± 0.3 | — |
| 3′-Methoxy-4′-hydroxymandelic acid | 3.3 ± 0.4 | 3.6 ± 0.3 | — |
| Phenoxyacetic acid | 1.2 ± 0.9 | 1.5 ± 1.3 | — |
| 3′-Hydroxyphenylacetic acid | 2.0 ± 0.4 | 2.7 ± 0.6 | — |
| 4′-Hydroxyphenylacetic acid | 18 ± 5 | 16.0 ± 2.1 | — |
| 3′,4′-Dihydroxyphenylacetic acid | 0.4 ± 0.1 | 0.9 ± 0.1* | 0.09 ± 0.02 |
| 3′-Methoxy-4′-hydroxyphenylacetic acid | 2.4 ± 0.3 | 3.4 ± 0.3* | 0.2 ± 0.1 |
| 3-Hydroxybenzoic acid | 0.03 ± 0.01 | 0.04 ± 0.01 | — |
| 4-Hydroxybenzoic acid | 1.0 ± 0.1 | 1.6 ± 0.2 | — |
| Pyrogallol | 2.2 ± 0.4 | 32 ± 6* | 5.7 ± 1.2 |
| 3-Methoxy-4-hydroxybenzoic acid | 0.2 ± 0.0 | 0.5 ± 0.1* | 0.06 ± 0.02 |
| Hippuric acid | 243 ± 38 | 352 ± 45* | 21 ± 6 |
| 3′-Hydroxyhippuric acid | 1.8 ± 0.5 | 9.2 ± 1.5* | 1.4 ± 0.3 |
| 4′-Hydroxyhippuric acid | 15 ± 6 | 47 ± 16* | 6.0 ± 1.9 |
| Tartaric acid | 1.8 ± 1.7 | 59 ± 14* | 11 ± 3 |
| Total | 298 ± 42 | 541 ± 55* | 46 ± 7 |
| Compounds | Baseline | 0–24 h | % Intakec |
|---|---|---|---|
| a Data expressed as mean values in μmol ± SE (n = 4), quantified using the available standard, unless otherwise stated. b Values followed by an asterisk denote a statistically significant increase in the amount excreted 0–24 h after juice consumption compared to excretion over the 24 h period before ingestion (paired t-test, p < 0.05). c Significant increases expressed as a percentage of (poly)phenolic compounds ingested in 350 ml of Concord grape juice (528 μmol), corrected for baseline excretion; nd, not detected. | |||
| 5-(3′,4′-Dihydroxyphenyl)-γ-valerolactone | nd | nd | — |
| 3-(3′-Hydroxyphenyl)propionic acid | nd | nd | — |
| 3-(3′,4′-Dihydroxyphenyl)propionic acid | nd | nd | — |
| 3-(4′-Hydroxyphenyl)lactic acid | 0.6 ± 0.3 | 1.2 ± 0.4 | — |
| 3-(3′-Hydroxyphenyl)hydracrylic acid | nd | nd | — |
| Mandelic acid | 0.09 ± 0.03 | 0.2 ± 0.0 | — |
| 4′-Hydroxymandelic acid | 1.7 ± 1 | 2.1 ± 0.7 | — |
| 3′-Methoxy-4′-hydroxymandelic acid | 1.8 ± 0.8 | 2.5 ± 0.7 | — |
| Phenoxyacetic acid | 0.1 ± 0.0 | 0.1 ± 0.1 | — |
| 3′-Hydroxyphenylacetic acid | 0.9 ± 0.5 | 1.0 ± 0.4 | — |
| 4′-Hydroxyphenylacetic acid | 8.5 ± 3.2 | 11 ± 2 | — |
| 3′,4′-Dihydroxyphenylacetic acid | 0.2 ± 0.1 | 0.4 ± 0.1 | — |
| 3′-Methoxy-4′-hydroxyphenylacetic acid | 1.2 ± 0.6 | 1.6 ± 0.3 | — |
| 3-Hydroxybenzoic acid | nd | nd | |
| 4-Hydroxybenzoic acid | 0.01 ± 0.01 | 0.04 ± 0.04 | — |
| 3-Methoxy-4-hydroxybenzoic acid | 0.05 ± 0.02 | 0.1 ± 0.0 | — |
| Pyrogallol | 2.6 ± 1.0 | 4.3 ± 2.1 | — |
| Hippuric acid | 46 ± 19 | 56 ± 17 | — |
| 3′-Hydroxyhippuric acid | nd | nd | — |
| 4′-Hydroxyhippuric acid | 3.5 ± 2.3 | 13 ± 2.9* | 1.8 ± 0.3 |
| Tartaric acid | 2.1 ± 1.7 | 67 ± 14* | 12 ± 3 |
| Total | 69 ± 27 | 161 ± 35* | 17 ± 6 |
3. Discussion
This study investigated the colonic catabolism of (poly)phenolic compounds in Concord grape juice, using an in vitro model of colonic fermentation and measurements of the urinary catabolites excreted by healthy individuals and ileostomists following acute intake of the juice.Following in vitro incubation of the juice with faecal slurries, 16 phenolic acids and aromatic compounds were identified and attributed to the degradation of (poly)phenolic compounds by the colonic microbiota (Table 2). The presence of tartaric acid, caffeic acid, p-coumaric acid and ferulic acid in the faecal incubations, as early as 5 min following addition of the juice in the slurries, suggests the microbiota have the capacity to rapidly hydrolyse the tartaric acid moiety from the hydroxycinnamate esters, with subsequent release of free hydroxycinnamates. The presence of tartaric acid excreted in similar amounts in the 24 h urine samples of healthy and ileostomy volunteers suggests, however, that in vivo metabolism of the tartaric esters of hydroxycinnamates occurs in the proximal rather than the distal gastro-intestinal tract. From the 155 μmol of tartaric esters ingested, 59–67 μmol of tartaric acid were excreted in urine, accounting for 37–42% of intake. This is in agreement with Stalmach et al.11 who found that 67% of the tartaric acid esters of hydroxycinnamates ingested from the juice were recovered intact in ileal effluent, leaving a potential 33% for absorption and metabolism in the upper gastro-intestinal tract.
In the faecal suspensions, caffeic acid, p-coumaric acid and ferulic acid released from the tartarate esters reached their maximum concentrations between 0 and 2 h after incubation of the juice, and were virtually absent from the medium after this period (Fig. 1). In parallel, increasing levels of 3-(3′-hydroxyphenyl)propionic acid, 3-(4′-hydroxyphenyl)propionic acid and 3-(3′,4′-dihydroxyphenyl)propionic acid, resulting from hydrogenation of the hydroxycinnamate side chain, were produced from baseline to reach peak concentrations after 6 h (Fig. 3). The presence of 3-(3′-hydroxyphenyl)propionic acid following incubation of trans-caftaric acid with faecal suspensions has previously been reported.15 The production of 3-(4′-hydroxyphenyl)propionic acid and 3-(3′,4′-dihydroxyphenyl)propionic acid by faecal slurries (Table 2) indicates the capacity of the microflora to carry out side chain hydrogenation and dehydroxylation of the phenyl ring of hydroxycinnamates.16–19
Excretion of tartaric acid in urine, without further conjugation, is likely to be related to the upper gastrointestinal cleavage of hydroxycinnamate tartaric acid esters. The presence of free and sulfated caffeic, p-coumaric and ferulic acids in the plasma of volunteers after acute intake of a single serving of the Concord grape juice reported by Stalmach et al.11 also supports this deconjugation step, and 0.5–1.8 h time for these compounds to reach peak plasma levels is indicative of metabolism in the proximal gastrointestinal tract. However, the presence of 3-(3′,4′-dihydroxyphenyl)propionic acid and 3-(3′-hydroxyphenyl)propionic acid in the urine of healthy but not ileostomy volunteers (Tables 3 and 4) suggests that part of the caffeic acid released in the small intestine is further metabolised in the colon. The previous detection of peak plasma levels of sulfated hydroxycinnamates 3.9–6.0 h after consumption of the juice,11 is also consistent with proximal metabolism.
Apart from a high content in hydroxycinnamate tartarate esters, Concord grape juice also contains substantial amounts of monomeric and oligomeric flavan-3-ols.12 Previous studies investigating the incubation of human faecal microbiota with (+)-catechin and (−)-epicatechin reported the production of 3-(3′,4′-dihydroxyphenyl)propionic acid, 3-(3′-hydroxyphenyl)propionic acid and 3-(phenyl)propionic acid,20,21 all of which were detected in the present study. Appeldoorn et al.22 proposed that the colonic catabolism of procyanidin dimers involved the production of 3′,4′-dihydroxyphenylacetic acid, yielding 3′-hydroxyphenylacetic acid from the degradation of the upper unit, whereas the lower unit resulted in the formation of 5-(3′,4′-dihydroxyphenyl)-γ-valerolactone and ultimately 3-(3′-hydroxyphenyl)propionic acid. In the 24 h urine samples collected after Concord grape juice consumption by healthy subjects free 5-(3′,4′-dihydroxyphenyl)-γ-valerolactone, 3-(3′,4′-dihydroxyphenyl)propionic acid (aka dihydrocaffeic acid), 3-(3′-hydroxyphenyl)propionic acid and 3′,4′-dihydroxyphenylacetic acid were found increasingly excreted after ingestion of the juice, together with 3′-methoxy-4′-hydroxyphenylacetic acid and the previously reported sulfated metabolites of 3-(3′,4′-dihydroxyphenyl)propionic acid and 3-(3′-methoxy-4′-hydroxyphenyl)propionic acid,11 suggesting methylation and sulfation of these catabolites either in the wall of the colon or post-absorption in the liver and possibly also kidneys.
As previously reported, the formation of 3,4-dihydroxybenzoic acid in the faecal incubations is likely to derive from the degradation of the B-ring of anthocyanidins, cyanidin derivatives in particular,23,24 or from the reduction of 3′,4′-dihydroxyphenylacetic acid produced from breakdown of quercetin derivatives.25–27 The dihydroxybenzoic acid was not detected in urine after juice intake, possibly as a consequence of its in vivo conversion to 3-methoxy-4-hydroxybenzoic acid (Table 3). Although increased levels of 3′,4′-dihydroxyphenylacetic acid was detected in both faecal slurries and the urine of healthy subjects, its 3′-methoxy derivative was detected only in urine, indicative of methylation in vivo in a similar manner to its benzoic acid counterpart.
Gallic acid was present in the juice, but it can also be produced from the B-ring of anthocyanidins, most probably from delphinidin derivatives.28 Gallic acid is degraded into pyrogallol, catechol and resorcinol and phloroglucinol can be derived from any flavonoid with hydroxyl groups at the 5- and 7 positions on the A-ring.23 In the current study, only pyrogallol was excreted in 24 h urine samples of healthy volunteers (30 ± 6 μmol accounting for 5.7 ± 1.2% of intake) after intake of Concord grape juice (Table 3).
Although not produced in significant amounts compared to the control faecal samples, benzoic acid has been identified as the end product of microbial degradation in a number of faecal incubations with various (poly)phenolic compounds.13,29 Benzoic acid and hydroxybenzoic acids are typically absorbed from the colon, and subsequently glycinated in the liver,18 forming hippuric acid derivatives, which in the current study were detected in urine in significantly greater amounts after intake of the mixture of (poly)phenolic compounds in the Concord grape juice.
Another compound detected in urine after grape juice intake, but not produced in vitro by colonic fermentations, was 3-(3′-hydroxyphenyl)hydracrylic acid. This compound was also identified in the urine of volunteers after they consumed a single intake of green tea, but did not accumulate when (−)-epicatechin was incubated with faecal microbiota.20 The production of 3-(3′-hydroxyphenyl)hydracrylic acid has been proposed to derive from the hydroxylation of 3-(3′-hydroxyphenyl)propionic acid in the liver.20
Incubation of Concord grape juice with faecal slurries resulted in the production of 3-(4′-hydroxyphenyl)lactic acid with a peak concentration of 20 ± 6 μM being attained after a 48 h incubation (Table 2). However, the phenylacetic acid was not excreted in significant amounts in urine of healthy subjects compared to baseline. This compound has been reported to be excreted in the urine of rats following ingestion of pelargonidin-3-O-glucoside, but was not been detected in subsequent studies with other types of anthocyanins or derivatives.30 3-(Phenyl)lactic acid has been reported to be produced from lactic acid bacteria, such as Lactobacillus plantarum,31 and production appeared to be stimulated by the presence of (poly)phenolic compounds in the faecal medium (Table 2). The levels of 3-(phenyl)lactic acid produced demonstrated a high inter-individual variation, with % CV values of ca. 60–70%. Such high inter-individual variations have been reported previously with catabolites produced from human faecal microbiota,32 as well as those formed in the colon in human feeding studies.25
From the current results obtained from the in vitro incubation of Concord grape juice with human faecal microbiota, urinary excretion of the phenolic acids and aromatic compounds derived from in vivo colonic catabolism, and previous work on the identification of catabolites produced from faecal incubations of individual (poly)phenolic compounds, we propose the tentative pathways for the colonic degradation of Concord grape juice (poly)phenolics, which are produced in situ, absorbed in the proximal colon and further metabolised in the liver prior to being excreted in urine (Fig. 5). The catabolic processes carried out by the colonic bacteria include hydrolysis, hydroxylation, hydrogenation, decarboxylation and dehydroxylation. This, in turn, results in increased bioavailability of ingested (poly)phenolic compounds, with urinary excretion of colonic catabolites corresponding to 46% of intake (Table 3) compared to a mere 3.6% of phase I and phase II metabolites resulting from an upper gastro-intestinal absorption being excreted in urine.11
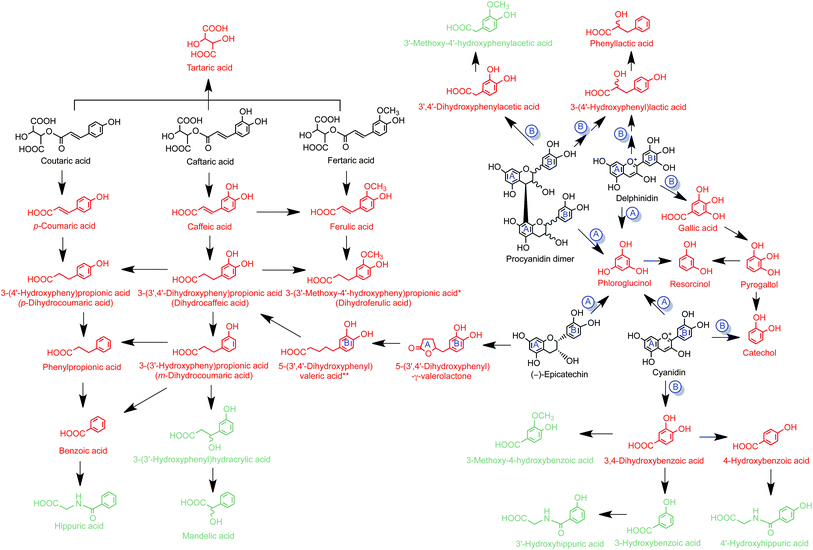 | ||
| Fig. 5 Proposed pathways for catabolism associated with the consumption of Concord grape juice anthocyanins, hydroxycinnamate esters, (−)-epicatechin and procyanidin dimers. Structures in black are parent compounds, those in red are their colonic microbiota catabolites and green structures are compounds detected in urine but not produced by faecal incubations, indicating they are likely to be formed by post absorption phase II metabolism in the wall of the colon and/or the liver prior to excretion. Possible flavonoid A and B ring-origin of catabolites are indicated. *Dihydroferulic acid has been detected in urine after consumption of Concord grape juice.11 **5-(3′,4′-dihydroxyphenyl)valeric acid is a potential intermediate that did not accumulate in detectable quantities. | ||
The potential health benefits associated with the production of the wide array of catabolites produced in the colon deserve detailed further investigation in view of the reported anti-inflammatory,3 antiglycative, neuroprotective7 and antiproliferative effects10 of these phenolic acids, as well as their impact on the EphA2–EphrinA1 system in human prostate cancer cells.8
4. Experimental
4.1. Grape juice and chemicals
The drink under investigation was 100% Concord grape juice supplied by Welch Foods Inc. (Concord, MA, USA). Standards of benzoic acid, 3-hydroxybenzoic acid, 4-hydroxybenzoic acid, 3,4-dihydroxybenzoic acid, 3-methoxy-4-hydroxybenzoic acid, pyrogallol, pyrocatechol, resorcinol, phloroglucinol, gallic acid, 3-(phenyl)propionic acid, phenylacetic acid, phenoxyacetic acid, 3′-hydroxyphenylacetic acid, 4′-hydroxyphenylacetic acid, 3′,4′-dihydroxyphenylacetic acid, 3′-methoxy-4′-hydroxyphenylacetic acid, 3-(3′-hydroxyphenyl)propionic acid, 3-(4′-hydroxyphenyl)propionic acid, 3-(3′,4′-dihydroxyphenyl)propionic acid, mandelic acid, 4′-hydroxymandelic acid, 3′-methoxy-4′-hydroxymandelic acid, 3-(phenyl)lactic acid, 3-(4′-hydroxyphenyl)lactic acid, 2′,4′,5′-trimethoxycinnamic acid, p-coumaric acid, ferulic acid, caffeic acid, hippuric acid, 3′-hydroxyhippuric acid, 4′-hydroxyhippuric acid, and tartaric acid, were purchased from Sigma-Aldrich Co Ltd (Poole, Dorset, UK) or AASC Ltd (Southampton, Hampshire, UK).Reagents used to prepare the buffer, macromineral, micromineral and reducing solutions for the in vitro fermentations (ammonium carbonate, sodium bicarbonate, disodium phosphate, potassium phosphate, magnesium sulfate, calcium chloride, manganese chloride, cobalt chloride, iron chloride, cysteine hydrochloride, sodium hydroxide and sodium sulfide) were purchased from Sigma-Aldrich Co Ltd (Poole, Dorset, UK) and Fisher Scientific Ltd (Loughborough, Leicestershire, UK).
Ethyl acetate and dichloromethane were purchased from Rathburn Chemicals Ltd (Walkerburn, Peeblesshire, UK). Anhydrous hexane, tryptone and resazurin were purchased from Sigma-Aldrich and hydrochloride and N,O-bis[trimethylsilyl]trifluoroacetamide + 10% trimethylchlorosilane (BSTFA + 10% TMCS) were obtained from Fisher Scientific Ltd.
4.2. Urine and faecal sample collection
Urine was collected for 24 h following acute intake of 350 ml of Concord grape juice by a group of healthy (n = 8) and a group of ileostomy volunteers (n = 4), as described previously.11 The study protocol was approved by the University of Glasgow Medical Faculty Ethics Committee (FM 00207 and FM 05308) and the subjects gave written informed consent. Prior to starting the study, volunteers followed a diet low in (poly)phenolic compounds for 2 days with urine being collected over the second 24 h period. Volunteers were subsequently fed 350 ml of juice after an overnight fast, and aliquots of total urine collected for 24 h, were stored at −80 °C for further analysis. Volunteers continued to follow a low (poly)phenolic diet during the 24 h urine collection period.Faecal samples were collected from three healthy donors, who were 22–34 years of age with no history of gastrointestinal conditions, no food allergies, not taking any vitamins or supplements and having taken no antibiotics for a year prior to the study. For 60 h prior to providing a faecal sample, volunteers followed a low (poly)phenolic diet consisting in the avoidance of fruits and vegetables, tea, coffee, wine and wholemeal foods. On the morning of the study, volunteers provided a stool sample in a fasted state, collected in a tub containing an AnaeroGen sachet (Oxoid, Basingstoke, Hampshire, UK) to generate anaerobic conditions. Samples were processed within 1 h of passage.
4.3. In vitro fermentation of Concord grape juice with faecal slurries
The fermentation medium used to produce the slurries was prepared as described previously.33 Briefly, 2.25 g of tryptone was mixed in 450 ml of distilled water and 112.5 μl of micromineral solution (13.2 g CaCl2·2H2O, 10 g of MnCl2·4H2O, 1 g of CoCl2·6H2O, 8 g of FeCl3·6H2O completed to 100 ml with distilled water). To this, 225 ml of buffer solution was added (2 g of NH4HCO3, 17.5 g of NaHCO3 completed to 500 ml with distilled water) as well as 225 ml of macromineral solution (2.85 g of Na2HPO4, 3.1 g of KH2PO4, 0.3 g of MgSO4 and completed to 500 ml with distilled water) and 1125 μl of 1% (w/v) resazurin solution. The medium was adjusted to pH 7 using 6 M HCl, boiled and allowed to cool under oxygen-free nitrogen (OFN). To 42 ml of the fermentation medium, 2 ml of a reducing solution was added (312.5 mg of cysteine hydrochloride, 2 ml of 1 M NaOH, 312.5 mg of Na2S·9H2O and completed to 50 ml with distilled water). The mixture was boiled, flushed with OFN until reaching anaerobic conditions and placed in 100 ml-fermentation bottles.Fresh faeces from each volunteer were mixed with 0.07 M of sodium phosphate buffer (pH 7) to make a 32% (w/v) faecal slurry, prior to being strained through a nylon mesh. For each volunteer, 5 ml of the strained slurry were added to the fermentation medium and reducing solution mixture. To this, 2 ml of concentrated Concord grape juice (10 ml of juice freeze-dried and reconstituted in 2 ml of distilled water) were added to each fermentation bottle. 2 ml of distilled water containing 0.5 g of glucose was added to faecal samples as a control. Bottles were flushed with OFN before incubation in a shaking water bath at 37 °C in darkness. Two ml aliquots were taken at 0 h, 2 h, 6 h, 24 h and 48 h, and stored at −80 °C prior to analysis by GC-MS.
4.4. Extraction and derivatization of phenolic acids and aromatic compounds in faecal slurries and urine samples
The method used to extract phenolic acids and aromatic compounds in urine and faecal slurries that had not been subjected to prior glucuronidase/sulphatase treatment was adapted from Grün et al.34 To 500 μl of urine or 900 μl of faecal slurries (in duplicate), 65 μl of 1 M HCl was added as well as 30 μl of 2′,4′,5′-trimethoxycinnamic acid (1 mg ml−1) used as the internal standard. Samples were extracted three times by adding 1.5 ml of ethyl acetate, followed each time by 30 s of vortexing and centrifugation for at 4000g for 10 min at 4 °C. Supernatants were pooled, placed in an amber glass vial and dried under a flow of nitrogen heated at 35 °C until dry. Dichloromethane (200 μl) was added to each vial, and further dried under nitrogen after which samples were derivatised by addition of 50 μl of BSTFA + 10% TMCS, and each vial were flushed with nitrogen prior to capping. The extracts were incubated at 70 °C for 4 h, with vortexing every 30 min to facilitate silylation. At the end of the incubation period, 350 μl of anhydrous hexane was injected into each vial, vortexed and left to cool to room temperature prior to 1 μl being analysed by GC-MS.4.5. GC-MS analysis of derivatized faecal slurries and urine samples
Derivatised phenolic acids and aromatic compounds in urine samples and faecal slurries, were analysed using a Trace DSQ single quadrupole GC-MS, equipped with an AI300 autosampler (Thermo Finnigan Ltd, Hempstead, Hertfordshire, UK) using a modification of previously used procedures.20 Samples were injected in the split mode with a 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. The injector temperature was maintained at 220 °C. The mass spectrometer was used in the positive ionization mode with the ion source and transfer line set at 180 °C and 310 °C, respectively. Separations were carried out on a fused silica capillary column (30 m × 0.25 mm i.d.) coated with cross-linked 5% phenylmethylsiloxane (film thickness 0.25 μm) (Phenomenex, Macclesfield, Cheshire, UK). Helium was the carrier gas with a flow rate of 1.2 ml min−1. The column temperature was initially set at 40 °C and raised to 160 °C at 20 °C min−1, 200 °C at 1.5 °C min−1 and 250 °C at 10 °C min−1 to a final temperature of 300 °C at 40 °C min−1, held for 5 min. Data acquisition was performed in full scan mode (m/z 50–470) with ionization energy of 70 eV, and analysis was carried out using Xcalibur software version 2.0 (Thermo Fisher Scientific UK, Hempstead, Hertfordshire, UK). Phenolic acids were identified according to the mass spectra and retention times obtained from authentic standards analysed under identical conditions. When standards were not commercially available, identification was achieved through the integrated NIST mass spectral library 2008 (Scientific Instruments Services Inc., Ringoes, NJ, USA), with a confidence of 70% or above. Calibration curves of the ratio between the target ion (m/z) of the standard compound of interest and the target ion of the internal standard (m/z 279) were computed, with concentrations ranging from 3–40 mg ml−1 (r2 > 0.95). Values for phenolic acids in the faecal slurries were expressed in μM as mean values ± SE (n = 3). Values for phenolic acids quantified in urine samples were expressed as mean values ± SE (n = 8 for healthy volunteers and n = 4 for ileostomy volunteers) in μmol.
1 ratio. The injector temperature was maintained at 220 °C. The mass spectrometer was used in the positive ionization mode with the ion source and transfer line set at 180 °C and 310 °C, respectively. Separations were carried out on a fused silica capillary column (30 m × 0.25 mm i.d.) coated with cross-linked 5% phenylmethylsiloxane (film thickness 0.25 μm) (Phenomenex, Macclesfield, Cheshire, UK). Helium was the carrier gas with a flow rate of 1.2 ml min−1. The column temperature was initially set at 40 °C and raised to 160 °C at 20 °C min−1, 200 °C at 1.5 °C min−1 and 250 °C at 10 °C min−1 to a final temperature of 300 °C at 40 °C min−1, held for 5 min. Data acquisition was performed in full scan mode (m/z 50–470) with ionization energy of 70 eV, and analysis was carried out using Xcalibur software version 2.0 (Thermo Fisher Scientific UK, Hempstead, Hertfordshire, UK). Phenolic acids were identified according to the mass spectra and retention times obtained from authentic standards analysed under identical conditions. When standards were not commercially available, identification was achieved through the integrated NIST mass spectral library 2008 (Scientific Instruments Services Inc., Ringoes, NJ, USA), with a confidence of 70% or above. Calibration curves of the ratio between the target ion (m/z) of the standard compound of interest and the target ion of the internal standard (m/z 279) were computed, with concentrations ranging from 3–40 mg ml−1 (r2 > 0.95). Values for phenolic acids in the faecal slurries were expressed in μM as mean values ± SE (n = 3). Values for phenolic acids quantified in urine samples were expressed as mean values ± SE (n = 8 for healthy volunteers and n = 4 for ileostomy volunteers) in μmol.
4.6. Statistical analysis
Statistical analysis of the data was performed using Minitab version 15 (Minitab Ltd, Coventry, West Midlands, UK). A paired t-test was used to compare the concentrations of individual phenolic acids from faecal incubations with the Concord grape juice vs. incubation with glucose alone. Comparisons of the amounts of phenolic acids excreted for 24 h in urine before and after acute intake of the juice were performed using a paired t-test (in groups of healthy and ileostomy volunteers). Statistical significance was set at p < 0.05.5. Conclusions
In healthy subjects with a functional colon, 40% of ingested (poly)phenolic compounds in Concord grape juice pass from the small to the large intestine.11 The current study investigated the fate of these undigested compounds on reaching the colon by (i) incubation of the juice using an in vitro model of colonic fermentation and (ii) through quantification of catabolites produced after colonic degradation and their subsequent absorption prior to urinary excretion by healthy subjects and ileostomy volunteers after the ingestion of Concord grape juice. A total of 16 phenolic acids and aromatic compounds derived from colonic metabolism of Concord grape juice (poly)phenolic compounds were identified in the faecal incubation samples. In urine samples, 13 phenolic acids and aromatic compounds were excreted in significantly increased amounts after intake of the juice by healthy volunteers, whereas only two of these compounds were excreted in elevated amounts by ileostomists. The production of phenolic acids and aromatic compounds by colonic catabolism contributes to the bioavailability of Concord grape (poly)phenolic compounds to a much greater extent than phase I and II metabolites originating from absorption in the upper gastrointestinal tract.11 Catabolic pathways are proposed, highlighting the impact of colonic microbiota and subsequent phase II metabolism prior to excretion of phenolic acids and other aromatic compounds derived from Concord grape juice (poly)phenolics that pass from the small to the large intestine.Acknowledgements
The research project was funded by Welch Foods Inc. The authors would like to thank the Welch Foods Inc. who funded the project and the volunteers who provided faecal samples and participated in the feeding studies.References
- A. Crozier, I. B. Jaganath and M. N. Clifford, Nat. Prod. Rep., 2009, 26, 1001–1043 RSC.
- C. D. Davis and J. A. Milner, J. Nutr. Biochem., 2009, 20, 743–752 CrossRef CAS.
- M. Larrosa, C. Luceri, E. Vivoli, C. Pagiuca, M. Lodovici, G. Moneti and P. Dolara, Mol. Nutr. Food Res., 2009, 53, 1044–1054 CrossRef CAS.
- D. Del Rio, L. G. Costa, M. E. J. Lean and A. Crozier, Nutr., Metab. Cardiovasc. Dis., 2010, 20, 1–6 Search PubMed.
- J. M. Laparra and Y. Sanz, Pharmacol. Res., 2010, 61, 219–225 CrossRef CAS.
- M. Monagas, M. Urpi-Sarda, F. Sánchez-Patán, R. Llorach, C. Gómez-Cordovés, C. Andres-Lacueva and B. Bartolomé, Food Funct., 2010, 1, 233–253 RSC.
- E. Verzelloni, C. Pellacani, D. Tagliazucchi, S. Tagliaferri, L. Calani, L. G. Costa, F. Brighenti, G. Borges, A. Crozier, A. Conte and D. Del Rio, Mol. Nutr. Food Res., 2011, 55, S35–S43 CrossRef CAS.
- M. Tognolini, C. Giorgio, I. H. Mohamed, E. Barocelli, L. Calani, E. Reynaud, O. Dangles, G. Borges, A. Crozier, F. Brighenti and D. Del Rio, J. Agric. Food Chem., 2012 DOI:10.1021/jf205305m.
- Y. Han, T. Haraguchi, S. Iwanaga, H. Tomotake, Y. Okazaki, S. Mineo, A. Moriyama, J. Inoue and N. Kato, J. Agric. Food Chem., 2009, 57, 8587–8590 CrossRef CAS.
- K. Gao, A. Xu, C. Krul, K. Venema, Y. Liu, Y. Niu, J. Lu, L. Bensoussan, N. P. Seeram, D. Heber and S. M. Henning, J. Nutr., 2006, 136, 52–57 CAS.
- A. Stalmach, C. A. Edwards, J. D. Wightman and A. Crozier, Mol. Nutr. Food Res., 2012, 56, 497–509 CrossRef CAS.
- A. Stalmach, C. A. Edwards, J. D. Wightman and A. Crozier, J. Agric. Food Chem., 2011, 59, 9512–9522 CrossRef CAS.
- A.-M. Aura, Phytochem. Rev., 2008, 7, 407–429 CrossRef CAS.
- A.-M. Aura, in Flavonoids and Related Compounds: Bioavaialability and Function, ed. J. P. E. Spencer and A. Crozier, CRC Press, Baco Raton, FL, USA, pp. 201–231 Search PubMed.
- M.-P. Gonthier, M.-A. Verny, C. Besson, C. Rémésy and A. Scalbert, J. Nutr., 2003, 133, 1853–1859 CAS.
- M. A. Peppercorn and P. Goldman, J. Bacteriol., 1971, 108, 996–1000 CAS.
- A. Chesson, G. J. Provan, W. R. Russell, L. Scobbie, A. J. Richardson and C. Stewart, J. Sci. Food Agric., 1999, 79, 373–378 CrossRef CAS.
- L. Poquet, M. N. Clifford and G. Williamson, Drug Metab. Dispos., 2008, 36, 190–197 CAS.
- L. Poquet, M. N. Clifford and G. Williamson, Biochem. Pharmacol., 2008, 75, 1218–1229 CrossRef CAS.
- S. Roowi, A. Stalmach, W. Mullen, M. E. J. Lean, C. A. Edwards and A. Crozier, J. Agric. Food Chem., 2010, 58, 1296–1304 CrossRef CAS.
- A.-M. Aura, I. Mattila, T. Seppänen-Laakso, J. Miettinen, K. M. Oksman-Caldentey and M. Orešič, Phytochem. Lett., 2008, 1, 8–22 Search PubMed.
- M. M. Appeldoorn, J. P. Vincken, A.-M. Aura, P. C. H. Hollman and H. Gruppen, J. Agric. Food Chem., 2009, 57, 1084–1092 CrossRef CAS.
- M. V. Selma, J. C. Espin and F. A. Tomas-Barberan, J. Agric. Food Chem., 2009, 57, 6485–6501 CrossRef CAS.
- A.-M. Aura, P. Martin-Lopez, K. A. O'Leary, G. Williamson, K. M. Oksman-Caldentey, K. Poutanen and C. Santos-Buelga, Eur. J. Nutr., 2005, 44, 133–142 CrossRef CAS.
- A. R. Rechner, M. A. Smith, G. Kuhnle, G. R. Gibson, E. S. Debnam, S. K. Srai, K. P. Moore and C. A. Rice-Evans, Free Radical Biol. Med., 2004, 36, 212–225 CrossRef CAS.
- I. B. Jaganath, W. Mullen, C. A. Edwards and A. Crozier, Free Radical Biol. Med., 2006, 40, 1035–1046 CAS.
- A.-M. Aura, K. A. O'Leary, G. Williamson, M. Ojala, M. Bailey, R. Puupponen-Pimia, A. M. Nuutila, K. M. Oksman-Caldentey and K. Poutanen, J. Agric. Food Chem., 2002, 50, 1725–1730 CrossRef CAS.
- M. Hidalgo, M. J. Oruna-Concha, S. Kolida, G. E. Walton, S. Kallithraka, J. P. Spencer and S. de Pascual-Teresa, J. Agric. Food Chem., 2012, 60, 3882–3890 CrossRef CAS.
- M. P. Gonthier, C. Rémésy, A. Scalbert, V. Cheynier, J. M. Souquet, K. Poutanen and A.-M. Aura, Biomed. Pharmacother., 2006, 60, 536–540 CrossRef CAS.
- M. N. Clifford, Anthocyanins – nature, occurrence and dietary burden, J. Sci. Food Agric., 2000, 80, 1063–1072 CrossRef CAS.
- E. Armaforte, S. Carri, G. Ferri and M. F. Caboni, J. Chromatogr., A, 2006, 1131, 281–284 CrossRef CAS.
- G. Gross, D. M. Jacobs, S. Peters, S. Possemiers, J. van Duynhoven, E. E. Vaughan and T. van de Wiele, J. Agric. Food Chem., 2010, 58, 10236–10246 CrossRef CAS.
- I. B. Jaganath, W. Mullen, M. E. J. Lean, C. A. Edwards and A. Crozier, Free Radical Biol. Med., 2009, 47, 1180–1189 CrossRef CAS.
- C. H. Grün, F. A. van Dorsten, D. M. Jacobs, M. Le Belleguic, E. J. J. van Velzen, M. O. Bingham, H. G. Janssen and J. P. van Duynhoven, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2008, 871, 212–219 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2013 |
