Tetraalkylammonium oleate and linoleate based ionic liquids: promising extractants for metal salts†
Dries
Parmentier
ab,
Sybrand J.
Metz
a and
Maaike C.
Kroon
*b
aWetsus, Center of excellence for sustainable water technology, Agora 1, 8900 CC Leeuwarden, The Netherlands
bDepartment of Chemical Engineering and Chemistry, Eindhoven University of Technology, Den Dolech 2, 5612 AZ Eindhoven, The Netherlands. E-mail: m.c.kroon@tue.nl; Fax: +31-40-2463966; Tel: +31-40-2475289
First published on 1st November 2012
Abstract
In this paper new extractants, that have the potential to be sustainably regenerated, are proposed for metal removal from aqueous phases. These extractants are a novel class of ionic liquids (ILs) with unsaturated fatty acids as anions (oleate and linoleate). The advantages of using these ILs are their simple synthesis, their sustainability (fatty acids anions are renewable), their biocompatibility and their non-toxicity. This makes these ILs “simpler and greener” compared to other solvents used for metal extraction. The newly synthesized ILs were evaluated for extraction of Li, Na, K, Mn(II), Fe(II), and Zn(II) chlorides from aqueous solutions. No or negligible extraction efficiencies were observed for the alkali metal salts, but excellent extraction (>99%) efficiencies were obtained for the period IV transition metal salts.
Introduction
The chemical and mining industries have produced numerous aqueous streams polluted with valuable (heavy) metal salts.1 As a result, groundwater is often polluted with these salts. Economically and technically, it is challenging to remove these impurities from the aqueous phase. Furthermore, also in the industrial metal extraction process it is difficult to selectively separate the different metal salts.2 Conventional methods to selectively recover and purify these metals use either volatile organic solvents, non-reusable absorbents, or harmful chemicals, such as sulfuric acid and cyanex, or a combination of these.3–5Liquid–liquid extraction is one of the most important techniques for metal ion separation. As well as in the laboratory as on an industrial scale it offers several advantages over competing techniques.2 Operation in a continuous mode, employment of relatively simple equipment and employment of only small quantities of the reagent are only some of these advantages. The biggest disadvantage of classical liquid–liquid extraction is the use of flammable, volatile or toxic water-immiscible organic solvents. One of the possibilities to improve the sustainability of this process is the use of ionic liquids (ILs) as water-immiscible extractants.6,7
ILs are defined as organic salts formed by the combination of bulky organic cations with a wide variety of anions that are liquid at room temperature.8,9 Whereas classical solvents are made up of neutral molecules, ILs are made up of large ions, which are held together by electrostatic interactions. Because of these interactions, the properties of ILs are considerably different from those of molecular liquids. They have a wide liquid range, negligible vapor pressure at room temperature, and high chemical and electrochemical stability.10
Recently, the deployment of ILs as extracting agents for the recovery of valuable metals from waste water has become an interesting alternative for waste water plants.11 This has been done in two different ways. The first option is based on using special extractants (e.g. crown ethers, cyanex) to extract the metals from the aqueous phase into the IL phase.12–15 However, in these systems also ion exchange occurs: the uptake of the metal ion is accompanied by dissolution of the cation from the ionic liquid into the aqueous phase.16 A preferred option is to use task-specific ionic liquids (TSILs) as extractants which are not prone to ion exchange.17–21 These ILs have an ion that has a functionality that favours interaction with metal ions. In the literature, the focus on TSILs for metal extraction is prominent for ILs with carboxylic acid, phosphinic acid, thiourea, urea, thioether, and thiol functionalities in their ions.
In this work, TSILs with unsaturated fatty acids were for the first time synthesized and evaluated for their salt extraction capabilities. Metal extraction functionalities for these anions are their double bond(s) together with their carboxylic group. From earlier research it was already known that the unsaturated fatty acids allowed removal of heavy metals, ammonium, and rare earth metals in a wide range of concentrations.22 On the other hand, unsaturated fatty acid soaps in combination with organic solvents and NaCl were also already used to extract europium via a microemulsion system.23 The disadvantage was that both processes were based on cation exchange, in which sodium or calcium ions were replaced by other metal ions. This made chemical regeneration necessary and this was done by adding sulfuric acid to form a metal sulfate, followed by the addition of sodium/calcium hydroxide to form the fatty acid soap again. In these articles, it was mentioned that the carboxylic group22,23 hydrophobisation effect22 and microemulsion formation23 have a large influence on the metal extraction using unsaturated fatty acids. On the other hand it has already been shown that double bonds show good d-orbital metal interactions.24 These interactions are even strengthened because, in addition to σ-bond interaction, there is also π-back donation.24
By working with fatty acid based TSILs we want to obtain full metal extraction so that the water phase is not polluted with less harmful ions and we want to avoid the use of chemical regeneration. The use of these anions, which are natural and biodegradable, for IL synthesis also brings us a step closer in making ILs more environmentally acceptable for industrial applications.25 Highly hydrophobic cations, with very low water solubility, were chosen as counter ions to minimize ion exchange and maximize metal extraction.
Experimental
Materials
The ILs used in this research were synthesized with reagents purchased from Sigma-Aldrich. Linoleic and oleic acids had a purity of ≥99%. Tetraoctylammonium chloride and methyltrioctylammonium chloride had a purity of 98% and 97%, respectively. Sodium hydroxide and ethanol (70%) were obtained from VWR-Prolabo®, and Toluene chromosolv® for HPLC (99%) was purchased from Sigma-Aldrich. MilliQ water (18.2 MΩ cm) used throughout the synthesis was obtained by a Millipore Milli-Q® biocel, which used a Q-grade® column. All chemicals were used as received, without any additional purification step. For composing the standard metal solutions, anhydrous LiCl (99%) was obtained from Sigma-Aldrich. NaCl (>99.9%), KCl (>99.9%), ZnCl2 (98%), FeCl2·4H2O (≥99.0%), and MnCl2·2H2O (99%) were purchased from VWR-Prolabo®.Synthesis
Four ILs, i.e. tetraoctylammonium linoleate, methyl-trioctylammonium oleate, methyltrioctylammonium linoleate and tetraoctylammonium oleate, were synthesized at the same experimental conditions by a two-step synthesis (Fig. 1). First, sodium fatty acid was made by adding an equimolar amount of sodium hydroxide to the fatty acid dissolved in ethanol by stirring it overnight at room temperature. Afterwards, a metathesis reaction, for 3 h at room temperature, was performed between the tetraalkylammonium chloride, dissolved in toluene and water, and the sodium fatty acid. A viscous liquid was obtained after purification of the organic phase. Detailed synthesis of the four ILs can be found in the ESI.† | ||
| Fig. 1 Ions that were used to synthesize the highly hydrophobic ionic liquids used in this article. | ||
Analysis
Analyses of our synthesized end products were performed on a 400 Bruker nuclear magnetic resonance (NMR) spectrometer for 1H NMR. Density and viscosity measurements were carried out on an Anton Paar SVM 3000/G2 type stabinger, with an uncertainty of ±0.0005 g cm−3 for the density, ±0.005 mPa s for the viscosity, and ±0.01 K for the temperature. A differential scanning calorimetry (DSC) Q 1000 and a Perkin Elmer Pyris 1 were used to obtain the melting temperature with a scan rate of 10 °C min−1, a sensitivity of 0.2 μW and a temperature precision of ±0.05 °C. Decomposition temperatures were measured with a thermographic analysis (TGA) Q 500 apparatus at a scan rate of 10 °C min−1 under a nitrogen flow with a weighing precision of ±0.01%, a sensitivity of 0.1 μg, and an isothermal temperature precision of ±0.1 °C. The spectra were analysed with TG instruments software. Determination of chloride impurities was done via ion chromatography (IC) as previously described,26 but problems occurred during this technique. The described procedure to prepare the IC sample by dissolving 1.1 g of IL in 2 mL acetonitrile before being diluted to 10 mL with water resulted in a phase separation. IC samples with less IL resulted in malfunction of the IC. Finally, the chloride content was determined to be negligibly low by washing with water and checking that no AgCl precipitate was formed after AgNO3 addition. The water contents of the ILs were determined by a coulometric Karl Fischer titrator (Mettler Toledo, model DL39).Experimental salt extraction procedure
First, two aqueous metal solutions were prepared. The group I (alkali) metal solution consists of LiCl, NaCl, and KCl. Of each of these salts, 1 gram was dissolved in 100 mL demineralised water. For the aqueous solutions containing period IV transition metals, we dissolved 0.5 gram of MnCl2·2H2O together with 0.5 gram of FeCl2·4H2O and 0.5 gram of ZnCl2 in 100 mL demineralised water. The four ILs synthesized were tested for both alkali metal extraction and period IV transition metal salt extraction. One gram of the selected IL was divided equally over two centrifuge tubes. 0.5 mL of metal solution was added to one tube and to the other 0.5 mL demineralized water was added as a reference (blank). Both samples were stirred for 2 h on a vortex mixer Heidolph multireax and afterwards placed in an Allegra TM X-12R centrifuge from Beckman Coulter for 10 min at 3750 rpm. All extraction experiments took place at 20 °C under controlled laboratory conditions. Afterwards, aqueous phases were analysed on IC and inductively coupled plasma (ICP) to detect if metal extraction was occurring. A Metrohm 761 Compact IC and 762 IC interface was used to analyse the anion concentration with a detection limit of 0.1 mg L−1. Cations were measured with a Perkin Elmer, precisely ICP, which used an optical emission spectrometer Optima 5300DV. Results were obtained via a Winlab 32 ICP continuous automated analysis. The ICP-AES has a detection limit of 25–250 μg L−1, with an uncertainty of 1.4–2%.Extraction efficiencies (E) were calculated by
| E(%) = (c0aq − c1aq)/c0aq × 100 |
Subsequently, electronspray-ionisation combined with mass spectrometry (ESI-MS) was used to detect whether ion exchange took place as well. In that case, part of our IL would dissolve in water after metal extraction. An Agilent Technologies 1200 series ESI-MS was used with acetonitrile![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water 90
water 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 as the eluent working with an Agilent masshunter workstation data acquisition.
10 as the eluent working with an Agilent masshunter workstation data acquisition.
Results and discussion
For this research, we first synthesized the ILs with fatty acid anions. To do this, tetraalkylammonium halide ILs were used in a metathesis reaction with the sodium salt of the fatty acids. The synthesis of these ILs was very simple and they were obtained in high (between 72 and 99%) yields. The physical properties of the synthesized ILs are outlined in Table 1. In the literature, it is mentioned that the density of ILs depends mostly on the molecular shape of the anion.27 Fatty acids which have already a low density were used as anions. We assume that this is the reason why these ILs have, in contrast to common ILs, a density lower than water. Viscosities were found to be more dependent on the cationic structure.8 Water and chloride are present in the synthetic route of these ILs and they can alter all the physical properties considerably.28 Water is present, but chloride was not found to be a large impurity.| ILs | ρ (g cm−3) | η (mPa s) | υ (mm2 s−1) | T m (°C) | T d (°C) | w H2O (ppm) |
|---|---|---|---|---|---|---|
N8888 C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
0.883 | 1280 | 1450 | <−60 | 170 | 6900 |
N8881 C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
0.889 | 1047 | 1178 | −37 | 150 | 9800 |
N8888 C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.875 | 1234 | 1410 | <−60 | 175 | 7200 |
N8881 C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.884 | 1321 | 1494 | −25 | 169 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 880 880 |
The synthesized ILs were evaluated for their metal extraction properties. As a result of the extraction experiment, a promising color change was noticed for the ILs after they were mixed with the period IV transition metal solution (Fig. 2). No color change was noticed for the ILs when they were mixed with the aqueous group I metal solution. A comparison of the aqueous metal concentrations was done for all four ILs after each extraction experiment. The extraction efficiencies of the ILs for the specific metals can be found in Table 2 for the alkali metal salts and in Table 3 for the period IV transition metal salts.
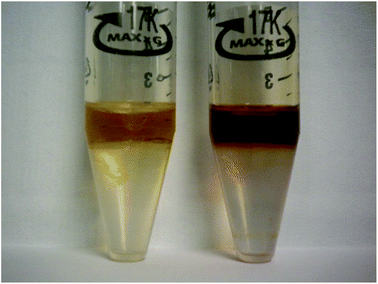 | ||
| Fig. 2 Color change observed during period IV transition metal extraction. The first tube shows us the IL tetraoctylammonium linoleate before metal extraction is shown (left). In the second tube we observe the same IL after metal extraction (right). | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), methyl-trioctylammonium oleate (E2,N8881 C18
2), methyl-trioctylammonium oleate (E2,N8881 C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), methyltrioctylammonium linoleate (E3,N8881 C18
1), methyltrioctylammonium linoleate (E3,N8881 C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), tetraoctylammonium oleate (E4,N8888 C18
2), tetraoctylammonium oleate (E4,N8888 C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)
1)
| E 1 (%) | E 2 (%) | E 3 (%) | E 4 (%) | |
|---|---|---|---|---|
| Chloride | 0 | 11.7 | 0 | 2 |
| Potassium | 0 | 0 | 0 | 0 |
| Sodium | 0 | 0 | 0 | 0 |
| Lithium | 0 | 22.4 | 0 | 4 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), methyl-trioctylammonium oleate (E2,N8881 C18
2), methyl-trioctylammonium oleate (E2,N8881 C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), methyltrioctylammonium linoleate (E3,N8881 C18
1), methyltrioctylammonium linoleate (E3,N8881 C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), tetraoctylammonium oleate (E4,N8888 C18
2), tetraoctylammonium oleate (E4,N8888 C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)
1)
| E 1 (%) | E 2 (%) | E 3 (%) | E 4 (%) | |
|---|---|---|---|---|
| Chloride | 89.5 | 84.1 | 73.1 | 90.8 |
| Iron | 88.8 | 96.5 | 71 | 98.4 |
| Manganese | 58.6 | 69.4 | 37.3 | 70.7 |
| Zinc | 99.8 | 94.9 | 99.6 | 99.8 |
No significant reductions in ion concentrations were observed when the ILs were mixed with the group I metal solution (Table 2). Only the oleate based ILs showed a minor reduction of lithium as well as a reduction of the chloride concentration. The reason why the reduction in total chloride concentration is smaller is because the amount of total chloride is higher (the solution also contains NaCl and KCl). The extraction efficiency of sodium and potassium is 0%. However, in some experiments the concentration of sodium and potassium slightly increased (leading to negative extraction efficiencies up to −3%), which could be explained by the sodium hydroxide used during synthesis of the ILs, and potassium is a major impurity in sodium hydroxide.
Table 3 shows an enormous extraction (up to >99%) for the iron and zinc concentrations using all four ILs. In addition, the concentration of manganese was significantly reduced (up to 71%). Comparison with extraction efficiencies with pure unsaturated fatty acids shows that the newly synthesized ILs perform equally well.22 The influence of the anion in our ILs on the extraction efficiency was dominant. It was found that the linoleate based ILs show better extraction of zinc but less extraction of iron and manganese compared to the oleate based ILs. The cation has a smaller influence on the extraction efficiency. The extraction efficiencies of methyl-trioctylammonium based ILs are slightly lower than the ones of tetraoctylammonium based ILs. The high extraction of period IV transition metals is the reason for the color change that was observed for all ILs. Together with the reduction of these metals, a reduction in chloride concentration was observed, which points out that for these systems ion extraction occurs instead of ion exchange.
A calculation was done to determine the ratio of extraction/exchange. Therefore, we divided the theoretical concentration of chloride from the removed metal concentration (based on the observed cation extraction) by the observed removed concentration of chloride. These values ranged from 0.88 to 0.93. This means that salt extraction is the dominant mechanism. There are 2 possible reasons why this value is slightly lower than 1 (indicating that more chloride than cations are extracted): (i) some ion exchange occurs, and (ii) oxidation of Fe(II) to Fe(III), followed by metal fatty acid complex formation. Oxidation of Fe(II) seems to be the most likely explanation, because over time a precipitate is formed indicating the occurrence of a chemical reaction. Moreover, ESI-MS results show that the concentration of the organic ions in the water phase (<10 ppm for tetraoctylammonium cations, <25 ppm for methyl-trioctylammonium cations, and the concentration of anions was below the detection limit) is similarly low for the aqueous period IV transition metal solution compared to the milliQ (blank) experiment after mixing with the ILs, indicating that no ion exchange occurs. Therefore, this process is a green alternative for metal extraction from aqueous phases.
It should be mentioned that after the extraction experiment we always detect some low organic ion concentrations in the water phase (<25 ppm). Methyltrioctyl ammonium based ILs were even able to form an emulsion with distilled water (blank) under vigorous shaking, while the tetraoctylammonium based ILs fortunately did not form an emulsion with the blank as well as with the metal solutions. Probably the asymmetry of the cation favors emulsion formation. ESI-MS results confirmed an increase of the cation methyltrioctyl ammonium in the water phase after emulsion formation by vigorous shaking. For this reason, it is obvious that the ions for the used ILs have to be biocompatible, non-toxic, and highly hydrophobic.
The challenge now is to sustainably regenerate the ILs. By sustainable we mean that the regeneration should be less electric energy consuming than current metal extraction processes and without the use of toxic chemicals. Electrodeposition and cementation look very promising for this task. Especially, if one could remove the different metals separately out of the ILs.
Conclusions
Ionic liquids with unsaturated fatty acids as anions were easily synthesized via a metathesis reaction. The advantages of these ILs are their simple synthesis, their sustainability (fatty acids anions are renewable), their biocompatibility and their non-toxicity. This makes these ILs “simpler and greener” compared to other TSILs used for metal extraction. While they showed no or negligible extraction efficiencies for alkali metal salts, very good extraction efficiencies (>99%) were observed for the period IV transition metal salts. The extraction process was based on ion extraction instead of ion exchange, which minimized IL leakages into the water phase.Further research is necessary to regenerate these ILs with electrodeposition or cementation. This would result in a very sustainable process to remove heavy metal salts from aqueous water solutions.
Acknowledgements
This work was performed in the TTIW-cooperation framework of Wetsus, centre of excellence for sustainable water technology (http://www.wetsus.nl). Wetsus is funded by the Dutch Ministry of Economic Affairs, the European Union Regional Development Fund, the Province of Fryslân, the City of Leeuwarden and the EZ/Kompas program of the “Samenwerkingsverband Noord-Nederland”. The authors would like to thank the participants of the research theme “Salt” for the fruitful discussions and their financial support. Furthermore, the authors would like to thank Ivan Ottenheijm and the lab analysts from Wetsus and the TU/e for the technical support.Notes and references
- J. F. Blais, Z. Djedidi, R. Ben Cheikh, R. D. Tyagi and G. Mercier, J. Hazard., Toxic, and Radioact. Waste, 2008, 12(3), 135–149 Search PubMed.
- S. Wellens, B. Thijs and K. Binnemans, Green Chem., 2012, 14, 1657–1665 RSC.
- N. B. Devi, K. C. Nathsarma and V. Chakravortty, Hydrometallurgy, 1998, 49(1–2), 47–61 Search PubMed.
- J. G. Dean, F. L. Bosqui and K. H. Lanouette, Environ. Sci. Technol., 1972, 6(6), 518–522 Search PubMed.
- A. G. Chmielewski, T. S. Urbanski and W. Migdal, Hydrometallurgy, 1997, 45(3), 333–344 Search PubMed.
- M. L. Dietz, Sep. Sci. Technol., 2006, 41(10), 2047–2063 CrossRef CAS.
- G. Huddleston and R. D. Rogers, Chem. Commun, 1998,(16), 1765–1766 RSC.
- J. P. Hallett and T. Welton, Chem. Rev., 2011, 111(5), 3508–3576 CrossRef CAS.
- U. Domańska and R. Bogel-lukasik, J. Phys. Chem. B, 2005, 109(24), 12124–12132 CrossRef CAS.
- J. S. Wilkes, Green Chem., 2002, 4(2), 73–80 RSC.
- A. P. Abbott, G. Frisch, J. Hartley and K. S. Ryder, Green Chem., 2011, 13, 471–481 RSC.
- V. A. Cocalia, J. D. Holbrey, K. E. Gutowski, N. J. Bridges and R. D. Rogers, Tsinghua Sci. Technol., 2006, 11(2), 188–193 Search PubMed.
- X. Sun, Y. Ji, L. Guo, J. Chen and D. Li, Sep. Purif. Technol., 2011, 81(1), 25–30 CAS.
- A. E. Visser and R. D. Rogers, J. Solid State Chem., 2002, 171(1–2), 109–113 Search PubMed.
- A. E. Visser, R. P. Swatloski, S. T. Griffin, D. H. Hartman and R. D. Rogers, Sep. Sci. Technol., 2001, 36(5–6), 785–804 CrossRef CAS.
- M. L. Dietz and D. C. Stepinski, Green Chem., 2005, 7(10), 747–750 RSC.
- Z. Li, Q. Wei, R. Yuan, X. Zhou, H. Liu, H. Shan and Q. Song, Talanta, 2007, 71(1), 68–72 CrossRef CAS.
- J. R. Harjani, T. Friščić, L. R. MacGillivray and R. D. Singer, Dalton Trans., 2008,(34), 4595–4601 RSC.
- P. Nockemann, B. Thijs, S. Pittois, J. Thoen, C. Glorieux, K. Van Hecke, L. Van Meervelt, B. Kirchner and K. Binnemans, J. Phys. Chem. B, 2006, 110(42), 20978–20992 CrossRef CAS.
- X. Sun, Y. Ji, F. Hu, B. He, J. Chen and D. Li, Talanta, 2010, 81(4–5), 1877–1883 Search PubMed.
- L. Fischer, T. Falta, G. Koellensperger, A. Stojanovic, D. Kogelnig, M. Galanski, R. Krachler, B. K. Keppler and S. Hann, Water Res., 2011, 45(15), 4601–4614 CrossRef CAS.
- J. Šiška, Hydrometallurgy, 2005, 76(3–4), 155–172 CrossRef CAS.
- W. Wang, Y. Yang, H. Zhao, Q. Guo, W. Lu and Y. Lu, J Radioanal. Nucl. Chem., 2011, 1–6 Search PubMed.
- H. Zhao, A. Ariafard and Z. Lin, Inorg. Chim. Acta, 2006, 359(11), 3527–3534 CrossRef CAS.
- M. Petkovic, K. R. Seddon, L. P. Rebelo and C. S. Pereira, Chem. Soc. Rev., 2011, 40(3), 1383–1403 RSC.
- C. Villagrán, M. Deetlefs, W. R. Pitner and C. Hardacre, Anal. Chem., 2004, 76(7), 2118–2123 CrossRef CAS.
- S. Seki, T. Kobayashi, Y. Kobayashi, K. Takei, H. Miyashiro, K. Hayamizu, S. Tsuzuki, T. Mitsugi and Y. Umebayashi, J. Mol. Liq., 2010, 152(1–3), 9–13 CrossRef CAS.
- K. R. Seddon, A. Stark and M. J. Torres, Pure Appl. Chem., 2000, 72(12), 2275–2287 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: General experimental procedures, characterization for ionic liquids and copies of NMR, ESI/MS spectra. See DOI: 10.1039/c2gc36458a |
| This journal is © The Royal Society of Chemistry 2013 |
