Production and upgrading of 5-hydroxymethylfurfural using heterogeneous catalysts and biomass-derived solvents†
Jean Marcel R.
Gallo
,
David Martin
Alonso
,
Max A.
Mellmer
and
James A.
Dumesic
*
Department of Chemical and Biological Engineering, University of Wisconsin-Madison, 1415 Engineering Drive, Madison, WI 53706, USA. E-mail: dumesic@engr.wisc.edu; Fax: +1-608-262-5434; Tel: +1-608-262-109
First published on 26th October 2012
Abstract
High yields of HMF from glucose can be achieved using biomass-derived solvents and a combination of solid Lewis and Brønsted catalysts in a salt-free reaction system. The HMF produced in this system can be oxidized to FDCA or hydrogenated to DMF, both being high-value chemicals.
The conversion of renewable biomass resources into chemicals and fuels traditionally obtained from petroleum is strategically important to improve the sustainability of the chemical industry. Lignocellulosic biomass is the non-edible portion of biomass, and extensive research has been carried out in its conversion into platform molecules. The platform molecule 5-hydroxymethylfurfural (HMF), produced from the Brønsted acid catalyzed dehydration of C6 sugars (hexoses), is considered to be one of the top value-added chemicals.1 Mechanistic studies have shown that HMF is formed from the dehydration of the hexoses in the furanose form (5-member ring).2–4 Although glucose is the most abundant and least expensive hexose, it presents low amounts of furanose isomer in solution (1% in water5), and its dehydration into HMF thus takes place with low selectivity.6 In contrast, fructose, which presents 21.5% of the furanose form in aqueous solution,5 can be dehydrated to HMF in higher yields using monophasic or biphasic solvent systems and using homogeneous and heterogeneous Brønsted acids.7–13 Dumesic and co-workers14,15 employed a biphasic system consisting of an aqueous layer saturated with NaCl and containing fructose and HCl or H2SO4 as catalysts, in combination with an extracting organic layer to protect HMF from degradation reactions. Several alcohols, ketones, and ethers were used as extracting organic layers, and yields for HMF as high as 70% were observed.14,15 In monophasic solvent systems using dimethyl sulfoxide or ionic liquids as solvents, HMF can be obtained with yields higher than 90%;7,11,16 however, the separation and purification of HMF from these solvents are complicated.
While glucose can be obtained from cellulose by hydrolysis with yields of 98–100%, isomerization of glucose to fructose is economically limited to 42%,17 requiring additional and expensive separation steps. As a consequence, the final market price of fructose is significantly higher than that of glucose. In order to obtain HMF in high yields from glucose, recent studies have aimed to use one-pot isomerization reactions to produce fructose by using a Lewis acid or Lewis base, followed by Brønsted acid-catalyzed dehydration of fructose to HMF (Fig. 1).
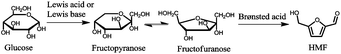 | ||
| Fig. 1 Conversion of glucose to HMF by a combined isomerization/dehydration reaction pathway. | ||
Zhao et al.11 first reported HMF yields of 68–70% from glucose in the ionic liquid 1-ethyl-3-methyl-imidazolium chloride using CrCl2 as the Lewis acid catalyst. In subsequent studies with ionic liquids, HMF was produced from glucose with yields higher than 90%.18 However, ionic liquids are not yet suitable for large scale applications due to their high cost and deactivation by small amounts of water.7 Binder and Raines12 reported that a system using dimethylacetamide (DMA), NaBr, and CrCl2 resulted in HMF yields of 81%, being as effective as ionic liquid systems. Other authors have explored biphasic systems. Huang et al.19 reported a 63% HMF yield in a biphasic reactor system with a two-step process involving the isomerization of glucose to fructose in the presence of glucose isomerase and borate ions, followed by the HCl-catalyzed dehydration of fructose to HMF. Dumesic and co-workers6 reported 62% yield of HMF from glucose using a biphasic reactor consisting of AlCl3·6H2O and HCl as catalysts in water saturated with NaCl, in contact with sec-butylphenol. In all of these systems, the main goal was to maximize HMF yield, while the upgrading and purification of HMF and the sustainability of the process remained as secondary problems. For example, reutilization of homogeneous catalysts can be an issue, and these catalysts lead to corrosion problems that require expensive materials of construction. Moreover, the replacement of these homogeneous catalysts with heterogeneous catalysts is not possible in the presence of salts, due to exchange of protons on the catalyst with cations in solution, leading to deactivation of the heterogeneous catalyst. Furthermore, the sustainability of biomass conversion process would be improved by the use of biomass-derived solvents, alleviating the need to purchase and transport petroleum-derived solvents to the biomass conversion site. These solvents must operate in presence of solid catalysts at reaction conditions favorable for glucose or fructose dehydration, and they must be compatible with upgrading processes, which typically involve oxidation, hydrogenation, or hydrogenolysis reactions.
Herein, we present an integrated process using solid acid catalysts and biomass-derived solvents, γ-lactones and tetrahydrofuran (THF), for the conversion of glucose to HMF, followed by the subsequent upgrading of HMF by hydrogenation or oxidation. As depicted in Fig. 2, γ-valerolactone (GVL) can be obtained from hydrogenation of levulinic acid, a platform molecule derived from monosaccharide dehydration. In addition, GVL is an important platform molecule used for the production of chemicals and fuels.20,21 Other γ-lactones with higher molecular weights can be obtained from GVL, as described elsewhere,22,23 or by ring closing of unsaturated acids.24 Thus, in addition to using GVL, we have studied γ-hexalactone (GHL), γ-octalactone (GOL), and γ-undecalactone (GUL). Similar to GVL, THF can be derived from biomass from the decarbonylation and hydrogenation of furfural, a product of xylose dehydration.25,26
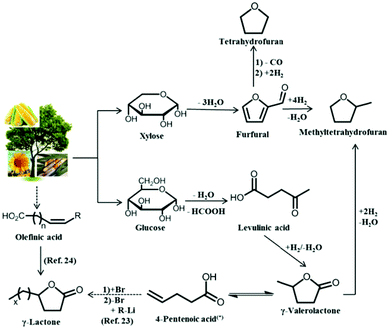 | ||
| Fig. 2 Pathways for the production of THF, GVL and other γ-lactones from biomass. (*)2- and 3-pentenoic acid are also formed in the isomerization, however, the consecutive reaction takes place with the 4-pentenoic acid isomer. | ||
To begin our studies of γ-lactones and THF as biomass-derived solvents for conversion of hexoses to HMF, we employed biphasic systems similar to those reported previously where THF and sec-butylphenol were used as the organic layers.6 In biphasic reactors, monosaccharide dehydration takes place in the aqueous layer, followed by the extraction of HMF by the organic layer, where it is protected from the catalysts, minimizing side reactions. The aqueous layer has to be saturated with NaCl to diminish the solubility of both the organic solvent and HMF, improving the efficiency of the organic extracting layer. Aluminium chloride is used as isomerization catalyst, while dehydration is catalyzed by HCl.
As seen in the results presented in Table 1, the selectivity for conversion of glucose to HMF using the γ-lactones in a biphasic system (with the exception of GUL) are comparable with systems using THF and SBP as the extracting solvent. However, as observed previously with THF, γ-lactones extract part of the HCl from the aqueous layer, which not only affects HMF separation or upgrading, but also increases process cost, because the aqueous layer has to be re-acidified and the organic layer has to be neutralized. For example, the molar ratio of HMF formed to HCl lost is approximately 200 when using THF and GVL as the extracting solvents. The use of SBP appears to be the most promising solvent for these biphasic systems, due to high HMF selectivity and negligible extraction of HCl; however, the high toxicity of this solvent could limit its application.
| Feed | Organic layer | Time/min | Conv./% | Selec./% | % HMF in org. layer | % HCl in org. layer |
|---|---|---|---|---|---|---|
| a Reaction conditions: 1.5 g of NaCl-saturated aqueous feed (5 wt% glucose, or fructose, 5.0 mmol L−1 AlCl3, and 3.17 mmol L−1 HCl); 3.0 g of organic solvent; temperature 170 °C. b Results from ref. 6. | ||||||
| Fructose | GVL | 20 | 94 | 84 | 94 | 30 |
| Glucose | GVL | 40 | 88 | 70 | 94 | 30 |
| Glucose | GHL | 40 | 88 | 65 | 92 | 20 |
| Glucose | GOL | 40 | 89 | 65 | 92 | 10 |
| Glucose | GUL | 40 | 92 | 54 | 83 | 0 |
| Glucoseb | THF | — | 80 | 71 | 93 | 30 |
| Glucoseb | SBP | 40 | 91 | 68 | 97 | 0 |
Of the solvents listed in Table 1, THF, GVL, and GHL can form a monophasic mixture with water. THF and GVL are completely miscible in water, while GHL can dissolve 7–10 wt% of water. The use of monophasic solvent systems alleviates potential mixing problems that may be encountered when scaling up biphasic systems. Accordingly, we next studied the production of HMF from glucose in a solvent system consisting of GVL, GHL, or THF with 10 wt% water using AlCl3 and HCl as catalysts. As shown in Table 2, the results obtained in these monophasic systems are similar to those shown in Table 1.
A potential advantage of using the above monophasic systems is that the addition of salt is not necessary (as it is to achieve a biphasic system with these solvents), allowing the homogeneous catalysts used for glucose isomerization and fructose dehydration to be replaced by solid acid catalysts. Accordingly, conversion of glucose to HMF would need two kinds of catalysts: a solid Lewis acid or Lewis base to substitute aluminium chloride and a solid Brønsted acid to substitute HCl.
Recent studies have shown that hydrotalcites27 and tin containing zeolites and silicas28 are active for glucose isomerization to fructose. Using a combination of Sn-β and HCl in a biphasic system, Nikolla et al.29 obtained HMF yields of 57% at 79% conversion of glucose. No tin leaching was observed.29 Takagaki et al.27 reported HMF yields of 42% at 73% conversion in a two-step process by combining the solid Brønsted acid catalyst, Amberlyst-70 (Amb-70), and a solid base catalyst, hydrotalcite, in N,N-dimethylformamide. The bifunctional catalyst Sulfated ZrO2–Al2O3 led to 47.9% yield at total conversion in DMSO.30
To establish the most appropriate isomerization catalyst for glucose isomerization, a leaching test was performed for Sn-β (Si![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sn = 400), Sn-SBA-15 (Si
Sn = 400), Sn-SBA-15 (Si![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sn = 40) and hydrotalcite. For this test, 0.1 g of catalyst was stirred in a mixture GVL–H2O (9
Sn = 40) and hydrotalcite. For this test, 0.1 g of catalyst was stirred in a mixture GVL–H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for 30 min at 130 °C. The catalyst was removed by filtering (while the solution was still hot), and glucose was added to the solvent to make a 2 wt% sugar solution. The mixture was stirred for 30 min at 130 °C. Conversion of glucose was only observed in the solution contacted with hydrotalcite, indicating leaching.
1) for 30 min at 130 °C. The catalyst was removed by filtering (while the solution was still hot), and glucose was added to the solvent to make a 2 wt% sugar solution. The mixture was stirred for 30 min at 130 °C. Conversion of glucose was only observed in the solution contacted with hydrotalcite, indicating leaching.
The solid Brønsted acid catalyst used for our studies in monophasic reactor systems was Amb-70, a sulfonic acid functionalized catalyst that was shown in a previous study to be more selective for HMF production from fructose than other solid acid catalysts, such as zeolites (mordenite, ZSM-5, Z-Y, USY, and Z-β), cubic and amorphous zirconium phosphate, titanium oxide, niobium oxide, and phosphated niobic acid.10,31 The same study also showed low deactivation of Amb-70 in the dehydration of fructose at 130 °C in a flow reactor system using THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (4
H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as solvent.10,31
1) as solvent.10,31
Because water is produced during glucose conversion to HMF and the presence of water increases the solubility of glucose, we studied the effect of water concentration for the conversion of glucose to HMF using GVL as the solvent and Amb-70 and Sn-SBA-15 as catalysts. Although water is known to promote side reactions in the dehydration of sugars,14 it can be seen in the results from Fig. S1† that water can be beneficial in low concentration. At similar conversion (∼70%), the system with mass ratio GVL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (9
H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) showed higher HMF selectivity than those with a GVL
1) showed higher HMF selectivity than those with a GVL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O ratio of 4
H2O ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or pure GVL.
1 or pure GVL.
Table 3 shows the results for conversion of fructose and glucose to HMF using Amb-70 or a combination of Amb-70 and Sn-based catalysts (Sn-β or Sn-SBA-15) in GVL, GHL or THF containing 10 wt% water. These experiments were carried out using different catalysts and solvents at similar conversions (∼90%). It can be seen that HMF was obtained from fructose using Amb-70 with selectivities between 80 and 85%. On the other hand, direct dehydration of glucose using Amb-70 led to selectivities lower than 35%, in agreement with early reports showing that direct dehydration of glucose with mineral acids leads to low selectivity to HMF.6,29 The combination of a Sn-based catalyst and Amb-70 leads to significant improvement in the selectivity to HMF from glucose. Systems using Sn-β show at least 9% higher selectivity than those using Sn-SBA-15. In this respect, Taarning and co-workers have shown that Sn-β displays higher Lewis acid strength than Sn-SBA-15 which gives it significantly higher catalytic activity.32 Using Sn-β/Amb-70 for the conversion of glucose, selectivities of 64, 59 and 70% were obtained, respectively, using GVL, GHL and THF as the solvent.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MTHF with water in a ratio (9
MTHF with water in a ratio (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)a
1)a
| Feed | Solvent | Lewis acid | Time/min | Conv./% | Selec./% |
|---|---|---|---|---|---|
a Reaction conditions: 1.5 g of feed (2 wt% glucose or fructose, organic![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water weight ratio (9 water weight ratio (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)); temperature 130 °C.
b Catalysts: 0.05 g Amb-70.
c Catalysts: 0.1 g Amb-70.
d Catalysts: 0.05 g of Sn-β and 0.05 g Amb-70.
e Catalysts: 0.05 g of Sn-SBA-15 and 0.05 g Amb-70.
f THF 1)); temperature 130 °C.
b Catalysts: 0.05 g Amb-70.
c Catalysts: 0.1 g Amb-70.
d Catalysts: 0.05 g of Sn-β and 0.05 g Amb-70.
e Catalysts: 0.05 g of Sn-SBA-15 and 0.05 g Amb-70.
f THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MTHF weight ratio (1 MTHF weight ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1).
|
|||||
| Fructoseb | GVL | — | 9 | 89 | 80 |
| Glucosec | GVL | — | 30 | 92 | 32 |
| Glucosed | GVL | Sn-β | 20 | 92 | 64 |
| Glucosee | GVL | Sn-SBA-15 | 15 | 90 | 51 |
| Fructoseb | GHL | — | 10 | 91 | 81 |
| Glucosec | GHL | — | 30 | 85 | 30 |
| Glucosed | GHL | Sn-β | 20 | 93 | 59 |
| Glucosee | GHL | Sn-SBA-15 | 15 | 90 | 50 |
| Fructoseb | THF | — | 10 | 91 | 85 |
| Glucosec | THF | — | 50 | 90 | 25 |
| Glucosed | THF | Sn-β | 30 | 90 | 70 |
| Glucosee | THF | Sn-SBA-15 | 20 | 90 | 40 |
| Glucosec | MTHF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) THFf THFf |
— | 60 | 85 | 26 |
| Glucosed | MTHF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) THFf THFf |
Sn-β | 40 | 91 | 66 |
Because methyltetrahydrofuran (MTHF) can be produced directly from biomass-derived furfural or GVL, we also explored the use of this solvent for conversion of glucose to HMF. MTHF is not miscible with water; therefore, to obtain a monophasic solvent system, MTHF was mixed with 50% THF. The solvent system consisting of THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MTHF in a weight ratio of 1
MTHF in a weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 10 wt% water produced a selectivity of 66% for HMF formation from glucose (Table 3), which is comparable to that obtained in GVL.
1 and 10 wt% water produced a selectivity of 66% for HMF formation from glucose (Table 3), which is comparable to that obtained in GVL.
Fig. 3 shows the conversion of glucose to fructose and HMF as a function of time in the presence of Amb-70 and Sn-β for GVL (Fig. 3A), GHL (Fig. 3B), THF (Fig. 3C), and THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MTHF (Fig. 3D).
MTHF (Fig. 3D).
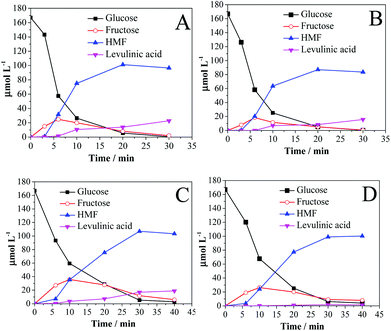 | ||
Fig. 3 Glucose dehydration in (A) GVL, (B) GHL, (C) THF, and (D) THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MTHF (1 MTHF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Solvents contain 10 wt% water. Reaction conditions: 2 wt% glucose; 0.05 g Sn-β; 0.05 g Amb-70; temperature 130 °C. 1). Solvents contain 10 wt% water. Reaction conditions: 2 wt% glucose; 0.05 g Sn-β; 0.05 g Amb-70; temperature 130 °C. | ||
In glucose conversion (Fig. 3), the product observed initially is fructose, and formation of HMF begins at approximately 5 min of reaction time. Comparing the extent of fructose formation in the different solvents, it can be concluded that the effectiveness of glucose isomerization controls the HMF selectivity (Table 3), i.e., THF > THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MTHF = GVL > GHL. Diminishing the mass ratio Amb-70/Sn-β in an attempt to increase the relative rate of glucose isomerization in GVL did not lead to any improvement in HMF selectivity (Fig. S2†). Thus, in addition to functioning as a catalyst for glucose isomerization, Sn-β also appears to catalyze fructose degradation to unidentified products. After HMF has been formed, it can undergo further reaction to form equimolar amounts of levulinic (LA) and formic acid (FA) generated from HMF hydrolysis (HMF = LA + FA). The carbon balances in the reactions carried out using GVL, GHL, THF
MTHF = GVL > GHL. Diminishing the mass ratio Amb-70/Sn-β in an attempt to increase the relative rate of glucose isomerization in GVL did not lead to any improvement in HMF selectivity (Fig. S2†). Thus, in addition to functioning as a catalyst for glucose isomerization, Sn-β also appears to catalyze fructose degradation to unidentified products. After HMF has been formed, it can undergo further reaction to form equimolar amounts of levulinic (LA) and formic acid (FA) generated from HMF hydrolysis (HMF = LA + FA). The carbon balances in the reactions carried out using GVL, GHL, THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MTHF (1
MTHF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and THF were, respectively, 80, 65, 70 and 85%. Common degradation products, as solid humins and soluble polymers, formed by the cross-polymerization of glucose and HMF33 were not quantified. However, it was shown before that GVL, for example, can solubilize humins,34 thereby facilitating the separation of the product and minimizing deposition of carbon on the solid catalyst surface.
1) and THF were, respectively, 80, 65, 70 and 85%. Common degradation products, as solid humins and soluble polymers, formed by the cross-polymerization of glucose and HMF33 were not quantified. However, it was shown before that GVL, for example, can solubilize humins,34 thereby facilitating the separation of the product and minimizing deposition of carbon on the solid catalyst surface.
Because HMF is a platform chemical and not a final product, it is important to find methods to separate HMF from the reaction medium or to integrate the production of HMF with subsequent upgrading reactions, thereby decreasing the cost of the final product. GVL and GHL have high boiling points that are similar to HMF; therefore, the separation of HMF from these solvents by distillation would require the use of low pressures and could lead to degradation of HMF. In contrast, MTHF and THF have a low boiling point (80 and 66 °C, respectively) and can be easily separated from HMF. In the reaction solvent consisting of THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MTHF (1
MTHF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) or THF with 10% water, organic solvents can be evaporated to yield an aqueous solution of HMF, or alternatively, water can be removed using a drying agent such as magnesium sulfate or calcium chloride, followed by distillation to obtain pure HMF. Another route to separate HMF from GVL or GHL is to convert HMF to a lower boiling point compound. For example, the high value chemical 2,5-dimethylfuran (DMF)14 has a boiling point significantly lower than GVL or GHL. Also, it is more stable than HMF, such that DMF can be removed by distillation. To achieve this separation route, we have shown that DMF can be produced with 46% yield by hydrogenolysis of HMF in the presence of lactones using a RuSn/C catalyst at 200 °C. Importantly, this catalyst produced DMF from HMF without conversion of the solvent (GVL or GHL), in accordance with previous literature.34
1) or THF with 10% water, organic solvents can be evaporated to yield an aqueous solution of HMF, or alternatively, water can be removed using a drying agent such as magnesium sulfate or calcium chloride, followed by distillation to obtain pure HMF. Another route to separate HMF from GVL or GHL is to convert HMF to a lower boiling point compound. For example, the high value chemical 2,5-dimethylfuran (DMF)14 has a boiling point significantly lower than GVL or GHL. Also, it is more stable than HMF, such that DMF can be removed by distillation. To achieve this separation route, we have shown that DMF can be produced with 46% yield by hydrogenolysis of HMF in the presence of lactones using a RuSn/C catalyst at 200 °C. Importantly, this catalyst produced DMF from HMF without conversion of the solvent (GVL or GHL), in accordance with previous literature.34
One of the most attractive compounds that can be produced from HMF is 2,5-furandicarboxylic acid (FDCA). FDCA can be produced by oxidation of HMF with molecular oxygen in an aqueous alkaline solution using supported gold, platinum or palladium catalysts.35–37 FDCA is a monomer that can be used to produce polymers similar to polyethylene terephthalate (PET). PET has a growing market, with more than 49 million tons produced in 2009.38 For this reason, FDCA has been rated as a top twelve value added chemical by the U.S. Department of Energy.39 For the production of FDCA, HMF has to be separated to avoid oxidation of the organic solvent. As mentioned above, HMF can be removed from THF and MTHF by distillation, and it can thus be used directly in the oxidation reaction. Removal of HMF from the less volatile γ-lactones can be achieved by contacting these solutions containing HMF with water and cyclopentane (CP) in different proportions, as shown in Table S1.†
Contacting 1 part of a solution containing HMF in GHL with 1 part of water and 16 parts of CP, leads to an aqueous layer containing 80 wt% of the initial HMF and 5 wt% of the initial GHL. Contacting the resultant aqueous layer twice with 6 parts of CP decreases the GHL concentration to 0.35 wt% of the initial value, while retaining all of the HMF in the aqueous layer. The HMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GHL molar ratio in the final aqueous layer is equal to 2.51. GVL is more soluble in water than GHL; therefore, extraction of HMF from GVL requires 4 consecutive extractions with 20 parts of CP to yield an aqueous solution with 80 wt% of the initial HMF amount and 0.5 wt% of the initial amount of GVL. The HMF
GHL molar ratio in the final aqueous layer is equal to 2.51. GVL is more soluble in water than GHL; therefore, extraction of HMF from GVL requires 4 consecutive extractions with 20 parts of CP to yield an aqueous solution with 80 wt% of the initial HMF amount and 0.5 wt% of the initial amount of GVL. The HMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GVL molar ratio in the final aqueous layer is equal to 1.52. The boiling point of CP (50 °C) is much lower than GVL (207 °C) and GHL (219 °C), hence it can be separated easily from the lactone by distillation.
GVL molar ratio in the final aqueous layer is equal to 1.52. The boiling point of CP (50 °C) is much lower than GVL (207 °C) and GHL (219 °C), hence it can be separated easily from the lactone by distillation.
Aqueous solutions of HMF and γ-lactones (that could be produced by the method outlined above using CP) were used as feed solutions for studies of HMF oxidation under the reaction conditions proposed by Davis et al.,36i.e., aqueous solution containing 0.1 mol L−1 HMF, 2 mol L−1 NaOH, 2000 kPA oxygen, 1 wt% Au/TiO2 (HMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Au = 100) at 22 °C. Using an aqueous solution of HMF with up to 0.5% of GVL or GHL, yields of 80% for FDCA and 20% for 2-hydroxymethylfurancarboxylic acid (HFCA) were observed, in accordance with previous literature in the absence of lactones.36 If the reaction is carried out in the presence of larger amounts of lactones (i.e. 5%), yields of 56% for FDCA and 44% for HFCA are observed, and 35% of the γ-lactone is converted to levulinic acid (from GVL) or 4-oxohexanoic acid (from GHL). Separation of FDCA was achieved by decreasing the pH of the reaction mixture to a value of one, leading to precipitation of FDCA, while HFCA and salt remained in solution. The precipitate was filtered and washed with ethanol.
Au = 100) at 22 °C. Using an aqueous solution of HMF with up to 0.5% of GVL or GHL, yields of 80% for FDCA and 20% for 2-hydroxymethylfurancarboxylic acid (HFCA) were observed, in accordance with previous literature in the absence of lactones.36 If the reaction is carried out in the presence of larger amounts of lactones (i.e. 5%), yields of 56% for FDCA and 44% for HFCA are observed, and 35% of the γ-lactone is converted to levulinic acid (from GVL) or 4-oxohexanoic acid (from GHL). Separation of FDCA was achieved by decreasing the pH of the reaction mixture to a value of one, leading to precipitation of FDCA, while HFCA and salt remained in solution. The precipitate was filtered and washed with ethanol.
The overall yields we have achieved for production of DMF and FDCA on a glucose basis for each of the solvent systems considered in this paper are shown in Fig. 4. Starting with THF as solvent, an overall yield of 50% is obtained for FDCA, while 38 and 35% are the yields when the process uses GVL and GHL as the solvents, respectively. If the target product is DMF, then yields of 27 and 25% can be obtained in the systems using GVL and GHL as solvents, respectively.
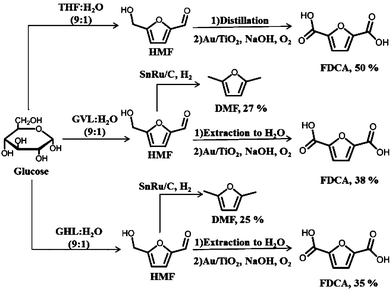 | ||
| Fig. 4 Overall yields for DMF and FDCA production from glucose starting with THF, GVL or GHL as solvent. | ||
Conclusions
The highest yields we have achieved for production of HMF from glucose using a combination of Amb-70 and Sn-β as solid acid catalysts are 59, 55, 60 and 63% using GVL, GHL, THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MTHF (1
MTHF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and THF as solvents, respectively. These results are comparable or higher than values obtained in the literature using a combination of Lewis and Brønsted acids. In addition to achieving high yields, the systems reported in this communication replace the use of homogeneous catalysts and salts, leading to a more sustainable and economically viable process. Separation and purification of HMF can be achieved by distillation for the case of THF as the solvent. In contrast, GVL and GHL have boiling points that are similar to HMF, requiring the isolation of HMF by different methods. In this respect, we show that HMF can be converted to DMF with 46% yield, and this low boiling point product can then be separated from the solvent by distillation. Alternatively, we show that HMF can be extracted to water assisted by cyclopentane. The oxidation of HMF to FDCA can then be carried out in the presence of small amounts of lactones, and yields of 80% were obtained. Thus, the catalytic systems proposed in this work not only replace the use of homogeneous catalysts and salts, but they also allow the integration of HMF production with processes for HMF upgrading to valuable products.
1) and THF as solvents, respectively. These results are comparable or higher than values obtained in the literature using a combination of Lewis and Brønsted acids. In addition to achieving high yields, the systems reported in this communication replace the use of homogeneous catalysts and salts, leading to a more sustainable and economically viable process. Separation and purification of HMF can be achieved by distillation for the case of THF as the solvent. In contrast, GVL and GHL have boiling points that are similar to HMF, requiring the isolation of HMF by different methods. In this respect, we show that HMF can be converted to DMF with 46% yield, and this low boiling point product can then be separated from the solvent by distillation. Alternatively, we show that HMF can be extracted to water assisted by cyclopentane. The oxidation of HMF to FDCA can then be carried out in the presence of small amounts of lactones, and yields of 80% were obtained. Thus, the catalytic systems proposed in this work not only replace the use of homogeneous catalysts and salts, but they also allow the integration of HMF production with processes for HMF upgrading to valuable products.
Acknowledgements
The authors thank Haldor Topsøe A/S, Denmark for the Sn-β and Fabiola Lárraga and Nestor Hernandez Lozada for their help with the experiments. This work was supported by the U.S. Department of Energy; the Center Enabling New Technologies through Catalysis (CENTC); and the Defense Advanced Research Projects Agency (DARPA). The views, opinions, and/or findings contained in this article are those of the author and should not be interpreted as representing the official views or policies, either expressed or implied, of the DARPA or the Department of Defense.Notes and references
- J. J. Bozell and G. R. Petersen, Green Chem., 2010, 12, 539–554 RSC.
- M. J. Antal Jr., W. S. L. Mok and G. N. Richards, Carbohydr. Res., 1990, 199, 91–109 CrossRef CAS.
- J. Guan, Q. A. Cao, X. C. Guo and X. D. Mu, Comput. Theor. Chem., 2011, 963, 453–462 CrossRef CAS.
- A. S. Amarasekara, L. D. Williams and C. C. Ebede, Carbohydr. Res., 2008, 343, 3021–3024 CrossRef CAS.
- J. F. Robyt, Essentials of Carbohydrate Chemistry, Springer Pub. Co., New York, 1998 Search PubMed.
- Y. J. Pagan-Torres, T. F. Wang, J. M. R. Gallo, B. H. Shanks and J. A. Dumesic, ACS Catal., 2012, 2, 930–934 CrossRef CAS.
- C. Moreau, A. Finiels and L. Vanoye, J. Mol. Catal. A: Chem., 2006, 253, 165–169 CrossRef CAS.
- Y. Roman-Leshkov, J. N. Chheda and J. A. Dumesic, Science, 2006, 312, 1933–1937 CrossRef CAS.
- J. N. Chheda, Y. Roman-Leshkov and J. A. Dumesic, Green Chem., 2007, 9, 342–350 RSC.
- A. J. Crisci, M. H. Tucker, M. Y. Lee, S. G. Jang, J. A. Dumesic and S. L. Scott, ACS Catal., 2011, 1, 719–728 CrossRef CAS.
- H. Zhao, J. E. Holladay, H. Brown and Z. C. Zhang, Science, 2007, 316, 1597–1600 CrossRef CAS.
- J. B. Binder and R. T. Raines, J. Am. Chem. Soc., 2009, 131, 1979–1985 CrossRef CAS.
- G. Yong, Y. Zhang and J. Y. Ying, Angew. Chem., Int. Ed., 2008, 47, 9345–9348 CrossRef CAS.
- Y. Roman-Leshkov, C. J. Barrett, Z. Y. Liu and J. A. Dumesic, Nature, 2007, 447, 982–U985 CrossRef CAS.
- Y. Román-Leshkov and J. Dumesic, Top. Catal., 2009, 52, 297–303 CrossRef.
- K.-i. Shimizu, R. Uozumi and A. Satsuma, Catal. Commun., 2009, 10, 1849–1853 CrossRef CAS.
- Y. Zhang, K. Hidajat and A. K. Ray, Biochem. Eng. J., 2004, 21, 111–121 CrossRef CAS.
- M. E. Zakrzewska, E. Bogel-Lukasik and R. Bogel-Lukasik, Chem. Rev., 2011, 111, 397–417 CrossRef CAS.
- R. L. Huang, W. Qi, R. X. Su and Z. M. He, Chem. Commun., 2010, 1115–1117 RSC.
- D. M. Alonso, J. Q. Bond and J. A. Dumesic, Green Chem., 2010, 12, 1493–1513 RSC.
- J. C. Serrano-Ruiz, R. Luque and A. Sepulveda-Escribano, Chem. Soc. Rev., 2011, 40, 5266–5281 RSC.
- L. E. Manzer, Appl. Catal., A, 2004, 272, 249–256 CrossRef CAS.
- T. M. Ugurchieva, A. V. Lozanova, M. V. Zlokazov and V. V. Veselovsky, Russ. Chem. Bull., 2008, 57, 657–659 CrossRef CAS.
- Y. Zhou, L. K. Woo and R. J. Angelici, Appl. Catal. A, 2007, 333, 238–244 CrossRef CAS.
- O. W. Cass, Ind. Eng. Chem., 1948, 40, 216–219 CrossRef CAS.
- E. J. Garcia-Suarez, A. M. Balu, M. Tristany, A. B. Garcia, K. Philippot and R. Luque, Green Chem., 2012, 14, 1434–1439 RSC.
- A. Takagaki, M. Ohara, S. Nishimura and K. Ebitani, Chem. Commun., 2009, 6276–6278 RSC.
- M. Moliner, Y. Roman-Leshkov and M. E. Davis, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 6164–6168 CrossRef CAS.
- E. Nikolla, Y. Roman-Leshkov, M. Moliner and M. E. Davis, ACS Catal., 2011, 1, 408–410 CrossRef CAS.
- H. P. Yan, Y. Yang, D. M. Tong, X. Xiang and C. W. Hu, Catal. Commun., 2009, 10, 1558–1563 CrossRef CAS.
- M. H. Tucker, PhD thesis, University of Wisconsin-Madison, 2011.
- C. M. Osmundsen, M. S. Holm, S. Dahl and E. Taarning, Proc. R. Soc. London, A, 2012, 468, 2000–2016 CrossRef CAS.
- H. M. Pilath, M. R. Nimlos, A. Mittal, M. E. Himmel and D. K. Johnson, J. Agric. Food Chem., 2010, 58, 6131–6140 CrossRef CAS.
- S. G. Wettstein, D. M. Alonso, Y. Chong and J. A. Dumesic, Energy Environ. Sci., 2012, 5, 8199–8203 CAS.
- B. N. Zope, S. E. Davis and R. J. Davis, Top. Catal., 2012, 55, 24–32 CrossRef CAS.
- S. E. Davis, B. N. Zope and R. J. Davis, Green Chem., 2012, 14, 143–147 RSC.
- S. E. Davis, L. R. Houk, E. C. Tamargo, A. K. Datye and R. J. Davis, Catal. Today, 2011, 160, 55–60 CrossRef CAS.
- In Eurasian Chemical Market International Magazine, 2011, 8, 56.
- T. A. Werpy and G. Petersen, U.S. Department of Energy, 2004.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental Section, Fig. S1, Fig. S2 and Table S1. See DOI: 10.1039/c2gc36536g |
| This journal is © The Royal Society of Chemistry 2013 |
