Brønsted acidic ionic liquids catalyze the high-yield production of diphenolic acid/esters from renewable levulinic acid†
Hai-Feng
Liu
ab,
Fan-Xin
Zeng
ab,
Li
Deng
*a,
Bing
Liao
a,
Hao
Pang
a and
Qing-Xiang
Guo
*c
aKey Laboratory of Cellulose and Lignocellulosics Chemistry, Guangzhou Institute of Chemistry, Chinese Academy of Sciences, Guangzhou, 510650, P. R. China. E-mail: dengli@gic.ac.cn; Fax: (+86) 2085232917; Tel: (+86) 2085232917
bUniversity of Chinese Academy of Sciences, Beijing, 100039, P. R. China
cDepartment of Chemistry, University of Science and Technology of China, Hefei 230026, P. R. China
First published on 30th October 2012
Abstract
The high-yielding synthesis of diphenolic acid (DPA) using Brønsted acidic ionic liquids (BAILs) has been demonstrated in this study. Importantly, p,p′-DPA was obtained as the preferential product over o,p′-DPA with an isomer ratio over 100. Moreover, diphenolic esters were also prepared in high yield through a one-pot method.
With the aim of achieving a sustainable future, growing efforts have been devoted to replacing fossil resources with renewable biomass. In this context, several bio-based platform chemicals and various routes based on these chemicals have been proposed.1 Among these routes, the condensation of levulinic acid (a versatile compound derived from carbohydrates) with phenols is of special interest because the product diphenolic acid (γ,γ-bishydroxyphenyl valeric acid, DPA) is not only an alternative for bisphenol A (BPA), which is widely used in the polycarbonate and epoxy resin industry, but also allows chemical modification on its intrinsic carboxylic group via esterification, reduction and amidation, potentially offering much more functionalized materials (Scheme 1).2
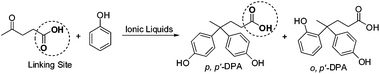 | ||
| Scheme 1 BAILs catalyzed condensation of levulinic acid and phenol. | ||
The synthesis of DPA is a traditional reaction, in which HCl and H2SO4 were usually employed as catalysts.3 Recently, this reaction has regained interest researchers and has been re-examined extensively with more robust and greener catalysts, i.e., solid acids instead of HCl or H2SO4, due to the attractive merits of levulinic acid and DPA. Clark and Guo et al. conducted the pioneering investigation on the synthesis of DPA using mesoporous silica-supported heteropolyacid catalysts and an excellent catalytic activity (TOF over 50 h−1) was achieved at 100 °C.4 To the same end, sulfonated hyperbranched polymer catalysts were prepared for this reaction by Sels et al.5 More importantly, they demonstrated the promoting effect of thiol compounds on the yield of DPA. Similar results can also be found in the study of BPA synthesis. Davis et al. systematically studied the enhanced catalytic activity caused by the combination of immobilized thiol groups and acid sites on the surface of solid acids and a possible mechanism for the role of the thiol group in the condensation was also proposed.6
Despite the recent advances in high TOFs and the role of thiol, the existing solid catalysts are only able to convert levulinic acid into DPA with a middle yield according to the literature.3–5 Therefore, an effective catalytic system for the high-yield production of DPA should be developed. Here, we prepared a series of BAILs (Fig. 1) for this condensation. BAILs are commonly recognized as robust, designable and eco-friendly solvents and catalysts for various organic reactions, especially for reactions involving bio-based chemicals.7 In terms of their application for DPA synthesis, they possess at least three advantages: (1) designable structure which enables the preparation of task-specific catalysts containing both acid sites and thiols, (2) low solubility in some organic extracts allowing easy recycling and (3) catalytic activity to catalyze esterification of DPA with alcohols into diphenolic esters. To our delight, the yield of DPA can be improved over 90 mol% in the presence of BAILs and diphenolic esters were also prepared with high yields in a one-pot method. Interestingly, an unexpected discrimination between the p,p′-DPA isomer and o,p′-DPA isomer, in the presence of BAILs, was observed. The ratio of p,p′-DPA to o,p′-DPA exceeds 100, which is the highest value reported to date.
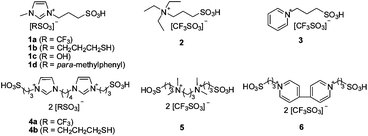 | ||
| Fig. 1 BAILs examined in this work. | ||
Table 1 shows the results of the ionic liquid 1a catalyzed condensation of levulinic acid with excessive phenol under different conditions. With 50 mol% 1a, the yield of DPA from levulinic acid and phenol (molar ratio = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) reached 33 mol% after 24 h at 60 °C, and extending the reaction time to 48 h afforded 55 mol% DPA and a p,p′-DPA to o,p′-DPA ratio of 16. Increasing the ratio of levulinic acid to phenol (1
4) reached 33 mol% after 24 h at 60 °C, and extending the reaction time to 48 h afforded 55 mol% DPA and a p,p′-DPA to o,p′-DPA ratio of 16. Increasing the ratio of levulinic acid to phenol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) gave lower yield of DPA (Table 1, entry 2). With higher loadings of 1a, the DPA yield was not improved but the isomer ratio rose to 20. Moreover, elevating the reaction temperature did not obviously increase the yield either (Table 1, entry 4) though higher TOFs could be achieved (Table S2†). A room temperature reaction could not provide DPA as a product, and only 38 mol% DPA was obtained at 50 °C. However, it should be noted that the low temperature is essential for the isomer ratio. Compared to the value at 60 or 70 °C, a higher ratio up to 40 was achieved at 50 °C. This also agrees with the experiments carried out at 80, 90 and 100 °C with much a lower ionic liquid loading (Table S2†), indicating the isomer ratio could be well tuned by the temperature together with the catalysts. Besides, the control experiment without catalyst also reveals that the reaction could not be auto-catalyzed by the carboxylic group of levulinic acid.
3) gave lower yield of DPA (Table 1, entry 2). With higher loadings of 1a, the DPA yield was not improved but the isomer ratio rose to 20. Moreover, elevating the reaction temperature did not obviously increase the yield either (Table 1, entry 4) though higher TOFs could be achieved (Table S2†). A room temperature reaction could not provide DPA as a product, and only 38 mol% DPA was obtained at 50 °C. However, it should be noted that the low temperature is essential for the isomer ratio. Compared to the value at 60 or 70 °C, a higher ratio up to 40 was achieved at 50 °C. This also agrees with the experiments carried out at 80, 90 and 100 °C with much a lower ionic liquid loading (Table S2†), indicating the isomer ratio could be well tuned by the temperature together with the catalysts. Besides, the control experiment without catalyst also reveals that the reaction could not be auto-catalyzed by the carboxylic group of levulinic acid.
| Entry | Loading of 1ab (mol%) | Temp. (°C) | Time (h) | Yield of DPAb (mol%) | Conversion of levulinic acid (mol%) | Ratio of p,p′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) o,p′ o,p′ |
|---|---|---|---|---|---|---|
| a Reaction conditions: levulinic acid (0.46 g, 4 mmol) and phenol (1.5 g, 16 mmol). b Based on the loading of levulinic acid. c Levulinic acid (0.46 g, 4 mmol) and phenol (1.13 g, 12 mmol). | ||||||
| 1 | 50 | 60 | 24 | 33 | 40 | 17 |
| 2c | 50 | 60 | 24 | 20 | 24 | 16 |
| 3 | 50 | 60 | 48 | 55 | 70 | 16 |
| 4 | 50 | 70 | 48 | 54 | 82 | 12 |
| 5 | 100 | 60 | 48 | 50 | 74 | 20 |
| 6 | 50 | 50 | 48 | 38 | 56 | 40 |
| 7 | 0 | 100 | 48 | 0 | 3 | — |
Next, another nine BAILs, which were readily prepared by adding acids to zwitterionic precursors (see ESI†), were examined in this reaction. Before the catalytic tests, their acidic strength was determined by UV-visible spectroscopy using nitroaniline as an indicator in methanol (Fig. S1 and Table S1†).8 However, no clear relationship could be found between the catalytic performance and acidic strength. The catalytic activities of the BAILs decreased in the order of 4b > 1a > 4a = 2 > 5 > 6 > 3 > 1c > 1d (Table 2, entries 1–8 and Table 1, entry 3). The thermal gravity analysis showed that the BAILs were stable at the temperature applied in this work (Fig. S2†). All the BAILs containing a triflic anion gave middle yields of DPA, between 44–55 mol%, which are lower than those catalyzed by the thiol-containing ionic liquid 4b (75 mol%, Table 2, entry 6). By comparison, H2SO4, triflic acid and 3-mercaptopropyl sulfonic acid were also tested and gave DPA in a yield of 64 mol%, 45 mol% and 80 mol%, respectively. The promoting effect of the thiol groups was confirmed again, which was in agreement with the results reported by Sels et al.5
| Entry | Catalysts | Additives | Yield of DPA (mol%) | Ratio of p,p′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) o,p′ o,p′ |
|---|---|---|---|---|
| a Reaction conditions: levulinic acid (0.46 g, 4 mmol), phenol (1.5 g, 16 mmol), catalysts (2 mmol) and additives (0.2 mmol), 60 °C for 48 h. b 1 mmol germinal dicationic BAILs. c 0.9 mmol 4a, 0.1 mmol 4b. d Only trace amount of o,p′-DPA was detected. e Without Ar protection. | ||||
| 1 | 1c | — | 35 | 32 |
| 2 | 1d | — | 13 | 30 |
| 3 | 2 | — | 50 | 21 |
| 4 | 3 | — | 44 | 33 |
| 5 | 4a | — | 50 | 32 |
| 6 | 4b | — | 75 | 50 |
| 7 | 5 | — | 48 | 33 |
| 8 | 6 | — | 47 | 33 |
| 9 | H2SO4 | — | 64 | 24 |
| 10 | CF3SO3H | — | 45 | 25 |
| 11 | HS(CH2)3SO3H | — | 80 | 30 |
| 12 | — | HSCH2COOH | — | — |
| 13 | 1a | HSCH2COOH | 79 | 9 |
| 14 | 2 | HSCH2COOH | 80 | 9 |
| 15 | 3 | HSCH2COOH | 87 | 9 |
| 16 | 4a | HSCH2COOH | 88 | 20 |
| 17 | 5 | HSCH2COOH | 79 | 13 |
| 18 | 6 | HSCH2COOH | 81 | 9 |
| 19 | H2SO4 | HSCH2COOH | 76 | 9 |
| 20 | 4a | 1b | 85 | >100d |
| 21 | 4a | 4b | 91 | >100d |
| 22 | 4a | 4b | 59 | 40 |
It is noteworthy that the isomer ratio rose up to 50 in the presence of 4b, revealing the advantage of combining thiol groups and an acidic ionic liquid. The ratio could also be increased to more than 30 by using 1c, 1d, 3 and geminal dicationic type BAILs (4a, 5 and 6).
Considering the promoting effect of thiol groups, mercaptoacetic acid was employed as an additive in order to achieve higher yields. The results (Table 2, entries 12–19) clearly show that the yields of DPA increased to 76–88 mol% with the addition of mercaptoacetic acid in the presence of BAILs as well as H2SO4, though mercaptoacetic acid alone exhibited no catalytic activity due to the low acidic strength of the carboxylic group. Nevertheless, it resulted in an undesired decline in the isomer ratio to a different extent. This is quite different from the former reported promotive performance of thiol compounds in the presence of solid acids.5,6 Although the exact reason for this was not clear, the BAIL catalytic system might be responsible for the unusual results.
Surprisingly, with 4a and 4b (molar ratio = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), this condensation could not only be improved with a DPA yield of 91%, but also offered a high selectivity for p,p′-DPA. Only a trace amount of o,p′-DPA was detected by high-performance liquid chromatography (HPLC, Fig. S3†). The results are better than those of 4b and 3-mercaptopropyl sulfonic acid used solely (Table 2, entries 6 and 11). Similar promoting effects of 1b were also found, revealing that the thiol-containing anion in BAILs played a key role in the high-yield and selective synthesis of DPA. Although a reasonable mechanism has not been obtained, the preference for p,p′-DPA should be attributed to the cooperation of thiol groups and cationic counterparts in the ionic liquid. Besides, it should be not ed that the reaction involving thiols should be conducted carefully under an inert atmosphere because the air could reduce the yield and isomer ratio significantly (Table 2, entry 22). A possible reason may be that the thiol groups were oxidized under air, although 4b is of good thermal stability according to the thermal gravity analysis (TGA, Fig. S2†).
1), this condensation could not only be improved with a DPA yield of 91%, but also offered a high selectivity for p,p′-DPA. Only a trace amount of o,p′-DPA was detected by high-performance liquid chromatography (HPLC, Fig. S3†). The results are better than those of 4b and 3-mercaptopropyl sulfonic acid used solely (Table 2, entries 6 and 11). Similar promoting effects of 1b were also found, revealing that the thiol-containing anion in BAILs played a key role in the high-yield and selective synthesis of DPA. Although a reasonable mechanism has not been obtained, the preference for p,p′-DPA should be attributed to the cooperation of thiol groups and cationic counterparts in the ionic liquid. Besides, it should be not ed that the reaction involving thiols should be conducted carefully under an inert atmosphere because the air could reduce the yield and isomer ratio significantly (Table 2, entry 22). A possible reason may be that the thiol groups were oxidized under air, although 4b is of good thermal stability according to the thermal gravity analysis (TGA, Fig. S2†).
On the basis of the above results, we attempted to study the preparation of diphenolic esters since esters are more convenient than carboxylic acids for further transformation in principle, such as amidation and hydrogenation. Moreover, the esterification can also facilitate the extraction of the product from BAILs by organic solvents due to the higher oleophilicity compared to DPA. Before performing this conversion, we proposed two strategies: (1) condensation of levulinates with phenol and (2) esterification of DPA after condensation. Both strategies can be realized through a one-pot reaction because the BAILs are good catalysts for esterification. The results reveal that by replacing ethyl levulinate for levulinic acid, the main product was ethyl diphenolic ester with middle yields, and DPA was formed as the co-product due to hydrolysis during the reaction. The total yield of condensation products was about 80 mol% (Table 3, entries 1–3). Similar results were obtained by using methyl levulinate and n-butyl levulinate, which afforded diphenolic esters with yields of 57 mol% and 65 mol%, respectively. On the other hand, the yields of diphenolic esters could be improved effectively and most DPA was transformed into the corresponding esters without obvious decomposition of DPA by adding alcohols for esterification at 40 °C for another 8 h after the condensation of levulinic acid with phenol. Based on this sequence (i.e., condensation followed by esterification), over 83 mol% diphenolic esters were obtained and the total yield of diphenolic esters and DPA was close to that of DPA before esterification (Table 3, entries 6–8).
| Entry | Substrates | Additives | DPA yield (mol%) | Diphenolic ester yield (mol%) | Total yield (mol%) |
|---|---|---|---|---|---|
| a Reaction conditions for entries 1–5: levulinates (4 mmol), phenol (1.5 g, 16 mmol), 4a (0.9 mmol) and additives (0.2 mol or 0.1 mmol 4b) at 60 °C for 48 h. Reaction conditions for entries 6–8: levulinic acid (0.46 g, 4 mmol), phenol (1.5 g, 16 mmol), 4a (0.9 mmol) and 4b (0.1 mol) at 60 °C for 48 h and 2 mL alcohol (methanol for entry 6, ethanol for entry 7 and n-butanol for entry 8) at 40 °C for 8 h. | |||||
| 1 | Ethyl levulinate | HSCH2COOH | 19 | 54 | 73 |
| 2 | Ethyl levulinate | 1b | 21 | 56 | 77 |
| 3 | Ethyl levulinate | 4b | 22 | 58 | 80 |
| 4 | Methyl levulinate | 4b | 22 | 57 | 79 |
| 5 | n-Butyl levulinate | 4b | 16 | 65 | 81 |
| 6 | Levulinic acid | 4b | 2 | 87 | 89 |
| 7 | Levulinic acid | 4b | 6 | 83 | 89 |
| 8 | Levulinic acid | 4b | 4 | 86 | 90 |
Finally, the BAILs recycling experiment was also carried out by extraction of DPA and diphenolic esters, as well as phenol with ethyl acetate (see ESI†). After extraction with 25 mL ethyl acetate 3 times, the recovery rate of the ethyl diphenolic ester was 82%, which was close to the above mentioned yield of ethyl diphenolic ester (Table 3, entry 7), indicating that most of the ethyl diphenolic ester was transferred into the organic phase from the BAILs catalytic system. By contrast, the recovery rate of DPA was only 65% under the same extraction conditions. This result evidently showed that the partition of ethyl diphenolic ester in the extraction could be enhanced effectively. Importantly, the regenerated BAILs also provided high catalytic performance for both the condensation and esterification reactions. Only a slight decrease in the yield of the ethyl diphenolic ester occurred after the first run and the following recycle catalytic tests afforded ethyl diphenolic ester with yields between 74–78 mol% (Fig. 2).
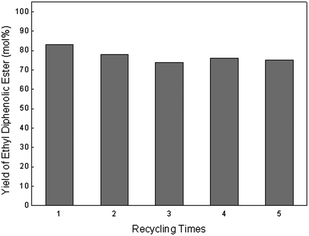 | ||
| Fig. 2 Yield of ethyl diphenolic ester in recycled catalytic runs. Reaction conditions: 4a (4.5 mmol), 4b (0.5 mmol), levulinic acid (2.32 g, 20 mmol) and phenol (7.52 g, 80 mmol) reacted at 60 °C for 48 h and ethanol (10 mL) at 40 °C for another 8 h. | ||
Conclusions
In summary, this communication has demonstrated the viability of the BAILs catalyzed condensation of renewable levulinic acid with phenol. Note that the addition of thiol-containing BAILs offers both a high yield of DPA (over 90 mol%) and a unique preference for the p,p′-DPA isomer. The successive esterification of DPA provides distinct advantages in terms of chemical modification and product separation. The recycling of BAILs has also been carried out in this study and the results suggest that the BAILs retain a high catalytic performance after 5 runs. Moreover, to extend the scope of the feedstocks and to explore the value of this catalytic system, further studies on the synthesis of other diphenolic compounds from levulinic acid, acetone, aldehydes and renewable phenols derived from lignin9 is in progress.Acknowledgements
The authors are grateful to the NFSC-Guangdong Joint Fund (U0834005), Youth Innovation Promotion Association, CAS and Foshan Centre for Functional Polymer Materials, CAS for financial support.Notes and references
- (a) T. Werpy and G. Petersen, Top Value Added Chemicals From Biomass, 2004, vol. I Search PubMed; (b) J. H. Clark, V. Budarin, F. E. I. Deswarte, J. J. E. Hardy, F. M. Kerton, A. J. Hunt, R. Luque, D. J. Macquarrie, K. Milkowski, A. Rodriguez, O. Samuel, S. J. Tavener, R. J. White and A. J. Wilson, Green Chem., 2006, 8, 853 RSC; (c) J. J. Bozell and G. R. Petersen, Green Chem., 2010, 12, 539 RSC; (d) A. M. Ruppert, K. Weinberg and R. Palkovits, Angew. Chem., Int. Ed., 2012, 51, 2564 CrossRef CAS; (e) S. V. Vyver, J. Geboers, P. A. Jacobs and B. F. Sels, ChemCatChem, 2011, 3, 82 CrossRef; (f) J. A. Geboers, S. V. Vyver, R. O. Beeck, P. A. Jacobs and B. F. Sels, Catal. Sci. Technol., 2011, 1, 714 RSC; (g) R. Rinaldi and F. Schuth, ChemSusChem, 2009, 2, 1096 CrossRef CAS; (h) J. Zhang, S. Wu, B. Li and H. Zhang, ChemCatChem, 2012, 4, 1230 CrossRef CAS.
- A. Corma, S. Iborra and A. Velty, Chem. Rev., 2007, 107, 2411 CrossRef CAS.
- (a) A. R. Bader and A. D. Kontowicz, J. Am. Chem. Soc., 1954, 76, 4465 CrossRef CAS; (b) Y. Isoda and M. Azuma, JP Pat., 08053390, 1994 Search PubMed; (c) H. Itsuda and M. Kawamura, JP Pat., 61186346, 1986 Search PubMed; (d) R. E. W. Romeo and Z. G. Gardlund, US Pat., 3567686, 1971 Search PubMed; (e) G. J. Willems and J. Liska, EP Pat., 933348, 1999 Search PubMed.
- (a) Y. H. Guo, K. X. Li and J. H. Clark, Green Chem., 2007, 9, 839 RSC; (b) Y. H. Guo, K. X. Li, X. D. Yu and J. H. Clark, Appl. Catal., B, 2008, 81, 182 CrossRef CAS; (c) X. D. Yu, Y. H. Guo, K. X. Li, X. Yang, L. L. Xu, Y. N. Guo and J. L. Hu, J. Mol. Catal. A: Chem., 2008, 290, 44 CrossRef CAS; (d) K. X. Li, J. L. Hu, W. Li, F. Y. Ma, L. L. Xu and Y. H. Guo, J. Mater. Chem., 2009, 19, 8628 RSC.
- (a) S. V. Vyver, J. Geboers, S. Helsen, F. Yu, J. Thomas, M. Smet, W. Dehaen and B. F. Sels, Chem. Commun., 2012, 48, 3497 RSC; (b) S. V. Vyver, J. Thomas, J. Geboers, S. Keyzer, M. Smet, W. Dehaen, P. A. Jacobs and B. F. Sels, Energy Environ. Sci., 2011, 4, 3601 RSC.
- (a) V. Dufaud and M. E. Davis, J. Am. Chem. Soc., 2003, 125, 9403 CrossRef CAS; (b) R. K. Zeidan, V. Dufaud and M. E. Davis, J. Catal., 2006, 239, 299 CrossRef CAS; (c) E. L. Margelefsky, R. K. Zeidan, V. Dufaud and M. E. Davis, J. Am. Chem. Soc., 2007, 129, 13691 CrossRef CAS; (d) E. L. Margelefsky, A. Bendjeriou, R. K. Zeidan, V. Dufaud and M. E. Davis, J. Am. Chem. Soc., 2008, 130, 13442 CrossRef CAS.
- (a) T. Welton, Chem. Rev., 1999, 99, 2071 CrossRef CAS; (b) J. P. Hallett and T. Welton, Chem. Rev., 2011, 111, 3508 CrossRef CAS; (c) M. E. Zakrzewska, E. Bogel-Lukasik and R. Bogel-Lukasik, Chem. Rev., 2011, 111, 397 CrossRef CAS; (d) H. B. Zhao, J. E. Holladay, H. Brown and Z. C. Zhang, Science, 2007, 316, 1597 CrossRef CAS; (e) G. Yong, Y. G. Zhang and J. Y. Ying, Angew. Chem., Int. Ed., 2008, 47, 9345 CrossRef CAS; (f) S. Q. Hu, Z. F. Zhang, J. L. Song, Y. X. Zhou and B. X. Han, Green Chem., 2009, 11, 1746 RSC.
- (a) C. Thomazeau, H. Olivier-Bourbigou, L. Magna, S. Luts and B. Gilbert, J. Am. Chem. Soc., 2003, 125, 5264 CrossRef CAS; (b) Y. Wang, D. Jiang and L. Dai, Catal. Commun., 2008, 9, 2475 CrossRef CAS; (c) H. Xing, T. Wang, Z. Zhou and Y. Dai, J. Mol. Catal. A: Chem., 2007, 264, 53 CrossRef CAS.
- H. A. Meylemans, T. J. Groshens and B. G. Harvey, ChemSusChem, 2012, 5, 206 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2gc36630d |
| This journal is © The Royal Society of Chemistry 2013 |
