Laser ablation (imaging) for mapping and determining Se and S in sunflower leaves
Marcelo Anselmo Oseas
da Silva
and
Marco Aurelio Zezzi
Arruda
*
Spectrometry, Sample Preparation and Mechanization Group (GEPAM) and National Institute of Science and Technology for Bioanalytics – Institute of Chemistry, University of Campinas – Unicamp, PO Box 6154, 13083-970, Campinas, SP, Brazil. E-mail: zezzi@iqm.unicamp.br; Fax: +55 19 3521 3023; Tel: +55 19 3521 3089
First published on 26th November 2012
Abstract
Qualitative and quantitative methods were developed for selenium and sulphur mapping/quantification in sunflower (Helianthus annuus L.) leaves. The plants were grown for 50 days in a greenhouse, being divided into two groups, one irrigated with only deionized water and the other treated with a daily dose of 7.58 mg of Na2SeO3. Leaves were collected during the growth period for directly evaluating the distribution of Se and S in these structures. For quantification analysis, pellets were produced from both CRM (100 and 1575a for Se and S, respectively) and the sunflower materials. The pellets were doped with 25 and 1200 μg g−1 Se and 5 and 20 mg g−1 S and analyzed by LA-ICP-MS. For accuracy purposes all the samples were also decomposed via microwave and analyzed by ICP-MS. To avoid polyatomic interferences Se and S were monitored as SeO+ and SO+ at m/z 96 and 48, respectively, 12C+ was used as an internal standard, and the ratios between SeO+/C+ and SO+/C+ were used for measurements. Statistic tests (t test at 95% confidence level) confirming good agreement between LA-ICP-MS and ICP-MS indicated the accuracy of this technique.
Introduction
Studies seeking to understand plant metal(oid) metabolism are important for nutrition (both plant and human), physiology, and toxicology, and have grown exponentially in number in the last decade. This can be attributed to new areas in science, such as metabolomics,1 and metallomics,2–4 and, in part, due to the development of new technologies, including those based on imaging.5–7 Depending on the imaging resolution, a tissue,8 organelle9 or cellular system10 can be mapped, enlarging the understanding of a studied system, and making in situ analysis possible.Imaging techniques applied to inorganic species include X-ray emission spectrometry,11 secondary ion mass spectrometry,12 autoradiography,13 and laser ablation imaging mass spectrometry – LA(i)-ICP-MS, the latter being the most recently developed technique, as demonstrated in a diversity of applications.14–20
Although great efforts are made to maximize the resolution as well as the quality of the images obtained through these systems, there are few applications in the literature focusing on quantitative analysis. Not least, the calibration and accuracy are challenging, such that it is often not possible to compare analysed samples with certified reference materials because of matrix differences, preventing conclusive in situ analysis.21 Then, strategies for maximizing the use of quantitative imaging techniques should be encouraged for solving these drawbacks, in order to fully explore the potential of these techniques.
This work reports a method for evaluating the distribution of selenium and sulphur in sunflower leaves through LA(i)-ICP-MS while quantitative analyses were also successfully carried out using this technique. For this task, different certified materials were used for validation of the method, including the use of a sunflower sample as a matrix, once the calibration curve was measured using doped Se or S pellets from these materials. Thus, imaging and direct determination of Se and S in sunflower leaves via LA-ICP-MS were possible.
Experimental
The reagents for pellet preparation and plant cultivation were from Merck (Darmstadt, Germany). All solutions were prepared in polypropylene flasks with deionized water (≥18.2 MΩ cm) from a Milli-Q water purification system (Millipore, Bedford, USA).Sunflower cultivation
The sunflower plants (Helianthus annuus L.) used in this study were cultivated in a greenhouse, and the protocols adopted for cultivation were previously described in the literature.22 Seeds were germinated in 1 L plastic pots, containing autoclaved-sterilized soil (400 mg per pot) with pH (5.5) and phosphate concentration adjusted to promote adequate growth of the sunflower plants.22 The germination took about 3 days and during the first seven days, the plants were irrigated using only deionized water. After this period, they were divided into two groups, one irrigated with deionized water only and the other treated with a daily dose of 7.58 mg of selenium (2200 mg L−1 Na2SeO3 solution was used as a selenium source). The daily volume of water added to the plants, and then the concentration of selenium solution, varied along the cultivation period and the needs of the sunflowers.Leaves were collected from the upper region of the plants throughout the cultivation period (after 29, 45 and 50 days of cultivation) for directly evaluating the distribution of selenium and sulfur in these structures as well as to validate the methodology using the cultivated vegetal material as a matrix source for preparation of standards. Considering the sunflower variety used in this work, flowering begins about 50 days after germination.
Methodology of validation
The proposed procedure for quantification of S and Se was carried out using pellets produced in our laboratory from certified reference materials as well as vegetal materials obtained during the cultivation of sunflowers.A certified reference material CRM 100 – minor and trace elements in beech leaves produced by the Institute for Reference Materials and Measurements – was used to prepare a calibration curve, allowing quantification. In this case, the CRM was spiked with 5.0, 10.0, 15.0 and 20.0 mg g−1 of S. The proposed procedure for pellet preparation consisted of the addition of 500 μL of NH4SO4 solution with different concentrations to 200 mg of CRM. The material was homogenized for 10 min and allowed to stand for 2 days. Then, the spiked portions of the solids were mixed using a mortar and pestle and pressed at 7 psi, producing pellets with different concentrations. Pellets were also prepared using the reference material 1575a, tomato leaves, produced by National Institute of Science and Technology, for validating the proposed analytical procedure for S.
Leaves of sunflowers obtained from plants treated with Se were dried, ground into fine powder in a mortar using liquid nitrogen, and dried at 40 °C until constant weight. The material was pressed under the same conditions as the standards.
For Se quantification, the certified reference material 1575a – tomato leaves – was selected for the calibration curve. Pellets with Se concentrations varying between 25 and 1200 μg g−1 were produced using the same analytical procedure described for S. Pellets were also prepared using the certified reference material CRM 281, trace elements in rye grass, produced by the Institute for Reference Materials and Measurements, in order to validate the analytical procedure for Se.
Sunflower leaves, obtained from control plants, were also used for preparing the pellets. In this case, the material was spiked with 100 μg g−1 of Se following the same procedure used to prepare the standards.
Based on the results obtained the pellets prepared using the standard reference materials CRM 100 – minor and trace elements in beech leaves – and 281 – trace elements in rye grass – were used for synthetic laboratory standard calibration of S and Se, respectively, during the direct elemental analysis of sunflower leaves.
After the analysis via LA-ICP-MS, the pellets prepared with sunflower leaf material as well as those with certified materials used for validating the analytical procedure were decomposed. For this, a mixture of 6.0 mL of sub boiling concentrated nitric acid and 0.5 mL of hydrogen peroxide was employed. A microwave oven (DGT, ProvectoAnalítica) with a nominal power of 1200 W was used to perform the procedure,23 comprised of three steps: 400 W at 5 min, 790 W at 8 min and 320 W at 4 min. Then, the samples were gently heated to evaporate the excess of nitric acid, and the volumes adjusted to 10 mL using 1.0% (v/v) nitric acid. Se and S determinations were performed with the ICP-MS operating with the standard sample introduction system, consisting of a cyclonic spray chamber and a Meinhard® nebulizer, according to the conditions shown in Table 1.
| LA-ICP-MS | ICP-MS (digested samples) | |
|---|---|---|
| RF power (W) | 1000 | 1200 |
| Carrier gas flow rate – argon (L min−1) | 1.1 | 0.91 |
| Auxiliary gas flow rate – argon (L min−1) | 1.8 | 1.2 |
| Reading mode | Peak hopping | Peak hopping |
| Dwell time (ms) | 63 | 60 |
| Integration time for each point (ms) | 315 | 1000 |
| Detector dead time (ns) | 60 | 60 |
| Sweeps | 5 | 20 |
| Conditions for the operation of the dynamic reaction cell | ||
| Element | Monitored species | Oxygen flow rate (L min−1) | RPq (V) | Rpa (V) |
|---|---|---|---|---|
| Selenium | 80Se16O+ | 0.70 | 0.50 | 0 |
| Sulfur | 32S16O+ | 0.70 | 0.50 | 0 |
| Carbon | 12C+ | 0.70 | 0.50 | 0 |
Sample preparation and analysis of sunflower leaves
Sunflower leaves from Se treated plants were harvested after 29 and 45 days of cultivation. For the control plants the leaves were harvested after 50 days of cultivation. The leaves were selected randomly and measured by LA-ICP-MS.To minimize the possibility of signal variations during the analyses, due to changes in water content, and also to increase the amount of ablated material introduced into the mass spectrometer with time, sunflower leaves were selected randomly, harvested and immediately dried at 40 °C until constant weight. After the drying process, the leaves were fixed onto acetate double-sided adhesive tape (3 M, Brazil) and placed into the ablation chamber for analysis.
A quadrupole-based ICP-MS (PerkinElmer ELAN DRC-e) coupled with a laser ablation system (New Wave UP 213) was used for imaging Se and S distribution in sunflower leaves. The analytical strategy adopted for carrying out the determination of Se and S free of interferences was based on the reaction of Se+ and S+ species formed in the plasma with oxygen in the reaction cell of the instrument. In this case, the monitored species consisted of the product ions SeO+ and SO+ at m/z 96 and 48, respectively. The parameters for the operation of the ICP-MS are also shown in Table 1. These conditions were previously optimized for monitoring S and Se using this hyphenation.22,24
To compensate for possible variations in the ablation process,25 which can be related to changes in the ablation process itself or to the heterogeneity of the prepared standards, 12C+ was chosen as the internal standard to normalize the analytical signals. Then, 12C+ is an interesting alternative for signal normalization since the reaction cell of the instrument is pressurized and optimized for the determination of Se and S. In this situation, the obtained signal for carbon was attenuated and ratios between SeO+/C+ and SO+/C+ were typically 0.001 and 1.0, respectively.
The conditions adopted for the analysis are described in Table 2, and adjusted for achieving the maximum material removal while avoiding cutting of the leaves by the laser during the analysis. Regarding resolution, the distance between successive lines was set to 300 μm.
| Wavelength of Nd:YAG laser (nm) | 213 |
| Frequency (Hz) | 20 |
| Laser intensity (%) | 80 |
| Average energy output (mJ) | 1.9 |
| Average fluence (J cm2) | 22 |
| Scan speed (μm s−1) | 70 |
| Laser beam diameter (μm) | 110 |
| Resolution – X axis (μm) | 22.05 |
| Resolution – Y axis (μm) | 300 |
The pellets prepared for the calibration of the instrument and for validating the method were scanned in five randomly selected regions, and for each one, 100 points were collected for each monitored ion. The average value was calculated and the ratios between SeO+/C+ and SO+/C+ were determined.
The software MatLab version 6.5 was used to construct the images of the scanned leaves and correlate the obtained signals with the concentrations of the elements in the leaves.
Results and discussion
Validation of the analytical procedure
The development of quantitative procedures for the evaluation of the elemental distribution in different materials, in special biological tissues, still presents a challenge because of the difficulty in finding a matrix that exactly matches the characteristics of the sample. In the present case, two other points hamper even more the determinations. The first consists of the fact that Se is considered a micronutrient and it is present at low concentrations in biological tissues,26 making difficult its determination. The second refers to the content of S in the certified reference material used for preparing the calibration curve. In this situation, the lowest limit for the calibration curve depends on the concentration of S present in the certified material.Taking into account these two points, the calibration curves obtained with the prepared pellets are shown in Fig. 1. The correlation coefficients obtained for Se and S were 0.9999 and 0.9992, respectively, indicating that the proposed procedure for pellet preparation as well as the strategy adopted for data collection and treatment were adequate.
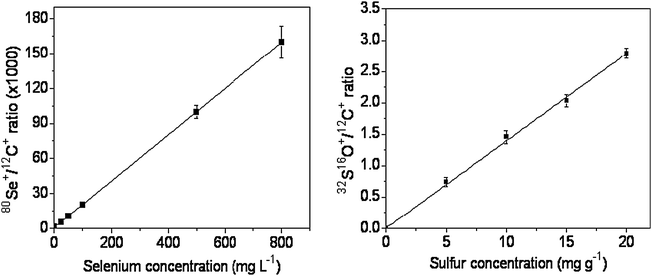 | ||
| Fig. 1 Calibration curves for Se and S, obtained after the analysis of certified reference materials pellets. | ||
The results related to the validation of the procedure can be seen in Tables 3 and 4 for Se and S, respectively. The precision of measurements using the hyphenation LA-ICP-MS is lower and explained in terms of the analysis of solid samples.27 In fact, all other data were obtained through techniques using analysis of solutions, which present homogeneity at the molecular level, thus explaining the higher precisions. The application of the t-test at 95% confidence level indicates good agreement between each group of the results.
Analysis of the images
Fig. 2 shows the results obtained for the distribution of Se and S in the leaves. For the leaves corresponding to plants treated with Se after 29 days of cultivation, Fig. 2A shows that Se is present mainly at the tip of the leaf. Additionally, the leaf itself (shown in the upper right corner) presents chlorosis regions caused by the excess of Se added during cultivation. However, a direct correlation between the accumulation of Se in a specific region of the leaf and morphological problems in the leaf was not observed. When the Se distribution is compared with the profile obtained for S (see Fig. 2B), a correlation between both species is noted. In a previous work,22 the authors reported that sunflower plants irrigated with Se preferentially promoted the translocation of this element to the leaves. In addition, the concentration of S in this region of the plant also increased, probably due to activation of the S uptake pathway. The literature reports that Se can replace S in amino acids,23,28 and this result indicates that the metabolism of S can change in the presence of high concentration of Se.21,29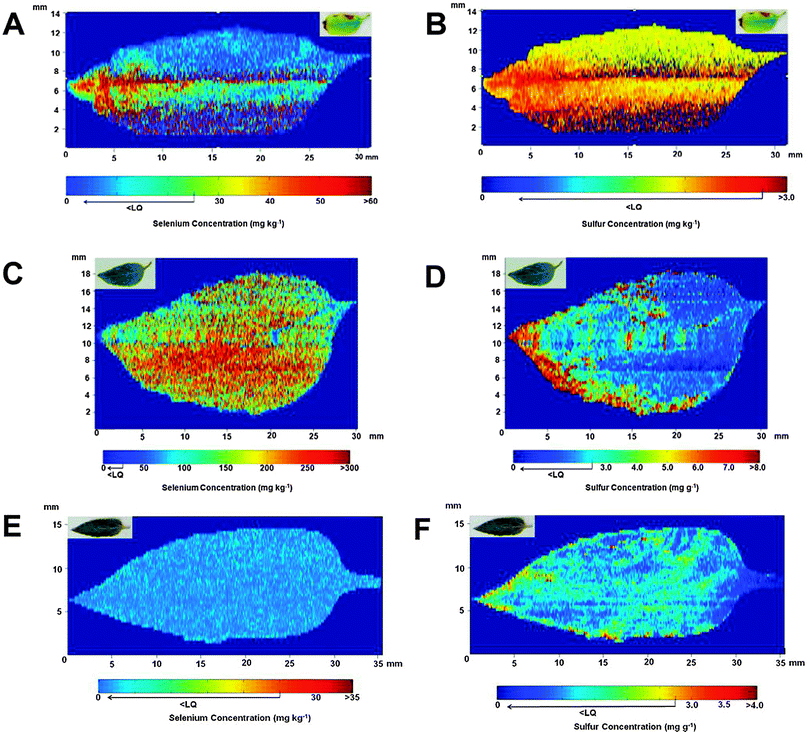 | ||
| Fig. 2 Se (images A, C and E) and S (images B, D and F) distributions observed in sunflower leaves after 29 (A and B), 45 (C and D) and 50 (E and F) days of cultivation. Leaves in A and B were irrigated with 106 mg of Se (sodium selenite), and in C and D with 174 mg of Se. Both Se and S distributions are shown in the sunflower leaves (E and F) collected from the control group after 50 days of cultivation. The picture of each leaf analyzed is shown on the upper left side of the image. | ||
The evaluation of a leaf after 45 days of cultivation reveals that the levels of Se increased ca. 5 times when compared with the leaf studied after 29 days, as can be seen in Fig. 2C. In this case, the distribution of Se was essentially homogeneous in all regions of the leaf. It is necessary to emphasize that under both studied conditions, part of the Se may have been lost during the sample preparation procedure since some volatile Se compounds, such as dimethylselenide and dimethyldiselenide, are supposed to be present in the leaves30 and could have been eliminated during the heating process. Related to the S distribution shown in Fig. 2D, although its level has been increased when compared with the leaf after 29 days of cultivation, this element is mainly concentrated at the tip of the structure and it is not homogeneously distributed in the leaf.
The comparison between Se treated plants and the control group reveals that both Se and S levels are lower in the control plants. The concentration of Se for the reference plants shown in Fig. 2E is lower than 25 μg g−1. The same profile is observed for S, as can be seen in Fig. 2F, but, in this case, the level of this element is close to the level found in the certified reference material, which is in the expected concentration of S in leaves for most of the plants.
Conclusions
The initial purpose of mapping and determining Se in leaves after adding sodium selenite to the sunflower plants was successfully attained. Besides good accuracy obtained by quantitative laser ablation, which is due not only to the optimization for SeO+ formation inside the reaction cell of the equipment for eliminating/avoiding polyatomic interferences, but also to the use of 12C+ as the internal standard for compensating signal fluctuations during ablation, this technique was shown to be useful for mapping Se and S in sunflower leaves, demonstrating different behavior in terms of Se and S distributions in the leaves. Additionally, through the LA(i) it was also be possible to conclude that Se is not present in the oxidized regions of the leaves. Another important point in the work was the lower than usual plasma radiofrequency used (1000 W), making possible good sensitivity even when working with a different mass–charge ratio from 80 (most abundant Se isotope).Finally, this work demonstrates that both LA(i)-ICP-MS and LA-ICP-MS are excellent techniques for macro or local analyses, since optimization conditions can be carefully evaluated.
Notes and references
- N. Verbruggen, C. Hermans and H. Schat, New Phytol., 2009, 181, 759 CrossRef CAS.
- S. C. Booth, M. L. Workentine, A. M. Weljie and R. J. Turner, Metallomics, 2011, 3, 1142 RSC.
- D. Esteban-Fernandez, E. Moreno-Gordaliza, B. Canas, M. A. Palacios and M. M. Gomez-Gomez, Metallomics, 2010, 2, 19 RSC.
- S. Mounicou, J. Szpunar and R. Lobinski, Chem. Soc. Rev., 2009, 38, 1119 RSC.
- M. Ha, J. H. Kwak, Y. Kim and O. P. Zee, Food Chem., 2012, 133, 1155 CrossRef CAS.
- Y. J. Lee, D. C. Perdian, Z. Song, E. S. Yeung and B. J. Nikolau, Plant J., 2012, 70, 81 CrossRef CAS.
- S. Kaspar, M. Peukert, A. Svatos, A. Matros and H. P. Mock, Proteomics, 2011, 11, 1840 CrossRef CAS.
- Y. Li, B. Shrestha and A. Vertes, Anal. Chem., 2008, 80, 407 CrossRef CAS.
- P. J. Horn, A. R. Korte, P. B. Neogi, E. Love, J. Fuchs, K. Strupat, L. Borisjuk, V. Shulaev, Y. J. Lee and K. D. Chapman, Plant Cell, 2012, 24, 622 CrossRef CAS.
- D. Holscher, R. Shroff, K. Knop, M. Gottschaldt, A. Crecelius, B. Schneider, D. G. Heckel, U. S. Schubert and A. Svatos, Plant J., 2009, 60, 907 CrossRef.
- K. J. Cloete, W. J. Przybylowicz, J. Mesjasz-Przybylowicz, A. D. Barnadas, A. J. Valentine and A. Botha, Plant, Cell Environ., 2010, 33, 1005 CrossRef CAS.
- N. Grignon, S. Halpern and J. Jeusset, J. Microsc. (Oxford, U. K.), 1997, 186, 51 CAS.
- S. N. Gray, J. Dighton, S. Olsson and D. H. Jennings, New Phytol., 1995, 129, 449 CrossRef CAS.
- J. S. Becker, Int. J. Mass Spectrom., 2010, 289, 65 CrossRef CAS.
- J. Lear, D. J. Harc, F. Fryer, P. A. Adland, D. I. Finkelstein and P. A. Doble, Anal. Chem., 2012, 84, 6707 CrossRef CAS.
- D. S. Urgast, D. G. Ellingsen, B. Berlinger, E. Eilertsen, G. Friisk, V. Skoug, Y. Thomassen, J. H. Beattie, I.-S. Kwuan and J. Feldmann, Anal. Bioanal. Chem., 2012, 404, 89 CrossRef CAS.
- B. Wu and J. S. Becker, Int. J. Mass Spectrom., 2012, 323–324, 34 CAS.
- I. Konz, B. Fernández, M. L. Fernández, R. Pereiro and A. Sanz-Medel, Anal. Bioanal. Chem., 2012, 403, 2113 CrossRef CAS.
- J. A. T. Pugh, A. G. Cox, C. W. McLeod, J. Bunch, M. J. Waiter, S. L. Hart, A. Bienemann, E. White and J. Bell, Anal. Bioanal. Chem., 2012, 403, 1641 CrossRef CAS.
- M. W. Bourassa and L. M. Miller, Metallomics, 2012, 4, 721 RSC.
- J. S. Becker, Inorganic Mass Spectrometry. Principles and Applications, Wiley, 2007 Search PubMed.
- M. A. O. da Silva, S. A. L. Andrade, P. Mazzafera and M. A. Z. Arruda, Int. J. Mass Spectrom., 2011, 307, 55 CrossRef.
- J. S. Garcia, P. L. Gratão, R. A. Azevedo and M. A. Z. Arruda, J. Agric. Food Chem., 2006, 54, 8623 CrossRef CAS.
- M. A. O. da Silva and M. A. Z. Arruda, Microchim. Acta, 2012, 176, 131 CrossRef.
- B. Wu, Y. Chen and J. S. Becker, Anal. Chim. Acta, 2009, 633, 165 CrossRef CAS.
- J. Lu and A. Holmgren, J. Biol. Chem., 2009, 284, 723 CrossRef CAS.
- B. Wu, M. Zoriy, Y. Chen and J. S. Becker, Talanta, 2009, 78, 132 CrossRef CAS.
- M. J. Hawkesford and L. J. De Kok, Sulfur in Plants: An Ecological Perspective, Springer, 2007 Search PubMed.
- M. J. Hawkesford and L. J. Kok, Plant, Cell Environ., 2006, 29, 382 CrossRef CAS.
- P. J. White, H. C. Bowen, P. Parmaguru, M. Fritz, W. P. Spracklen, R. E. Spiby, M. C. Meacham, A. Mead, M. Harriman, L. J. Trueman, B. M. Smith, B. Thomas and M. R. Broadley, J. Exp. Bot., 2004, 55, 1927 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2013 |
