New benzotrithiophene derivative with a broad band gap for high performance polymer solar cells†
Xiaoli
Zhao
abc,
Dalei
Yang
ad,
Hongying
Lv
abc,
Li
Yin
d and
Xiaoniu
Yang
*ab
aPolymer Composites Engineering Laboratory, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, 5625 Renmin Street, Changchun 130022, P. R. China. E-mail: xnyang@ciac.jl.cn
bState Key Laboratory of Polymer Physics and Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, 5625 Renmin Street, Changchun 130022, P. R. China
cGraduate School of the Chinese Academy of Sciences, Beijing 100049, P. R. China
dChangchun University of Technology, Changchun 130012, P.R. China
First published on 25th October 2012
Abstract
A new planar conjugated polymer, poly{benzo(1,2-b:3,4-b′:5,6-d′′)trithiophene-alt-4,4′-dihexyl-2,2′-bithiazole} (BTT-BTz), with a broad band gap and higher Voc based on benzotrithiophene and bithiazole units, was designed and synthesized. This copolymer possesses a deeper HOMO (−5.65 eV), and after 1,8-diiodoctane treatment, the power conversion efficiency of the photovoltaic device based on the BTT-BTz/PC71BM photoactive layer reaches 5.06% with 0.81 V of open-circuit voltage, 10.9 mA cm−2 of short-circuit current and 0.57 of fill factor, which is the highest one among the conjugated polymers based on BTT or BTz derivatives. This new conjugated copolymer seems a promising candidate for the application in tandem solar cells.
The development of bulk-heterojunction (BHJ) polymer solar cells has received considerable attention because of their unique advantages such as low cost, light weight and mechanical flexibility through solution manufacturing techniques.1–3 Among numerous approaches to increase power conversion efficiency (PCE), the design and synthesis of new low band gap (LBG) conjugated polymers have attracted the researchers' interest due to their effectiveness in maximizing the short-circuit current (Jsc) and the PCE of the photovoltaic devices.4–6 However, it is also important to explore broad band gap conjugated polymers for high efficient photovoltaic devices, because most of the conjugated materials often suffer from a relatively narrow adsorption width which significantly limits the use of solar radiation effectively.7 One solution to this problem is the tandem solar cell technique, which consists of a broad band gap material as the front cell and a low band gap material as the rear cell to absorb different parts of the solar spectrum. The two cells in the tandem cell are connected in series, and the Voc is the sum of the two cells while the current of tandem solar cells is limited by the lower one.8,9 Since considerable progress has been achieved for low band gap materials, it is urgent to design and synthesize a highly efficient and broad band gap conjugated material with high Voc to realize higher efficiency for the tandem solar cells.
Benzotrithiophene (BTT) monomer was first reported by McCulloch and coworkers in 2011.10 Its highly planar and electron rich unit makes it a potential candidate as the donor compound in donor–acceptor (D–A) copolymers for organic photovoltaic devices. The extended aromatic core is beneficial for the intermolecular π–π* stacking and charge transport. Although BTT has been proved to be one of the most promising candidates for high performance photovoltaic devices with a deeper HOMO,11,12 the efficiency needs to be further optimized. As one of the thiazole derivatives, bithiazole (BTz) has widely been used as the acceptor compound for D–A low band gap polymers due to its electron deficient nature resulting from the electron-withdrawing nitrogen of imine (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) in thiazole.13 It was found that the introduction of BTz into organic photovoltaic materials could effectively decrease the highest occupied molecular orbital (HOMO) energy level and improve the stability. Although many groups have reported the device performance based on BTz copolymers, to date, the PCEs remain at a low level of around 3%, and the potential of BTz as an acceptor unit in the copolymer needs to be further explored.14,15
N) in thiazole.13 It was found that the introduction of BTz into organic photovoltaic materials could effectively decrease the highest occupied molecular orbital (HOMO) energy level and improve the stability. Although many groups have reported the device performance based on BTz copolymers, to date, the PCEs remain at a low level of around 3%, and the potential of BTz as an acceptor unit in the copolymer needs to be further explored.14,15
In this communication, we first designed and synthesized a BTT-distannylated monomer containing C11 and C12 alkyl side chains, which would improve the solubility, and then copolymerized it with brominated BTz (BTz-dibromo). A new copolymer, poly{benzo(1,2-b:3,4-b′:5,6-d′′)trithiophene-alt-4,4′-dihexyl-2,2′-bithiazole} (BTT-BTz), was obtained with a good yield. The copolymer BTT-BTz holds an absorption range similar to that of the standard broad band gap polymer P3HT used in tandem solar cells, but the deeper HOMO energy level with respect to P3HT significantly increases Voc from 0.60 V to 0.81 V which will be beneficial to the total Voc of the tandem solar cells and the performance of the device. The PCE of the photovoltaic device based on this BTT-BTz copolymer as the donor material and PC71BM as the acceptor material reaches 5.06%, which is the highest one among the conjugated polymers based on BTT or BTz derivatives to the best of our knowledge so far.11,12,16,17
The synthesis and characterization of BTT-distannylated and BTz-dibromo monomers, and BTT-BTz copolymer, are given in the ESI (Schemes S1 and S2, Fig. S1–S3†), and Scheme 1 shows the chemical structures and the BTT-BTz synthesis route for convenience.
![The synthesis and the structure of poly{benzo[1,2-b:3,4-b′:5,6-d′′]trithiophene-alt-4,4′-dihexyl-2,2′-bithiazole} (BTT-BTz).](/image/article/2013/PY/c2py20786a/c2py20786a-s1.gif) | ||
| Scheme 1 The synthesis and the structure of poly{benzo[1,2-b:3,4-b′:5,6-d′′]trithiophene-alt-4,4′-dihexyl-2,2′-bithiazole} (BTT-BTz). | ||
The thermal stability of BTT-BTz copolymer was characterized by thermogravimetric analysis (TGA) under a nitrogen flow, and the thermogram shows (Fig. S4†) the decomposition temperature of over 420 °C for a 5% weight loss, indicating a good thermal stability.
The optical properties of BTT-BTz copolymer were examined by UV-Vis analysis both in chlorobenzene (CB) solution and in a solid film, and the corresponding spectra are given in Fig. 1. From this figure it can be seen that BTT-BTz copolymer shows a maximum absorption at λmax = 532 nm in solution, while the absorption is slightly red shifted from 532 nm to 538 nm for the BTT-BTz copolymer film, indicating the ordering of the molecules or the π–π* interchain interaction in the solid film.
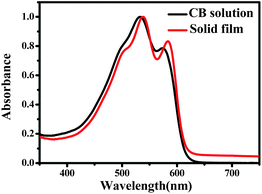 | ||
| Fig. 1 Normalized absorption spectra of BTT-BTz copolymer in CB solution and a solid film spin-cast from CB. | ||
The HOMO energy level of BTT-BTz copolymer was investigated by ultraviolet photoelectron spectroscopy (UPS) and was found to be −5.65 eV (Fig. S5†).12,18 The lower HOMO energy level of BTT-BTz is beneficial for the Voc of the photovoltaic devices and makes BTT-BTz a promising candidate due to its the broad band gap in the tandem solar cells, which is determined by the difference of the acceptor's LUMO and donor's HOMO.1,5,12,19,20 In combination with 2.05 eV of the optical band gap (ΔEopt), which was obtained from the absorption edge (λ = 610 nm) of the copolymer film shown in Fig. 1, the LUMO energy level of the copolymer was calculated to be −3.60 eV. The high LUMO provides the larger difference (>0.3 eV) between the LUMOs of BTT-BTz and PC71BM, which is sufficient for the charge separation in photovoltaic devices.
Photovoltaic properties of BTT-BTz copolymer were investigated with the standard device structure of ITO/PEDOT:PSS/copolymer:PC71BM/LiF/Al under the illumination of AM 1.5G at 100 mA cm−2. As is well recognized, addition of processing additives, such as 1-chloronaphthalene (CN), 1,8-octanedithiol (ODT), or 1,8-diiodooctane (DIO), was proven to be an effective method to improve the device efficiency.21,22 Stimulated by previous studies, in this work 1–3 vol% of DIO was selected as the additive and mixed with the primary solvent CB to optimize the device performance. The typical current density–voltage curves of the devices with and without DIO additive treatment are shown in Fig. 2a, and the corresponding values are listed in Table 1. For the pristine device, in which the copolymer/PC71BM active layer was cast directly from CB solution, a PCE of 2.49% was obtained. This efficiency is mainly resulted from the higher Voc (0.83 V) due to the low HOMO energy level of the BTT-BTz copolymer. However, the relatively low Jsc (6.2 mA cm−2) and FF (0.48) are disadvantageous. If the copolymer/PC71BM solution was treated with the DIO additive (1–3 vol%), the efficiency was significantly improved, and the highest PCE of 5.06% was obtained for the device treated with a 1 vol% DIO additive, which is two times higher than that for the pristine device. From Table 1 it can be seen that the high efficiency is attributed to the higher Jsc (10.9 mA cm−2) and FF (0.57). The incident photon-to-electron conversion efficiency (IPCE) curves (Fig. 2b) also show that the device treated with the 1 vol% DIO additive displays much higher conversion efficiency in the whole range of 400 nm to 600 nm, which reaches maximum 75% at around 550 nm, while only 50% of the maximum is obtained for the pristine device at the same wavelength.
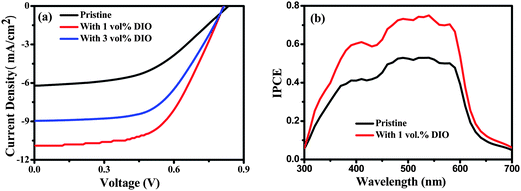 | ||
| Fig. 2 (a) Current density–voltage characteristics of photovoltaic devices based on the BTT-BTz copolymer/PC71BM active layer without (pristine) and with 1–3 vol% of DIO processing additive in CB solution. (b) Corresponding IPCE of the devices. | ||
| Sample | V oc (V) | J sc (mA cm−2) | FF | PCE (%) |
|---|---|---|---|---|
| Pristine | 0.83 | 6.22 | 0.48 | 2.49 |
| 1 vol% | 0.81 | 10.9 | 0.57 | 5.06 |
| 3 vol% | 0.82 | 8.96 | 0.56 | 4.11 |
To ascertain the effect of the photoactive layer on the device performance, the morphologies of the BTT-BTz/PC71BM blend films cast from only CB and the CB/ 1 vol% DIO mixture solvent were investigated by AFM and TEM, respectively, as shown in Fig. 3. Fig. 3a shows the AFM image of the blend film cast from CB solution, showing a surface roughness of 3.38 nm. The corresponding TEM image (Fig. 3b) displays the film prepared without DIO additive clearly showing the phase separation of larger PC71BM domains with the size of 100–200 nm, which is much greater than the exciton diffusion length and thus leads to a lower Jsc (6.22 mA cm−2) and PCE (2.49%) for the pristine photovoltaic device. If the BTT-BTz copolymer/PC71BM blend is treated with the CB/ 1 vol% DIO mixture solvent, the blend film shows smoother topography, of which the surface roughness is reduced to 2.58 nm (Fig. 3c). Meanwhile, the TEM image also displays a significantly different morphology, as shown in Fig. 3d. From this figure it can be seen that after treatment with the DIO additive, the morphology of the blend film is greatly optimized. The larger phase separation disappears and instead, a nanoscale phase separation and an interpenetrating network are constructed. The formation of this morphology is of benefit for the exciton dissociation and charge transportation, and therefore, the Jsc and the PCE of the device are dramatically increased to 10.9 mA cm−2 and 5.06%.
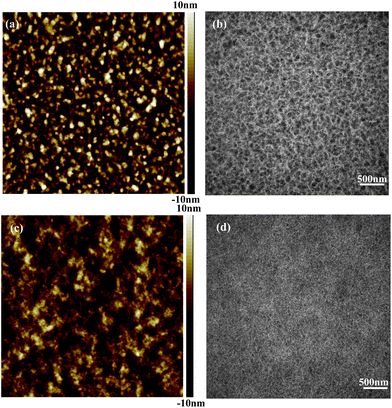 | ||
| Fig. 3 Morphologies of the BTT-BTz copolymer/PC71BM films cast from CB solution (a and b) and CB/ 1 vol% DIO solution (c and d). (a and c) The topographies by AFM tapping mode showing a 5μm × 5μm surface area, and (b and d) the BF-TEM images. | ||
In summary, a new semiconducting polymer, BTT-BTz copolymer, with a broad band gap and high Voc, was designed and synthesized. The pristine photovoltaic device based on the BTT-BTz copolymer/PC71BM photoactive layer shows a PCE of 2.49% with a higher Voc (0.83 V). After DIO additive treatment, the morphology of the blend film is greatly optimized, and the optimal device efficiency reaches 5.06% due to the increase in Jsc (10.9 mA cm−2) and FF (0.57). This efficiency is the highest one among the conjugated copolymers based on BTT or BTz derivatives. Considering the broad band-gap (2.05 eV) and high PCE (5.06%), this BTT-BTz semiconducting copolymer seems a promising candidate as one of the active layers in the tandem cell structure to capture different parts of the solar spectrum.
Acknowledgements
This work was funded by the National Natural Science Foundation of China (20990233), Hi-Tech Research and Development Program (863) of China (2011AA050524), and Solar Energy Initiative (KGCX2-YW-399 + 9) of the Chinese Academy of Sciences. X.Y. would also like to thank the Fund for Distinguished Young Scholars (20925415) and the Fund for Creative Research Groups (50921062) of NSFC for financial support.Notes and references
- H. Y. Chen, J. H. Hou, S. Q. Zhang, Y. Y. Liang, G. W. Yang, Y. Yang, L. P. Yu, Y. Wu and G. Li, Nat. Photonics, 2009, 3, 649–653 CrossRef CAS.
- C. J. Brabec, S. Gowrisanker, J. J. M. Halls, D. Laird, S. J. Jia and S. P. Williams, Adv. Mater., 2010, 22, 3839–3856 CrossRef CAS.
- L. J. Huo and J. H. Hou, Polym. Chem., 2011, 2, 2453–2461 RSC.
- J. Y. Zou, H. L. Yip, Y. Zhang, Y. Gao, S. C. Chien, K. O'Malley, C. C. Chueh, H. Z. Chen and A. K. Y. Jen, Adv. Funct. Mater., 2012, 22, 2804–2811 CrossRef CAS.
- Y. Zhang, S. C. Chien, K. S. Chen, H. L. Yip, Y. Sun, J. A. Davies, F. C. Chen and A. K. Y. Jen, Chem. Commun., 2011, 47, 11026–11028 RSC.
- S. Hellstrom, L. J. Lindgren, Y. Zhou, F. Zhang, O. Inganas and M. R. Andersson, Polym. Chem., 2012, 1, 1272–1280 RSC.
- L. Huo, X. Guo, S. Zhang, Y. Li and J. Hou, Macromolecules, 2011, 44, 4035–4037 CrossRef CAS.
- L. Dou, J. You, J. Yang, C.-C. Chen, Y. He, S. Murase, T. Moriarty, K. Emery, G. Li and Y. Yang, Nat. Photonics, 2012, 6, 180–185 CrossRef CAS.
- L. T. Dou, J. Gao, E. Richard, J. B. You, C. C. Chen, K. C. Cha, Y. J. He, G. Li and Y. Yang, J. Am. Chem. Soc., 2012, 134, 10071–10079 CrossRef CAS.
- C. B. Nielsen, J. M. Fraser, B. C. Schroeder, J. Du, A. J. P. White, W. Zhang and I. McCulloch, Org. Lett., 2011, 13, 2414–2417 CrossRef CAS.
- B. C. Schroeder, C. B. Nielsen, Y. J. Kim, J. Smith, Z. G. Huang, J. Durrant, S. E. Watkins, K. Song, T. D. Anthopoulos and I. McCulloch, Chem. Mater., 2011, 23, 4025–4031 CrossRef CAS.
- C. B. Nielsen, B. C. Schroeder, A. Hadipour, B. P. Rand, S. E. Watkins and I. McCulloch, J. Mater. Chem., 2011, 21, 17642–17645 RSC.
- Y. Z. Lin, H. J. Fan, Y. F. Li and X. W. Zhan, Adv. Mater., 2012, 24, 3087–3106 CrossRef CAS.
- M. J. Zhang, H. J. Fan, X. Guo, Y. J. He, Z. G. Zhang, J. Min, J. Zhang, G. J. Zhao, X. W. Zhan and Y. F. Li, Macromolecules, 2010, 43, 8714–8717 CrossRef CAS.
- Y. Zhang, J. Y. Zou, C. C. Cheuh, H. L. Yip and A. K. Y. Jen, Macromolecules, 2012, 45, 5427–5435 CrossRef CAS.
- C. B. Nielsen, R. S. Ashraf, B. C. Schroeder, P. D'Angelo, S. E. Watkins, K. Song, T. D. Anthopoulos and I. McCulloch, Chem. Commun., 2012, 48, 5832–5834 RSC.
- J. Lee, B. J. Jung, S. K. Lee, J. I. Lee, H. J. Cho and H. K. Shim, J. Polym. Sci., Part A: Polym. Chem., 2005, 43, 1845–1857 CrossRef CAS.
- R. J. Davis, M. T. Lloyd, S. R. Ferreira, M. J. Bruzek, S. E. Watkins, L. Lindell, P. Sehati, M. Fahlman, J. E. Anthony and J. W. P. Hsu, J. Mater. Chem., 2010, 21, 1721–1729 RSC.
- D. Gendron and M. Leclerc, Energy Environ. Sci., 2011, 4, 1225–1237 CAS.
- N. Kleinhenz, L. Q. Yang, H. X. Zhou, S. C. Price and W. You, Macromolecules, 2011, 44, 872–877 CrossRef CAS.
- J. S. Moon, C. J. Takacs, S. Cho, R. C. Coffin, H. Kim, G. C. Bazan and A. J. Heeger, Nano Lett., 2010, 10, 4005–4008 CrossRef CAS.
- J. K. Lee, W. L. Ma, C. J. Brabec, J. Yuen, J. S. Moon, J. Y. Kim, K. Lee, G. C. Bazan and A. J. Heeger, J. Am. Chem. Soc., 2008, 130, 3619–3623 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2py20786a |
| This journal is © The Royal Society of Chemistry 2013 |
