Biocompatible well-defined chromophore–polymer conjugates for photodynamic therapy and two-photon imaging†
Cristina
Cepraga‡
abcg,
Thibault
Gallavardin‡
c,
Sophie
Marotte‡
abdg,
Pierre-Henri
Lanoë
*c,
Jean-Christophe
Mulatier
c,
Frédéric
Lerouge
cg,
Stéphane
Parola
cg,
Mikael
Lindgren
ce,
Patrice L.
Baldeck
cf,
Jacqueline
Marvel
dg,
Olivier
Maury
c,
Cyrille
Monnereau
c,
Arnaud
Favier
*abg,
Chantal
Andraud
c,
Yann
Leverrier
*dg and
Marie-Thérèse
Charreyre
abg
aÉcole Normale Supérieure de Lyon, Laboratoire Joliot-Curie, USR CNRS 3010, F-69364 Lyon, France. E-mail: arnaud.favier@ens-lyon.fr; Fax: +33 4 72 72 80 80; Tel: +33 4 72 72 88 70
bINSA-Lyon, Laboratoire Ingénierie des Matériaux Polymères, UMR CNRS 5223, F-69621 Villeurbanne, France
cÉcole Normale Supérieure de Lyon, CNRS UMR 5182, Université Lyon 1, Site Monod, 46 allée d'Italie, F-69364, Lyon, France. E-mail: lanoe.ph@gmail.com
dINSERM, U851, 21, Avenue Tony Garnier, Lyon, F-69007, France. E-mail: yann.leverrier@inserm.fr
eNorwegian University of Science and Technology, Department of Physics, N-7491 Trondheim, Norway
fUniversité Joseph Fourier, Laboratoire de Spectrométrie Physique, UMR CNRS 5588, F-38402 Saint Martin d'Hères, France
gUniversité Lyon 1, 43 boulevard du 11 Novembre 1918, F-69622 Villeurbanne, France
First published on 24th August 2012
Abstract
A versatile approach is introduced for the synthesis of well-defined, biocompatible conjugates combining two-photon chromophores and hydrophilic multifunctional polymers synthesized by RAFT controlled radical polymerization. As an illustration, two different classes of conjugates carrying multiple fluorophores (based on an anthracene moiety, Anth) or photosensitizers (based on a dibromobenzene moiety, DBB) along the polymer chain were elaborated for bioimaging and photodynamic therapy (PDT) applications, respectively. In both cases, the polymer greatly improved the solubility in biorelevant media as well as the cell uptake. Anth conjugates provided high quality fluorescence microscopy images using both one- and two-photon excitation. DBB conjugates potently induced the death of cancer cells upon photoactivation.
Introduction
Two-photon absorption (TPA) chromophores have been used in various photonic application fields1 and have more recently raised increasing interest for bioimaging and therapy applications.2–4 Further progress in fluorescence imaging and some photodynamic cancer therapy schemes requires improved fluorophores and photosensitizers (PS).5 Such improvement could arise from chromophores especially designed to exhibit high TPA.5 Indeed, two-photon excitation (TPE) is confined at the focal point of the laser (ca. 1 μm3) and allows high 3D resolution of the excitation that reduces out-of-focus damage and would improve the spatial targeting of tumors. Moreover, TPE is generally performed under near-infrared (NIR) irradiation in a wavelength range of 700–1100 nm where absorption, scattering of light and auto-fluorescence of the biological tissues are low, enabling a better penetration of the incident light and improving the signal-to-noise ratio.The use of TPA chromophores in vitro or in vivo requires a fine control of their behavior under biorelevant conditions, i.e. solubility, absence of toxicity, concentration at the desired locus of action, and conjugation with bioactive molecules. Unfortunately, efficient TPA chromophores are inherently highly conjugated hydrophobic molecules and thus poorly soluble in aqueous media.6,7 Therefore, a common strategy relies on their incorporation into biocompatible polymeric carriers. TPA chromophores can be encapsulated into micelles,8–11 or inside nanoparticles obtained by microemulsion12 or formulation processes.13 However, since chromophore leaking can progressively occur, covalent bonds may be preferred. This can be achieved either by (co)polymerization of chromophore-containing monomers14 (that should not interfere with the polymerization mechanism, nor be degraded) or by post-modification of functional polymers.15
In this context, chromophore–polymer conjugates of well-defined compositions are required to provide a reliable and reproducible effect. However, control over the polymer structure and the number of incorporated chromophores is often limited. Then, a strategy consists in grafting chromophores at the periphery of dendrimers,16–18 leading to nano-sized conjugates that exhibit very high extinction coefficients and cross-section values (generally determined in organic solvents). Nevertheless, dendrimer synthesis is rather complex, and their use is likely to be restricted to very high value purposes.
To overcome most of the abovementioned limitations, our goal was to generate highly efficient TPA bioimaging probes and PDT photosensitizers by developing a straightforward synthesis of chromophore–polymer conjugates exhibiting a well-defined and tunable structure. We decided to use a polymer platform presenting the following characteristics: (i) being water-soluble and biocompatible; (ii) presenting multiple reactive sites for the binding of a controlled number of chromophores per polymer chain to get enhanced TPA cross-section and PS-associated phototoxicity; (iii) featuring a controlled molecular weight (MW), dispersity (Đ), composition and microstructure to provide very homogeneous conjugates; and (iv) easy to synthesize via a versatile polymerization process.
Thus, we directed our choice to a copolymer consisting of water-soluble and biocompatible N-acryloylmorpholine (NAM) units copolymerized with reactive N-acryloxysuccinimide (NAS) units (chromophore binding sites). Our previous studies on the RAFT copolymerization of this monomer pair resulted in an excellent control over the architecture of the P(NAM-co-NAS) chains in terms of MW and dispersity but also composition and microstructure. Not only the microstructure is identical from one chain to another – which is inherent to a living copolymerization – but, for most NAM/NAS molar ratios, the intrachain compositional drift is very low. Moreover, at the so-called azeotropic composition (60/40 molar ratio for this monomer pair), there is no compositional drift, resulting in a very regular spacing of the NAS units along the polymer chains.19
Novel TPA chromophore–polymer conjugates were elaborated by a combination of those multifunctional copolymers with two different TPA chromophores (based on an anthracene (Anth) and a dibromobenzene (DBB) moiety) designed for two-photon imaging and photodynamic therapy, respectively, that had recently given encouraging results (after encapsulation in Pluronic).11 For the present study, these chromophores were functionalized in order to carry (i) two short PEG chains to improve compatibility with aqueous media and (ii) a primary amino group for the covalent binding to the hydrophilic P(NAM-co-NAS) copolymer (Scheme 1). To the best of our knowledge, the resulting conjugates constitute the first example of TPA multichromophoric probes based on a polymer chain synthesized by controlled radical polymerization for both two-photon bio-imaging with excitation in the NIR regime and PDT.
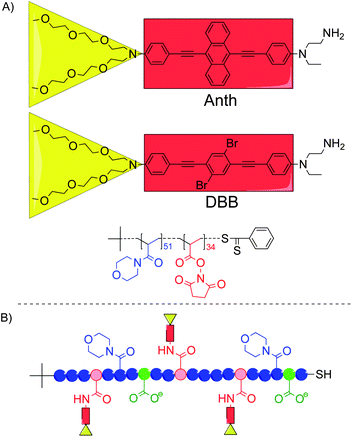 | ||
| Scheme 1 (A) Structures of the two TPA chromophores and the P(NAM-co-NAS) random copolymer used in this study. (B) General structure of the chromophore–polymer conjugates with the NAM units (blue circles), the NAS units after binding of the TPA chromophores (red circles), the hydrolyzed NAS units (green circles), the Anth or DBB chromophoric centers (red rectangles) and the oligo-ethyleneglycol side-chains (yellow triangles). | ||
Herein, we report on their synthesis and spectroscopic characterization in various solvents, including water. In addition, we present a thorough biological evaluation of these conjugates in cellulo that demonstrates their ability to serve as TPA bioimaging probes (Anth conjugate) and PDT photosensitizers (DBB conjugate), and highlights the role of the polymer in their behavior and efficiency under biorelevant conditions.
Results and discussion
Synthesis of TPA chromophore–polymer conjugates
The chromophore–polymer conjugates were synthesized in high yield (80–90%) from the same copolymer backbone (Mn = 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 g mol−1; Đ = 1.05) and the amino-functionalized chromophores (Scheme 1). After hydrolysis of the remaining activated ester functions and thorough purification, two anionic (carboxylated) conjugates were obtained with an average of 6.1 Anth (6Anth-H) and 4.4 DBB (4DBB-H) per polymer chain, respectively (Table 1).
900 g mol−1; Đ = 1.05) and the amino-functionalized chromophores (Scheme 1). After hydrolysis of the remaining activated ester functions and thorough purification, two anionic (carboxylated) conjugates were obtained with an average of 6.1 Anth (6Anth-H) and 4.4 DBB (4DBB-H) per polymer chain, respectively (Table 1).
| Conjugates | Y (%) | n c | n COO− | M n |
|---|---|---|---|---|
| a Y: coupling yield (%); nc: average number of chromophores per polymer chain determined by SEC/UV (nc = Y × n0 chromophores/n0 polymer chains, with n0 being the initial mole number of chromophores and polymer chains that were reacted). The error associated with nc, determined through this method was lower than 10%; nCOO−: average number of carboxylate charges per polymer chain (33.9 − nc); Mn: number average molecular weight of the chromophore–polymer conjugates (g mol−1) calculated assuming that the conjugates were in their sodium carboxylate form after dialysis and lyophilization. | ||||
| 6Anth-H | 90 | 6.1 | 27.8 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
| 4DBB-H | 81 | 4.4 | 29.5 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
Spectroscopic characterization
In contrast to the free chromophores that were only soluble in organic solvents, all conjugates were soluble in aqueous media as well as in polar organic solvents. In all cases, neither macroscopic nor microscopic aggregation (using dynamic light scattering, DLS) was observed. Thanks to the very good water-solubility of the chromophore–polymer conjugates, their spectroscopic parameters could be determined in water (Table 2). Measurements were also performed in dioxane, a low polarity solvent known to exhibit physical characteristics close to biological micro-environments.20 Moreover, dioxane enabled a comparison between the conjugates and the free chromophores.| Solvent | λ max (abs; em) | ε | ϕ | |
|---|---|---|---|---|
| a λ max (abs; em): maximal absorption and emission wavelengths (nm); ε: molar extinction coefficient (cm−1 M−1); ϕ: fluorescence quantum yield. b Anth chromophore was analyzed in its amine-protected form. | ||||
| Anth b | CHCl3 | 490; 557 | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.57 |
| Dioxane | 484; 548 | 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.61 | |
| 6Anth-H | Dioxane | 476; 554 | 108![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.08 |
| Water | 476; 609 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.01 | |
| DBB | CHCl3 | 400; 460 | 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.23 |
| Dioxane | 394; 448 | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.18 | |
| 4DBB-H | Dioxane | 389; 460 | 202![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.10 |
| Water | 420; 505 | 177![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.01 | |
Absorption spectra of the conjugates were similar to those of the free chromophores, except for a slight broadening of the absorption bands in water (thus a concomitant decrease of the extinction coefficient). Yet, the maximum absorption wavelength remained almost unchanged (ESI, Fig. S2†). In contrast, the fluorescence emission of both conjugates was clearly red-shifted (by about 50 nm) in water compared to dioxane (increasing solvent polarity) resulting in larger Stokes shifts. Their fluorescence quantum yield (ϕ) was found to decrease significantly as the protic character of the solvent increased (ESI, Table S2†). Thus, ϕ values were much higher in dioxane than in water for both conjugates. This indicated that the nature of the solvent critically impacts the conformation of the conjugates and the probability of fluorescence self-quenching (due to chromophore–chromophore interactions) which is indeed a well-known phenomenon associated with a high local chromophore concentration.21 TPA cross-section (σTPA) values of 420 and 400 GM were determined in chloroform for Anth and DBB free chromophores, respectively. Concerning the conjugates, σTPA (as well as the molar extinction coefficient, ε, Table 2) could be measured in water which was not possible for the free chromophores. Values were as high as 790 GM (6Anth-H) and 1380 GM (4DBB-H) at 740 nm, corresponding to 130 and 310 GM per bound chromophore, respectively (lower σTPA values are generally observed in aqueous media compared to organic solvents22). In summary, besides providing water-solubility, multifunctional hydrophilic polymers led to conjugates with high ε and σTPA values in water.
Biological evaluation
Biological evaluation of the conjugates was then conducted in cellulo. The objectives were to address the following questions: (i) Are the conjugates non-toxic in the absence of photoactivation? (ii) Is their cellular internalization favored compared to free chromophores? (iii) Can 6Anth-H conjugate be used as a probe for fluorescence microscopy under one- and two-photon excitation? (iv) Can 4DBB-H conjugate induce death of cancer cells upon photoactivation?Two photon excited fluorescence microscopy using 6Anth-H probe
To assess the cytotoxicity and the uptake of 6Anth-H, we first used Baf3 cells since they are very sensitive to a wide spectrum of cell death inducing treatments.23 Baf3 cells were incubated for various lengths of time with 6Anth-H (10−5 mol L−1 chromophore) and their fluorescence was recorded by flow cytometry (ESI, Fig. S3†). At this concentration, conjugates were not cytotoxic over a 24-hour period (ESI, Fig. S4†). Increase of fluorescence intensity over time was indicative of a continuous uptake of 6Anth-H (ESI, Fig. S5†). To test whether cellular uptake of 6Anth-H could be detected by one- and two-photon microscopy, we used a B16-F10 melanoma cell line (adherent cells, easily examined by microscopy). Upon one-photon excitation at 488 nm, 6Anth-H emission was detected throughout the cytoplasm of the cells (not in the nucleus) and concentrated in the perinuclear region (Fig. 1A, top panels). 6Anth-H was also successfully visualized upon two-photon excitation with a maximal signal and a minimal auto-fluorescence at 750 nm excitation (NIR) (Fig. 1A, bottom panels).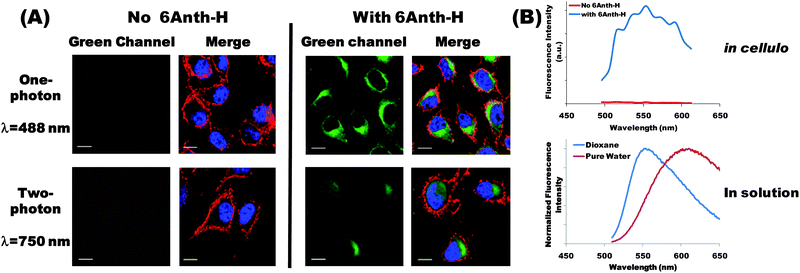 | ||
| Fig. 1 (A) One-photon (top panels) and two-photon (bottom panels) fluorescence images of 6Anth-H conjugate. Adherent B16-F10 melanoma cells were cultured for 24 h in the absence (left panel) or in the presence of 6Anth-H (right panels, 10−5 mol L−1 chromophore). Plasma membrane (red) and nuclei (blue) were visualized in all cases after one-photon excitation using anti-CD44 antibody (405 nm) and DRAQ5 DNA dye (633 nm) respectively. 6Anth-H (green) was detected using one-photon (488 nm) or two-photon excitation (750 nm). Scale bar is 10 μm. (B) (Top graph) Fluorescence emission spectrum measured in cellulo after two-photon excitation at 750 nm in the absence or in the presence of 6Anth-H (image acquisition at multiple wavelengths). (Bottom graph) Spectrum of the same conjugate in dioxane and pure water (fluorescence spectroscopy). | ||
The results obtained by fluorescence microscopy clearly demonstrate the potential of 6Anth-H conjugate to be used as a fluorescent probe. From a general perspective, ionic strength, pH and polarity can vary from one micro-environment to another. Important solvatochromism effects and ϕ variation may be observed depending on the localization of the probe inside the cell. As an illustration, we compared the 6Anth-H fluorescence emission spectrum in cellulo with the spectra recorded in pure water and in dioxane (Fig. 1B). In the absence of 6Anth-H, background auto-fluorescence was negligible. In the presence of 6Anth-H, the fluorescence emission spectrum in cellulo exhibited a maximum close to 550–560 nm and a very good signal-to-noise ratio. This emission spectrum differed from the one in pure water (maximum significantly blue-shifted compared to 609 nm in water), confirming that the conjugate experienced a different environment. Conversely, the observed emission profile exhibited a maximum close to that of the conjugate in dioxane solution, reflecting that the conjugates were mainly in interaction with low polar regions of the cells (e.g. lipid membranes, proteins). The fluorescence spectrum in cellulo together with higher fluorescence quantum yield of 6Anth-H in dioxane than in water probably explains the very good quality of the images recorded upon one- and two-photon excitation.
Photo-induced cell death using 4DBB-H photosensitizer
DBB derivatives are potential two-photon photosensitizers that were previously shown to induce cell death upon one-photon photoactivation.11 However, internalization into cells was limited when the free PS was solubilized in DMSO (due to its hydrophobic nature).11 Interestingly, when bound onto the hydrophilic polymer (4DBB-H conjugate), cellular uptake was greatly enhanced (Fig. 2A). Compared with PS encapsulated in Pluronic,11 cellular uptake was faster and more efficient.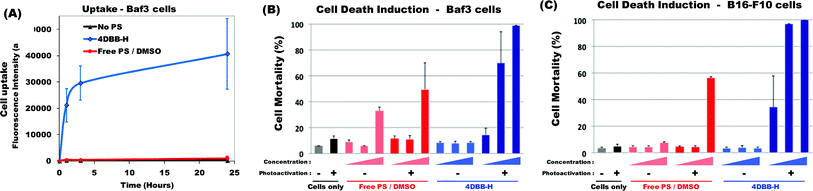 | ||
| Fig. 2 (A) Kinetics of 4DBB-H uptake. Baf3 cells were incubated without any PS (black line) or with the free PS in DMSO (red line, 10−5 mol L−1) or with 4DBB-H conjugate containing the equivalent of 10−5 mol L−1DBB (blue line) for the indicated period of time. Uptake was evaluated by flow cytometry. Results are expressed as the average of the mean fluorescence intensity ± SD of 2 independent experiments. (B) Induction of cell death upon photoactivation. Baf3 cells and B16-F10 melanoma cells were incubated for 24 h without any PS (black) or with increasing amount of free PS in DMSO (red) or 4DBB-H conjugate (blue) (4 × 10−7, 2 × 10−6 and 10−5 mol L−1DBB). Then, cells were submitted (+) or not (−) to irradiation (365 nm, 8 J cm−2). The percentage of cell mortality was assessed 5 h after irradiation by staining with propidium iodide followed by flow cytometry analysis. Results show the mean mortality ± SD of 2 to 4 independent experiments. | ||
In the absence of light irradiation, 4DBB-H was not toxic. Hence, Baf3 or B16-F10 cells did not show signs of increased mortality when incubated with 4DBB-H for 24 h (Fig. 2B) or 48 h (data not shown). In contrast, irradiation of cells previously incubated for 24 h with 4DBB-H rapidly induced cell death in a dose-dependent manner. Induction of cell death upon photoactivation was very efficient for concentration as low as 2 × 10−6 mol L−1DBB, especially in the case of B16-F10 melanoma cells. In contrast, the free PS in DMSO did not induce significant Baf3 cell death (toxicity at high concentration being mainly due to DMSO rather than photoactivation) and only induced B16-F10 cell death at the highest concentration (10−5 mol L−1) upon irradiation.
Altogether, this comparison between 4DBB-H conjugate and free PS showed compelling evidence that the copolymer strongly enhances the photosensitizer cell uptake and its ability to induce cell death. It has to be stressed that such comparison was possible thanks to the controlled number of PS bound onto the conjugate. This is not always feasible for other systems. These encouraging results will lead to further investigations, particularly regarding cell death induction under two-photon irradiation.
Conclusions
In conclusion, a versatile and controlled approach for the synthesis of well-defined TPA chromophore–polymer conjugates is described. Our results demonstrate that highly water-soluble polymer conjugates bearing multiple chromophores along the chain could be elaborated and provided valuable imaging probes for one- and two-photon excitation fluorescence microscopy as well as novel photosensitizers with significantly improved efficiency for photodynamic cancer therapy. In particular, cellular uptake was considerably increased when chromophores were covalently bound onto the polymer. We thus believe that this modular system could be an attractive candidate for numerous applications in biology and medicine ranging from diagnostics to therapeutics. We are currently working on extending the strategy to other systems thanks to the ability to finely tune the nature of the polymer and the chromophores as well as the architecture and the functionality of the conjugates.Experimental section
Materials
Detailed synthesis of TPA chromophores is described in the ESI.† Synthesis of the free PS, solubilized in DMSO for the phototoxicity assays, was previously described in the literature.11 All chemicals and solvents were purchased from Sigma Aldrich or Acros at the highest purity available and used without further purification.Analytical methods
Fluorescence quantum yield. Fluorescence quantum yields, ϕ, were calculated according to eqn (1) for diluted solutions having an optical density lower than 0.1, where A is the absorbance at the excitation wavelength λ, n is the refractive index and D is the integrated luminescence intensity. “r” and “x” stand for reference and sample. References were rubrene (ϕ = 0.27 in methanol) and Coumarin 153 (ϕ = 0.45 in methanol) for Anth and DBB compounds, respectively.
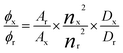 | (1) |
| ITwoPhotonFl ∝ σTPA × Ilaser2 × ϕ × c × Klaser | (2) |
TPA cross-section. TPA cross-section values (σTPA) were obtained using eqn (2), where IFl represents the two-photon fluorescence intensity; Ilaser the LASER intensity at 50 mW, Klaser a constant depending on the measurement set-up; ϕ the fluorescence quantum yield and c the concentration of chromophore (c = 10−4 mol L−1). The measurements were conducted in an intensity regime where the fluorescence signal showed a quadratic dependence on the intensity of the excitation beam, as checked for all samples. The uncertainty in the measured cross-sections is about 15% in CHCl3 and 20% in water.
Synthesis of reactive copolymers using RAFT polymerization
Poly(N-acryloylmorpholine-co-N-acryloxysuccinimide), P(NAM-co-NAS), with a 60/40 NAM/NAS molar ratio (Mn = 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 g mol−1; Đ = 1.05; average number of activated ester units per chain = 33.9) (Scheme 1) was synthesized according to the previously reported RAFT polymerization procedure.19
900 g mol−1; Đ = 1.05; average number of activated ester units per chain = 33.9) (Scheme 1) was synthesized according to the previously reported RAFT polymerization procedure.19
TPA chromophore–polymer conjugates synthesis
After coupling, the reaction medium was analyzed by SEC/UV in order to determine the coupling yield (Table 1). The conjugate was then precipitated in a large volume of diethyl ether and isolated from the supernatant by centrifugation. The procedure was repeated until complete discoloration of the supernatant, ensuring the absence of free unreacted chromophore. Purified orange conjugates were finally dried under vacuum up to constant weight.
Determination of the average number of chromophores per polymer chain, nc
Coupling yields, Y, and the average number of chromophores per polymer chain, nc, were determined before hydrolysis from SEC/UV chromatograms. Using UV detection (at the absorption maximum of the chromophores), two different peaks could be distinguished (Fig. 3): (A) chromophores bound to the copolymer chains (lower elution volume), (B) free unbound chromophores. A and B being the areas under each of these peaks, the coupling yield (Y) was determined using eqn (3) assuming that chromophore absorption properties remained unchanged upon coupling onto the copolymer.| Y = A/(A + B) | (3) |
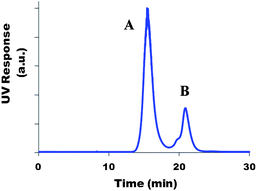 | ||
| Fig. 3 Example of a SEC/UV chromatogram of a sample withdrawn from the reaction medium during coupling of the chromophore onto the P(NAM-co-NAS) copolymer. | ||
The latter assumption was validated by determination of the extinction coefficients (ε) in DMF (SEC eluent) of (i) the free chromophores and (ii) the chromophores bound onto the polymer. Similar values were obtained within 10% error. For DBB compounds in DMF at λ = 400 nm, εDBB = 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1 M−1 and ε4DBB-H = 224
000 cm−1 M−1 and ε4DBB-H = 224![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1 M−1, i.e. ε = 51
000 cm−1 M−1, i.e. ε = 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1 M−1 per bound chromophore (4.4 chromophores per chain).
000 cm−1 M−1 per bound chromophore (4.4 chromophores per chain).
n c values were further confirmed from UV absorption spectra of the final conjugates by comparison of the total integrated absorption strength for the free chromophore and the corresponding conjugate. Using this alternative method, nc values were 6.3 and 4.4 for Anth and DBB conjugates respectively (compared to 6.1 and 4.4 from the SEC/UV method). This determination was associated with a 10% error.
Biological evaluation
To measure cell mortality, cells were collected and incubated with propidium iodide (PI, Sigma, 2 mg L−1) a standard flow cytometry viability probe and at least 5000 cells were analyzed immediately by flow cytometry. This allows us to distinguish between viable cells with the intact plasma membrane that exclude PI and non-viable cells that are permeable to PI. Baf3 cells are non-adherent and were collected by pipetting. Adherent cells loosen their attachment or detach from the substratum during apoptosis. Therefore cell mortality among B16-F10 cells was analyzed by pelleting floating cells and adherent cells harvested by trypsinization. Cells were then stained with PI and analyzed by flow cytometry.
Acknowledgements
We acknowledge the contribution of the imaging facility (PLATIM) and the cytometry platforms (Biosciences Gerland – Lyon Sud, UMS3444, US8), Jean Bernard for technical support in two-photon absorption measurements and Christophe Place for fruitful discussion about this manuscript. This work was supported by grants from INSERM, the ARC, the Ligue du Rhône, the Région Rhône-Alpes, Université Lyon 1. We also thank the ANR for PHL post-doctoral grant and financial support (NanoPDT, no. ANR-09-NANO-027) as well as LyonBiopole. CC acknowledges a PhD grant from the French Ministry of Research and Education.Notes and references
- G. S. He, L.-S. Tan, Q. Zheng and P. N. Prasad, Chem. Rev., 2008, 108, 1245 CrossRef CAS.
- W. R. Zipfel, R. M. Williams and W. W. Webb, Nat. Biotechnol., 2003, 21, 1369 CrossRef CAS.
- A. Diaspro, G. Chirico and M. Collini, Q. Rev. Biophys., 2005, 38, 97 CrossRef CAS.
- S. Brown, Nat. Photonics, 2008, 2, 394 CrossRef CAS.
- J. P. Celli, B. Q. Spring, I. Rizvi, C. L. Evans, K. S. Samkoe, S. Verma, B. W. Pogue and T. Hasan, Chem. Rev., 2010, 110, 2795 CrossRef CAS.
- M. Pawlicki, H. A. Collins, R. G. Denning and H. L. Anderson, Angew. Chem., Int. Ed., 2009, 48, 3244 CrossRef CAS.
- M. Rumi, J. E. Ehrlich, A. A. Heikal, J. W. Perry, S. Barlow, Z. Hu, D. McCord-Maughon, T. C. Parker, H. Röckel, S. Thayumanavan, S. R. Marder, D. Beljonne and J.-L. Brédas, J. Am. Chem. Soc., 2010, 122, 9500 CrossRef.
- Q. Zheng, T. Y. Ohulchanskyy, Y. Sahoo and P. N. Prasad, J. Phys. Chem. C, 2007, 111, 16846 CAS.
- M. Maurin, L. Vurth, J.-C. Vial, P. L. Baldeck, S. R. Marder, B. Van der Sanden and O. Stéphan, Nanotechnology, 2009, 20, 235102 CrossRef.
- C. D. Andrade, C. O. Yanez, M. A. Qaddoura, X. Wang, C. L. Arnett, S. A. Coombs, J. Yu, R. Bassiouni, M. V. Bondar and K. D. Belfield, J. Fluoresc., 2011, 21, 1223 CrossRef CAS.
- T. Gallavardin, M. Maurin, S. Marotte, T. Simon, A.-M. Gabudean, Y. Bretonnière, M. Lindgren, F. Lerouge, P. L. Baldeck, O. Stéphan, Y. Leverrier, J. Marvel, S. Parola, O. Maury and C. Andraud, Photochem. Photobiol. Sci., 2011, 10, 1216 CAS.
- D. Gao, R. R. Agayan, H. Xu, M. A. Philbert and R. Kopelman, Nano Lett., 2006, 6, 2383 CrossRef CAS.
- X. Shen, F. He, J. Wu, G. Q. Xu, S. Q. Yao and Q.-H. Xu, Langmuir, 2011, 27, 1739 CrossRef CAS.
- J. Pecher, J. Huber, M. Winterhalder, A. Zumbusch and S. Mecking, Biomacromolecules, 2010, 11, 2776 CrossRef CAS.
- S. Biswas, X. Wang, A. R. Morales, H.-Y. Ahn and K. D. Belfield, Biomacromolecules, 2011, 12, 441 CrossRef CAS.
- A. Adronov, J. M. J. Fréchet, G. S. He, K.-S. Kim, S.-J. Chung, J. Swiatkiewicz and P. N. Prasad, Chem. Mater., 2000, 12, 2838 CrossRef CAS.
- M. A. Oar, J. M. Serin, W. R. Dichtel, J. M. J. Fréchet, T. Y. Ohulchanskyy and P. N. Prasad, Chem. Mater., 2005, 17, 2267 CrossRef CAS.
- O. Mongin, R. K. Thatavarathy, M. H. V. Werts, A.-M. Caminade, J.-P. Majoral and M. Blanchard-Desce, Chem. Commun., 2006, 915 RSC.
- A. Favier, F. D'Agosto, M.-T. Charreyre and C. Pichot, Polymer, 2004, 45, 7821 CrossRef CAS.
- N. I. Zahid, O. K. Abou-Zied, R. Hashim and T. Heidelberg, J. Phys. Chem. C, 2011, 115, 19805 Search PubMed.
- J. Baumann and M. D. Fayer, J. Chem. Phys., 1986, 85, 4087 CrossRef CAS.
- H. Y. Woo, B. Liu, B. Kohler, D. Korystov, A. Mikhailovsky and G. C. Bazan, J. Am. Chem. Soc., 2005, 127, 14721 CrossRef CAS.
- J. Thomas, Y. Leverrier and J. Marvel, Oncogene, 1998, 16, 1399 CAS.
- C. Xu and W. W. Webb, J. Opt. Soc. Am. B, 1996, 13, 481 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Detailed synthesis of both TPA chromophores, complementary spectroscopic data, flow cytometry analyses, kinetics of cell uptake and cytotoxicity evaluation for 6Anth-H. See DOI: 10.1039/c2py20565c |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2013 |
