A porous coordination framework for highly sensitive and selective solid-phase microextraction of non-polar volatile organic compounds†
Chun-Ting He, Jing-Yu Tian, Si-Yang Liu, Gangfeng Ouyang, Jie-Peng Zhang* and Xiao-Ming Chen
MOE Key Laboratory of Bioinorganic and Synthetic Chemistry, State Key Laboratory of Optoelectronic Materials and Technologies, School of Chemistry & Chemical Engineering, Sun Yat-Sen University, Guangzhou 510275, P. R. China. E-mail: zhangjp7@mail.sysu.edu.cn
First published on 27th September 2012
Abstract
A porous metal azolate framework [Zn(mpba)] (MAF-X8, H2mpba = 4-(3,5-dimethylpyrazol-4-yl)benzoic acid) with large, hydrophobic, one-dimensional channels and good thermal/chemical stability was synthesized and characterized. High-quality MAF-X8 thin films were grown on stainless-steel fibers for solid-phase microextraction (SPME), which showed high sensitivity and selectivity towards non-polar volatile organic compounds.
Introduction
Porous coordination polymers (PCPs) or metal-organic frameworks (MOFs) have demonstrated prominent properties in gas storage, separation, heterogeneous catalysis,1 and other applications,2 mainly in the bulk form. In order to utilize their special properties more efficiently, devices based on PCP thin films have attracted intense attention recently.3 Inspiringly, a few PCPs have been found to be useful in analytical technologies such as solid-phase microextraction (SPME).4 As a highly effective sample pretreatment and enrichment method,5 SPME has been widely used in environment, biology, clinical medicine, food industry, etc.6 Nevertheless, due to the lack of mature adsorption materials, so far commercial and custom-made SPME fibers still face problems of low sensitivity and selectivity, poor stability, and/or poor anti-interference ability. For example, conventional adsorbent materials show poor non-polar/polar selectivity for size-similar molecules (e.g., non-polar and polar benzene derivatives), since the polar molecules interact strongly with the adsorbents.The first PCP coated SPME device was fabricated by Yan et al. using microporous Cu(II) trimesate (HKUST-1), which showed high enrichment and low limit of detection for benzene homologues. However, owing to the poor water stability of HKUST-1, the extraction efficiency of this device was very low when working in an environment with a relative humidity over 30%.4 It has been demonstrated that metal azolate frameworks (MAFs) constructed by imidazolate or pyrazolate derivatives can show very high thermal and chemical stability.7 Recently, SPME devices based on highly water-stable Zn(II) 2-methylimidazolate (MAF-4)8 and benzimidazolate (MAF-3)9 were reported by Yan et al.10 Due to the very small aperture size, MAF-4 (3.2 Å) shows superior selectivity for n-alkanes over branched alkanes, but requires a relatively long extraction time (ca. 20 min). With even smaller aperture size, MAF-3 (2.9 Å) can adsorb neither n-alkanes nor branched alkanes. At the same extraction conditions, benzene homologues can be only adsorbed on the outer surfaces of both MAF-4 and MAF-3.10 These examples highlight the importance of both ligand functionality and pore size of PCPs for SPME applications. Considering that generally carboxylate ligands can facilitate pore size modulation while azolate ligands can enhance framework stability, rational combination of these coordination groups may be a simple strategy for constructing new PCPs suitable for SPME applications. Here, we report the synthesis, structure, film fabrication and SPME application of a highly porous and stable Zn(II) pyrazolate–carboxylate framework showing excellent sensitivity and selectivity for non-polar benzene homologues.
Results and discussion
Synthesis and structure
Solvothermal reaction of 4-(3,5-dimethylpyrazol-4-yl)benzoic acid (H2mpba) and Zn(NO3)2 yielded crystals of a porous metal azolate framework compound [Zn(mpba)]·guest (MAF-X8). Single-crystal X-ray structural analysis (Table S1 and Fig. S1, ESI†) showed that MAF-X8 is a three-dimensional (3D), quasi-tetragonal, pillared-column, porous framework (void volume = 50%) constructed by tetrahedral Zn2+ and exo-tetradentate mpba2− (Fig. 1a). There are large 1D channels with widest and narrowest cross section area of 8.8 × 8.8 Å2 and 6.7 × 6.7 Å2, respectively, running along the crystallographic a-axis (Fig. 1b). The methyl groups of the ligand play an important role in the pore structure of MAF-X8, which not only reduce the channel size and make the ligand non-coplanar to change the channel shape, but also almost entirely block the Zn2+ ions and partially block the carboxylate O atoms, so as to increase the pore-surface hydrophobicity (Fig. 1c). It is worth pointing out that the non-stoichiometrically adsorbed mesitylene guest molecules are quite ordered in the crystal structure, indicating very good size matching between the channels of MAF-X8 and the aromatic molecules (Fig. S2, ESI†). Similar coordination and framework structures have been reported for Zn(II) and Co(II) 1,4-benzenedipyrazolates, which display straight, square channels with uniform cross section area of 10 × 10 Å2,11 because the ligand is longer and is planar.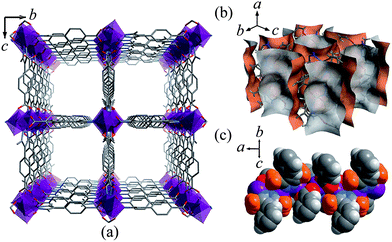 | ||
| Fig. 1 (a) 3D quasi-tetragonal pillared-column framework structure of MAF-X8 viewed along the a-axis (hydrogen atoms are omitted for clarity). (b) Perspective view of the pore surface of MAF-X8. (c) Side view of the Zn-carboxylate chain in space-filling mode (methyl groups are highlighted in orange). | ||
Framework stability and sorptive properties
Thermogravimetric analysis and powder X-ray diffraction (PXRD) studies of MAF-X8 showed that guest molecules can be removed below 220 °C and the host framework was stable up to 450 °C (Fig. S3 and S4, ESI†). The single-crystal structure of guest-free MAF-X8 was measured to confirm that the framework is rigid and not distorted after complete removal of guests. The N2 sorption isotherm of MAF-X8 measured at 77 K shows type-I characteristics with saturation uptake of 306 cm3 g−1 (Fig. S5, ESI†), corresponding to a pore volume of 0.47 cm3 g−1, which is slightly lower than the theoretical value of 0.52 cm3 g−1 as calculated from the crystal structure of MAF-X8. Fitting the isotherm gave a Brunauer–Emmett–Teller (BET) surface area of 1161 m2 g−1 and a Langmuir surface area of 1306 m2 g−1.As monitored by PXRD patterns, guest-free MAF-X8 is stable for 5 h in saturated water vapor. Prolonged exposure caused some slight changes in peak position and decrease of peak intensity but the original highly crystalline phase could be recovered by direct heating of the sample to 250 °C or exposure to methanol vapor at room temperature (Fig. S6, ESI†). This behavior suggests that water molecules do not destroy the chemical bonds but simply induce some reversible changes in the lattice conformation of the host framework.
Fabrication of SPME fibers
To investigate the potential SPME applications, we fabricated MAF-X8 thin films on the surface of stainless-steel fibers by an in situ hydrothermal growth method (see Experimental section in ESI†). Scanning electron micrographs showed that the stainless-steel fibers were coated by dense and quite regular MAF-X8 crystals (Fig. 2). The coating thickness was approximately 30 μm, being suitable for SPME. A PXRD pattern of the thin film confirmed that the crystals mostly grow along the a-axis with highly exposed (100) facets (Fig. 3), which will favor the adsorption and desorption of guests. It should be noted that highly oriented crystalline thin films are very important and useful,12 while the crystallites (HKUST-1, MAF-3 and MAF-4) were randomly oriented in known PCP-coated SPME fibers.4,10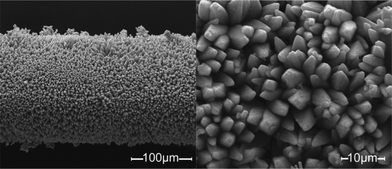 | ||
| Fig. 2 Scanning electron micrographs of MAF-X8 crystals grown on stainless-steel fibers. | ||
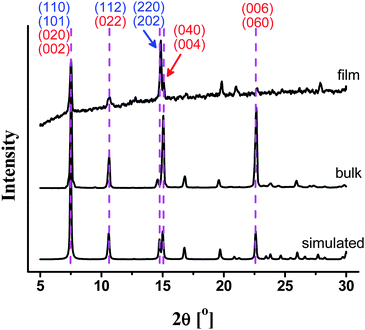 | ||
| Fig. 3 Comparison of the PXRD patterns of thin film and bulk microcrystals of MAF-X8. The indices of crystal faces highlighted in red and blue indicate that the intensity of the reflections should decrease (or even disappear) and increase, respectively, when the crystals are highly oriented along the a-axis. | ||
SPME applications
Volatile organic compounds are of wide concern in terms of pollution because of their possible serious damage on the brain, kidneys, liver or respiratory system.13 Four primary non-polar volatile organic compounds, benzene, toluene, ethylbenzene and o-xylene, are generally mixed and used as standard analytes (referred to as BTEX) in SPME and related studies.14 Clearly the highly efficient detection of the BTEX compounds is very important. Similarly, 2-chlorophenol, p-cresol, 2-nitrophenol, 2,4-dichlorophenol and 2,4,6-trichlorophenol are also highly controlled air or water pollutants.15 These phenol derivatives are used as standard polar analytes (referred to as phenols). In practical applications, BTEX and phenols generally coexist, which can bring competitive adsorption on the SPME fibers, and so reduce the sensitivity of the analysis. Moreover, the molecular sizes of these BTEX and phenols are very similar, which make these compounds difficult to separate.In our research, the analytes were extracted from the headspace of the sample matrix, which is generally believed to be able to reduce interference from poorly volatile substances.16 A saturated water solution of NaCl (relative humidity 75%) was used as the solvent. The extraction time needed for SPME fibers is a crucial parameter for quantitative analysis.4,5 The extraction time should be equal to or longer than the shortest time required for reaching the sorption equilibrium. The adsorption kinetics of the MAF-X8 coated SPME fibers were tested by 200 ng mL−1 BTEX, in which the chromatographic peak area was plotted against the trial extraction time. The result showed that the four analytes rapidly reached adsorption equilibrium in ca. 7 min (Fig. S7, ESI†). The extraction time of reported fibers, coated by materials such as organic modified silica,17 carbon nanotube,18 PCP4 and polymer19 are mostly longer than 15 min. The faster adsorption kinetics of MAF-X8 may due to the highly regular and large channels of the structure as well as the ordered and suitable crystal directions in the SPME thin films. To obtain both high efficiency and good reproducibility, we chose 8.0 min as the operating extraction time. Similary, the required desorption time is also a crucial parameter of SPME fibers. If the desorption is not complete, the correctness and sensitivity of analysis will be affected. On the other hand, long desorption time not only reduces the efficiency but also leads to short lifetime of the fibers (high temperature thermal desorption). In conventional gas chromatography conditions, complete desorption of BTEX from MAF-X8 film was very fast within 0.5 min. To avoid a carry-over effect, we chose 2.0 min as desorption time for further experiments.
To investigate the analytical figures of merit for our new SPME devices, the linear range, limits of detection (LODs), repeatability and the reproducibility were determined under the optimized conditions for BTEX analysis (Table S2, ESI†). First of all, the MAF-X8 fibers exhibited wide linearity over three orders of magnitude for BTEX with good correlation coefficients (R2 > 0.9976). Also they have very low LODs, i.e. 0.006–0.060 μg L−1 for the BTEX compounds. The relative standard deviation (RSD) of repeatability for six replicated extractions of standard solutions was lower than 4.1%, and the RSD for fiber-to-fiber reproducibility obtained with three fibers fabricated under the same conditions was below 8.0%. Furthermore, no decrease of extraction performance was observed even after more than 120 replicated extractions, indicating the high stability of MAF-X8 and its thin films. Coexistence of such high thermal stability, short extraction time and very low LODs has been rarely reported for other coating materials (Table S3 and Fig. S8, ESI†).
We compared the extraction efficiency of the MAF-X8 coated fibers with commercial PDMS/DVB (65 μm) and PDMS (100 μm) fibers (PDMS = polydimethylsiloxane, DVB = divinylbenzene), which have been proven to be the most efficient for enrichment of medium-polar and non/less-polar compounds, respectively. Generally, the PDMS/DVB fibers with porous structure shows better performance than the PDMS fibers. In view of the different thickness of the coatings, we compared the performances of MAF-X8 and commercial fibers by calculating the normalized adsorptive capacity per unit coating volume (normalized to the maximum chromatographic peak area), which is referred to as the “normalized extraction efficiency” hereafter. As presented in Fig. 4, MAF-X8 showed an excellent enrichment for the single BTEX compounds. The relative extraction efficiency of MAF-X8 fibers is 18 to 157 times higher than the PDMS fibers, or 2 to 8 times that of PDMS/DVB fibers. Such excellent performance of MAF-X8 fibers may result from the large specific surface area and the relatively hydrophobic pore surface of the material.
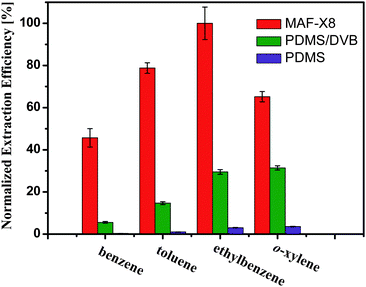 | ||
| Fig. 4 Comparison of the normalized extraction efficiencies of MAF-X8 coated fibers and commercial SPME fibers for BTEX. The error bar shows the standard deviation of the mean. | ||
It is universally acknowledged that selectivity of sorbent is another vital factor for SPME, because the real sample matrix may contain numerous kinds of pollutants, sometimes even the impurities are at a higher concentration than the target analytes. To further investigate the selectivity and the anti-interference ability of the MAF-X8 fibers, mixed BTEX–phenols standards were used as analytes, which are more similar to real samples. Interestingly, even when the BTEX–phenols ratio was increased to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, the extraction efficiencies for BTEX compounds were not reduced, although there was a slight increase in the adsorbed amount of phenols (Fig. 5), which indicated good anti-interference ability of the MAF-X8 fibers. By contrast, the commercial PDMS/DVB fibers showed gradually increased adsorption for phenols and obvious decrease in the extracted amounts of BTEX compounds when the concentration of phenols was increased. As expected, the PDMS fibers have a poorer selectivity at the same conditions with a rapidly increased adsorption of phenols. Presumably, the BTEX enrichment of MAF-X8 relative to the commercial fibers will be higher than that shown in Fig. 4 when a large proportion of phenols are present (Fig. S9, ESI†).
5, the extraction efficiencies for BTEX compounds were not reduced, although there was a slight increase in the adsorbed amount of phenols (Fig. 5), which indicated good anti-interference ability of the MAF-X8 fibers. By contrast, the commercial PDMS/DVB fibers showed gradually increased adsorption for phenols and obvious decrease in the extracted amounts of BTEX compounds when the concentration of phenols was increased. As expected, the PDMS fibers have a poorer selectivity at the same conditions with a rapidly increased adsorption of phenols. Presumably, the BTEX enrichment of MAF-X8 relative to the commercial fibers will be higher than that shown in Fig. 4 when a large proportion of phenols are present (Fig. S9, ESI†).
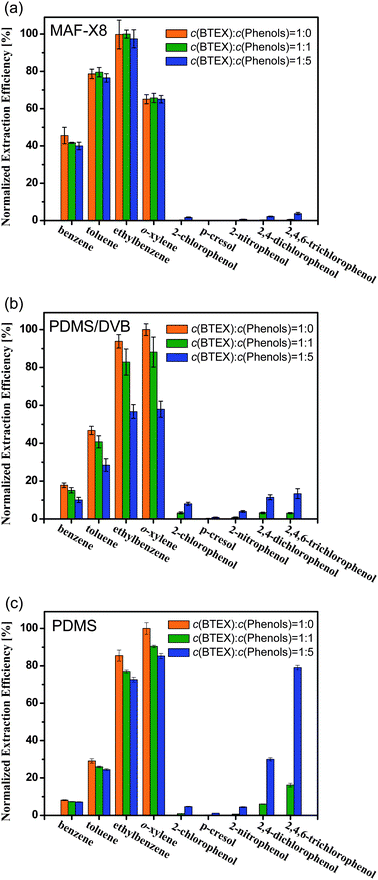 | ||
| Fig. 5 Normalized extraction efficiencies for single BTEX and BTEX–phenols mixtures at different mixed ratios. (a) MAF-X8 fibers, (b) commercial 65 μm PDMS/DVB fibers and (c) commercial 100 μm PDMS fibers. The error bar shows the standard deviation of the mean. | ||
Mechanism of selectivity
Because the molecular sizes of BTEX and phenols are both smaller than the pore size of MAF-X8, the high selectivity of BETX over phenols is not likely based on the size exclusive effect commonly observed for molecular sieves. To elucidate the mechanism of selective extraction, we used thermogravimetric analysis to examine the sorption property of MAF-X8 powder sample toward saturated vapors of representative nonpolar (toluene) and polar (2-chlorophenol) compounds. After long-time exposure, both toluene and 2-chlorophenol can be adsorbed in large amounts (Fig. S10, ESI†). For rigid adsorbents, the theoretical saturation/maximum guest uptakes can be empirically calculated from the crystallographic pore volume of adsorbent and density of adsorbate. Generally, the observed saturation/maximum uptakes are slightly lower than the theoretical ones due to the size/shape incompatibility between the pores and the guest molecules, especially when their sizes are similar (such as for MAF-X8 and BTEX–phenols). More accurate theoretical saturation/maximum adsorption amounts can be obtained by molecular simulation methods (see ESI†). As shown in Table S5 (ESI†) the observed toluene and mesitylene uptakes are well consistent with the molecular simulation results and slightly lower than the empirical results. The observed maximum uptake of 2-chlorophenol is larger than both the molecular simulation and empirical values, revealing that 2-chlorophenol not only can readily enter and occupy the 1D channels, but is also significantly adsorbed on the outer crystal surface of MAF-X8.While thermodynamic differences cannot account for the mechanism of selectivity, we further studied the adsorption kinetics of toluene and 2-chlorophenol. The adsorption of toluene can rapidly reach saturation/equilibrium within 8 min, which was consistent with the extraction time of the SPME fibers. On the contrary, the adsorption of 2-chlorophenol can not reach saturation even after 4000 min, and the adsorption amounts can be almost neglected in the first 15 min, consistent with the excellent selectivity of MAF-X8 (Fig. 6 and S10, ESI†) towards BTEX. Considering that the vapor pressure of 2-chlorophenol in headspace SPME experiment is much lower than the saturation vapor pressure, the adsorption of 2-chlorophenol might be slower and even virtually absent in headspace SPME experiments.
 | ||
| Fig. 6 Adsorption kinetics of saturated toluene and 2-chlorophenol vapors on microcrystalline MAF-X8 (molecular simulation suggested saturation uptake of 33.0 and 46.0% for toluene and 2-chlorophenol, respectively). | ||
To further explain the different adsorption kinetics, host–guest interactions were investigated by computer modelling (see ESI†). The molecular mechanics simulated diffusion barrier of 2-chlorophenol in the channel is much higher than that of toluene, probably due to the relatively small channel size of MAF-X8 and the dipole–dipole interaction between 2-chlorophenol and the partially exposed carboxylate O atoms. For comparison, we also performed the same simulation for isostructural Zn(II) 1,4-benzenedipyrazolate,11a which showed very small diffusion barriers for both 2-chlorophenol and toluene due to its large pore size and inert pore surface (without carboxylate oxygen atoms) (Fig. 7). Moreover, the periodic density functional theory results showed that 2-chlorophenol forms a strong O–H⋯O hydrogen bond (O⋯O 2.68 Å, O–H⋯O 165°, binding energy −15.88 kcal mol−1) with the uncoordinated carboxylate oxygen on the crystal surface, which blocks the entrance of the 1D channels. In contrast, toluene prefers entering the channel (binding energy −6.14 kcal mol−1) rather than staying at the channel entrance (Fig. 8). The different binding behaviors of guest molecules on the outer crystal surface of MAF-X8 are also consistent with the SPME performance on toluene and 2-chlorophenol.
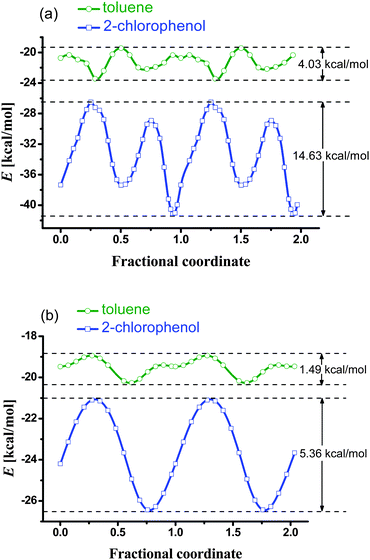 | ||
| Fig. 7 Potential profiles and diffusion barriers of guest molecules passing through the 1D channels of (a) MAF-X8 and (b) Zn(II) 1,4-benzenedipyrazolate. | ||
 | ||
| Fig. 8 Perspective view of the optimized (periodic density functional theory) structures of 2-chlorophenol and toluene on the outer crystal surface of MAF-X8 (the two structures are projected along the channel direction and are superimposed for comparison). | ||
Conclusions
In summary, a highly stable PCP has been synthesized using a bifunctional pyrazolate-carboxylate ligand, which can be readily fabricated as high-performance SPME devices showing short extraction and desorption time, high enrichment factors and low detection limits for non-polar, toxic, environment-unfriendly benzene homologues even in the presence of large proportion of polar interferents with similar molecular sizes. This work demonstrates that PCPs might be rationally designed to serve as promising adsorbent materials for specific SPME applications.Acknowledgements
This work was supported by the “973 Project” (2012CB821706) and NSFC (21121061 & 21001120).Notes and references
- (a) E. D. Bloch, W. L. Queen, R. Krishna, J. M. Zadrozny, C. M. Brown and J. R. Long, Science, 2012, 335, 1606–1610 CrossRef CAS; (b) H. Deng, S. Grunder, K. E. Cordova, C. Valente, H. Furukawa, M. Hmadeh, F. Gándara, A. C. Whalley, Z. Liu, S. Asahina, H. Kazumori, M. O'Keeffe, O. Terasaki, J. F. Stoddart and O. M. Yaghi, Science, 2012, 336, 1018–1023 CrossRef CAS; (c) M. P. Suh, H. J. Park, T. K. Prasad and D.-W. Lim, Chem. Rev., 2012, 112, 782–835 CrossRef CAS; (d) M. C. Das, Q. Guo, Y. He, J. Kim, C.-G. Zhao, K. Hong, S. Xiang, Z. Zhang, K. M. Thomas, R. Krishna and B. Chen, J. Am. Chem. Soc., 2012, 134, 8703–8710 CrossRef CAS; (e) J. Park, D. Yuan, K. T. Pham, J.-R. Li, A. Yakovenko and H.-C. Zhou, J. Am. Chem. Soc., 2012, 134, 99–102 CrossRef CAS; (f) M. Yoon, R. Srirambalaji and K. Kim, Chem. Rev., 2012, 112, 1196–1231 CrossRef CAS.
- (a) P. Horcajada, R. Gref, T. Baati, P. K. Allan, G. Maurin, P. Couvreur, G. Férey, R. E. Morris and C. Serre, Chem. Rev., 2012, 112, 1232–1268 CrossRef CAS; (b) N. C. Jeong, B. Samanta, C. Y. Lee, O. K. Farha and J. T. Hupp, J. Am. Chem. Soc., 2012, 134, 51–54 CrossRef CAS; (c) T. Uemura, N. Uchida, A. Asano, A. Saeki, S. Seki, M. Tsujimoto, S. Isoda and S. Kitagawa, J. Am. Chem. Soc., 2012, 134, 8360–8363 Search PubMed; (d) C. Wang, T. Zhang and W. Lin, Chem. Rev., 2012, 112, 1084–1104 CrossRef CAS.
- (a) L. E. Kreno, K. Leong, O. K. Farha, M. Allendorf, R. P. Van Duyne and J. T. Hupp, Chem. Rev., 2012, 112, 1105–1125 CrossRef CAS; (b) M. D. Allendorf, R. J. T. Houk, L. Andruszkiewicz, A. A. Talin, J. Pikarsky, A. Choudhury, K. A. Gall and P. J. Hesketh, J. Am. Chem. Soc., 2008, 130, 14404–14405 CrossRef CAS; (c) S. Achmann, G. Hagen, J. Kita, I. M. Malkowsky, C. Kiener and R. Moos, Sensors, 2009, 9, 1574–1589 CrossRef CAS; (d) E. Biemmi, A. Darga, N. Stock and T. Bein, Microporous Mesoporous Mater., 2008, 114, 380–386 CrossRef CAS; (e) R. Ameloot, L. Stappers, J. Fransaer, L. Alaerts, B. F. Sels and D. E. De Vos, Chem. Mater., 2009, 21, 2580–2582 CrossRef CAS; (f) Z.-Y. Gu, C.-X. Yang, N. Chang and X.-P. Yan, Acc. Chem. Res., 2012, 45, 734–745 CrossRef CAS.
- X.-Y. Cui, Z.-Y. Gu, D.-Q. Jiang, Y. Li, H.-F. Wang and X.-P. Yan, Anal. Chem., 2009, 81, 9771–9777 CrossRef CAS.
- C. L. Arthur and J. Pawliszyn, Anal. Chem., 1990, 62, 2145–2148 CrossRef CAS.
- (a) S. A. S. Wercinski, Solid Phase Microextraction: A Practical Guide, Marcel Dekker, New York, 1999 Search PubMed; (b) G. F. Ouyang, D. Vuckovic and J. Pawliszyn, Chem. Rev., 2011, 111, 2784–2814 Search PubMed.
- J.-P. Zhang, Y.-B. Zhang, J.-B. Lin and X.-M. Chen, Chem. Rev., 2012, 112, 1001–1033 CrossRef CAS.
- (a) X.-C. Huang, Ph.D. thesis, Sun Yat-Sen University, Guangzhou, China, 2004; (b) X.-C. Huang, Y.-Y. Lin, J.-P. Zhang and X.-M. Chen, Angew. Chem., Int. Ed., 2006, 45, 1557–1559 CrossRef CAS.
- X.-C. Huang, J.-P. Zhang and X.-M. Chen, Chin. Sci. Bull., 2003, 48, 1531–1534 CrossRef CAS.
- N. Chang, Z.-Y. Gu, H.-F. Wang and X.-P. Yan, Anal. Chem., 2011, 83, 7094–7101 CrossRef CAS.
- (a) H. J. Choi, M. Dincă and J. R. Long, J. Am. Chem. Soc., 2008, 130, 7848–7850 CrossRef CAS; (b) H. J. Choi, M. Dinca, A. Dailly and J. R. Long, Energy Environ. Sci., 2010, 3, 117–123 RSC.
- (a) D. Bradshaw, A. Garai and J. Huo, Chem. Soc. Rev., 2012, 41, 2344–2381 RSC; (b) A. Bétard and R. A. Fischer, Chem. Rev., 2012, 112, 1055–1083 CrossRef CAS.
- J. J. Shah and H. B. Singh, Environ. Sci. Technol., 1988, 22, 1381–1388 CAS.
- C. L. Arthur, L. M. Killam, K. D. Buchholz, J. Pawliszyn and J. R. Berg, Anal. Chem., 1992, 64, 1960–1966 CrossRef CAS.
- (a) K. D. Buchholz and J. Pawliszyn, Environ. Sci. Technol., 1993, 27, 2844–2848 CAS; (b) P. Barták and L. Čáp, J. Chromatogr., A, 1997, 767, 171–175 CrossRef CAS.
- Z. Zhang and J. Pawliszyn, Anal. Chem., 1993, 65, 1843–1852 CrossRef CAS.
- R. G. da Costa Silva and F. Augusto, J. Chromatogr., A, 2005, 1072, 7–12 CrossRef.
- (a) A. Sarafraz-Yazdi, A. Amiri, G. Rounaghi and H. E. Hosseini, J. Chromatogr., A, 2011, 1218, 5757–5764 CrossRef CAS; (b) A. Sarafraz-Yazdi, H. P. Moghadam, Z. Es'haghi and S. Sepehr, Anal. Methods, 2010, 2, 746–752 RSC; (c) R. Jiang, F. Zhu, T. Luan, Y. Tong, H. Liu, G. Ouyang and J. Pawliszyn, J. Chromatogr., A, 2009, 1216, 4641–4647 CrossRef; (d) Q. Li, X. Ma, D. Yuan and J. Chen, J. Chromatogr., A, 2010, 1217, 2191–2196 CrossRef CAS.
- A. Gaujac, E. S. Emídio, S. Navickiene, S. L. C. Ferreira and H. S. Dórea, J. Chromatogr., A, 2008, 1203, 99–104 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, thermogravimetric curves, PXRD, N2 sorption isotherm, additional structural plot, computational details, additional SPME data, and X-ray crystallographic files in CIF format. CCDC 892987 and 892988. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c2sc21181e |
| This journal is © The Royal Society of Chemistry 2013 |
