Synthesis and characterization of non-hydrolysable diphosphoinositol polyphosphate messengers†
Mingxuan
Wu
,
Barbara E.
Dul‡
,
Alexandra J.
Trevisan‡
and
Dorothea
Fiedler
*
Department of Chemistry, Princeton University, Washington Rd, Princeton, NJ 08544, USA. E-mail: dfiedler@princeton.edu; Tel: +1 609 258 1025
First published on 8th October 2012
Abstract
The diphosphoinositol polyphosphates (PP-IPs) are a central group of eukaryotic messengers. They regulate numerous processes, including cellular energy homeostasis and adaptation to environmental stresses. To date, most of the molecular details in PP-IP signalling have remained elusive, due to a lack of appropriate methods and reagents. Here we describe the expedient synthesis of methylene-bisphosphonate PP-IP analogues. Their characterization revealed that the analogues exhibit significant stability and mimic their natural counterparts very well. This was further confirmed in two independent biochemical assays, in which our analogues potently inhibited phosphorylation of the protein kinase Akt and hydrolytic activity of the Ddp1 phosphohydrolase. The non-hydrolysable PP-IPs thus emerge as important tools and hold great promise for a variety of applications.
Introduction
Cellular decision-making is governed by the concerted actions of signalling proteins and small molecule second messengers. Among the eukaryotic second messengers, the diphosphoinositol polyphosphates (PP-IPs) constitute an important class. These molecules have captured the attention of both chemists and biologists, because of their unique chemical structure and their ability to mediate a plethora of interesting phenotypes.1The PP-IPs are a group of highly phosphorylated signalling molecules, based on the myo-inositol scaffold, that contain one or two high-energy pyrophosphate groups. These phosphate groups are installed in a biosynthetic pathway that is well conserved from yeast to mammals (a simplified pathway diagram is shown in Fig. 1).1
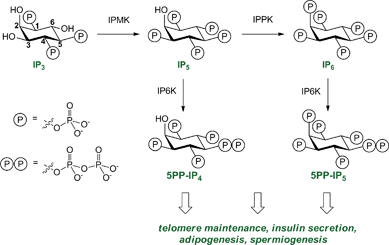 | ||
| Fig. 1 The diphosphoinositol polyphosphate biosynthetic pathway, in abbreviated form. Phosphorylation of inositol 1,4,5-triphosphate (IP3) by inositol multikinase (IPMK) and inositol pentakisphosphate 2-kinase (IPPK) results in formation of inositol pentakisphosphate (IP5) and inositol hexakisphosphate (IP6). Both IP5 and IP6 are substrates for IP6K (inositol hexakisphosphate kinase) to yield the diphosphoinositol polyphosphates 5PP-IP4 and 5PP-IP5, respectively. The numbering of the ring positions is indicated in the IP3 structure. | ||
The successive phosphorylation reactions are carried out by a set of dedicated small molecule kinases. Genetic perturbation of these kinases in yeast revealed important functions for PP-IPs in many cellular processes, including telomere maintenance,2 response to oxidative stress,3 and nutrient sensing.4 Mice that lack the kinase IP6K1 (inositol hexakisphosphate kinase 1) are not able to produce sufficient amounts of 5PP-IP5 – the best characterized PP-IP to date – in various tissues (Fig. 1).5 These mice exhibit severe defects in insulin secretion, increased peripheral insulin sensitivity, and resistance to age and diet-induced obesity.5,6 While these phenotypes are truly remarkable, the underlying molecular mechanisms have remained enigmatic.
Traditionally, small diffusible messengers bind to particular target proteins to control their activity or localization. It is therefore commonly assumed that PP-IPs utilize an allosteric mechanism to regulate protein function. However, only a handful of targets have been identified to date, and in some cases the biological relevance is not clear.4,5b,7 An alternative signalling mechanism for the PP-IPs involves the transfer of the high-energy β-phosphate group onto a phospho-serine residue, yielding a pyrophosphorylated protein.8 But due to technical challenges, it has only been possible to identify pyrophosphorylated proteins in biochemical assays and not from complex cell lysates.9 Overall, progress in decoding PP-IP signalling has been hindered by a lack of suitable methods and reagents. As current approaches rely on standard biochemical and genetic techniques, there is a pressing need for chemical tools that can help to decipher the discrete PP-IP signalling functions.
Here we report the synthesis and characterization of a number of non-hydrolysable methylene-bisphosphonate PP-IP analogues (Fig. 2). We demonstrate that the analogues are good mimics of the natural counterpart with regard to their conformation in solution and their protein binding properties. Our analogues thus represent an important set of mechanistic probes that will be of great use for the inositol signalling community, and we highlight a number of possible applications.
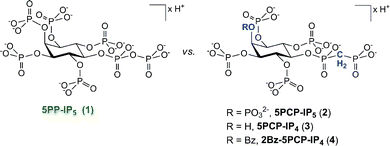 | ||
| Fig. 2 Comparison of the naturally occurring signalling molecule 5PP-IP5 (1) with the analogues described in this study. | ||
Results and discussion
Expedient synthesis of PP-IP analogues
The methylene-bisphosphonate moiety has been used widely as a stable substitute for diphosphate groups; an important example is AMPPCP (β,γ-methyleneadenosine 5′-triphosphate), a non-hydrolysable analogue of ATP.10 Among the PP-IP family members, the 5PP-IP5 messenger is the most prominent one and we therefore decided to focus on the synthesis of the non-hydrolysable methylene-bisphosphonate 5PCP-IP5 (2, Fig. 2) using the synthetic route outlined in Scheme 1.11 The protected myo-inositol 5 can be obtained from myo-inositol in three steps.12 The bisphosphonate group was then installed in the 5-position, to provide intermediate 6a in good yield. Removal of the benzoyl- and acetonide protecting groups furnished the pentaol 7b, which was subsequently phosphitylated and oxidized (8b). The phosphate groups were unveiled as the free phosphates via hydrogenation in the presence of NaHCO3, and 5PCP-IP5 (2) was isolated as the sodium salt. Compared to the synthesis of the natural molecule 5PP-IP5,13 the non-hydrolysable analogue is accessible in good quantity and high purity, while requiring fewer synthetic steps.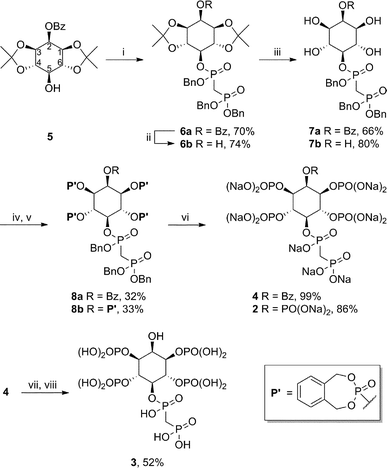 | ||
| Scheme 1 Synthesis of non-hydrolysable PP-IP analogues. Reagents and conditions: (i) Benzyl((bis(benzyloxy)phosphoryl)methyl)phosphonochloridate, KHMDS, THF, −78 °C to rt, overnight; (ii) NaOMe, MeOH, rt, overnight; (iii) H2O, p-TsOH, acetone, overnight; (iv) N,N-diethyl-1,5-dihydro-2,4,3-benzodioxaphosphepin-3-amine, 1H-tetrazole, CH3CN, 0 °C to rt, 36 h; (v) mCPBA, CH3CN, 0 °C to rt, 3 h; (vi) H2, Pd black, NaHCO3, t-BuOH/H2O, rt, overnight; (vii) conc. aq. NH3, rt, 4 days; (viii) Dowex-H+. | ||
Another PP-IP family member is 5PP-IP4 (Fig. 1), a molecule that has been linked to telomere maintenance and DNA damage repair.2 The corresponding bisphosphonate analogue 5PCP-IP4 (3) was synthesized using a slightly modified route: Removal of the acetonide groups from 6a was followed by phosphitylation, and oxidation (8a). Subsequent hydrogenation afforded a diphosphoinositol phosphate analogue that is benzoyl-protected in the 2-position (4). The benzoyl group was cleaved using aqueous ammonia and yielded the non-hydrolysable analogue 5PCP-IP4 (3). Both 5PCP-IP4 (3) and 5PCP-IP5 (2) showed no signs of decomposition in aqueous solution at neutral pH, after 40 days at room temperature.
5PP-IP5 and 5PCP-IP5 exhibit a similar pH dependent conformational change
The pKa's of a pyrophosphate group differ slightly from those of a bisphosphonate group,14 and it was important to determine that 5PP-IP5 (1), and the non-hydrolysable analogue 5PCP-IP5 (2), exhibit the same properties in solution. Since the conformation of the inositol ring depends on the ionization state of the individual phosphate groups, 1H and 31P NMR titration curves can be used to compare the solution properties of 1 and 2.15To do so, 5PP-IP5 (1) was synthesized according to a modified literature procedure (Scheme S1†),13,16 and both 5PP-IP5 and 5PCP-IP5 were titrated in D2O (containing 140 mM K+, 10 mM Na+, and 1 mM Mg2+ to mimic cellular metal cation content) at room temperature. Fig. 3 depicts a subset of the 1H NMR titration data of the two molecules (for all data see Fig. S1 and S2†). The ring inversion, which converts 5 equatorial/1 axial substituents to 1 equatorial/5 axial substituents, occurred around pD 8.8 for both molecules.17 While these data do not provide a direct measurement of the pKa values for 1 and 2, the titration curves highlight the similarity of the overall ionization state of the two molecules.18
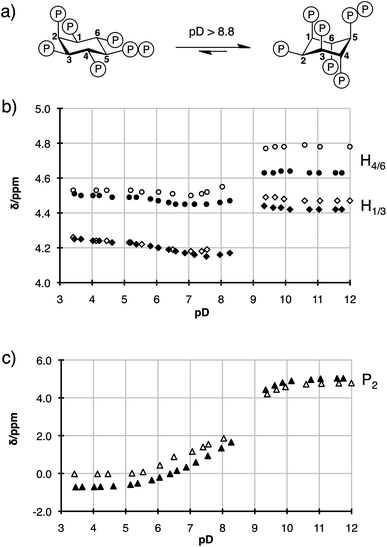 | ||
| Fig. 3 (a) 5PP-IP5 and 5PCP-IP5 undergo a conformational change around pD 8.8. (b) 1H NMR titration curves for 5PP-IP5 (hollow circles for H4/6 and hollow diamonds for H1/3) and 5PCP-IP5 (filled circles for H4/6 and filled diamonds for H1/3). Between pD 8.4 and pD 9.2 peaks could not be assigned due to severe broadening of the resonances (see Fig. S1†). (c) 31P NMR titration curves for 5PP-IP5 (hollow triangles for P2) and 5PCP-IP5 (filled triangles for P2). | ||
5PCP-IP5 exhibited much higher stability compared to 5PP-IP5. Even at a low or high pD (2.0 or 13.0), no detectable decomposition occurred after 40 days at room temperature. The non-hydrolysable PP-IP analogues will therefore be of great use to characterize the physicochemical properties of the PP-IP messengers. Furthermore, our lab is interested in the rich metal-coordination chemistry of these molecules.19 The presence of highly Lewis acidic metal cations, such as Mg2+ and Fe3+, promotes hydrolysis of the PP-IPs, but our bisphosphonate analogues circumvent this problem and can be used as surrogates to delineate the metal-binding properties of PP-IP molecules.
PP-IP analogues potently inhibit Akt phosphorylation in vitro
It is thought that PP-IPs can control protein activity or localization via allosteric regulation.4,5b,7 If 5PCP-IP5 indeed closely mimics 5PP-IP5, it should exert the same allosteric control over a given protein binding partner.Recently, it was reported that 5PP-IP5 binds to the pleckstrin homology (PH) domain of the protein kinase Akt (also known as protein kinase B).5b This binding event was proposed to stabilize Akt in an inactive conformation, which precludes Akt from becoming phosphorylated on threonine 308 by the upstream kinase PDK1 (3-phosphoinositide-dependent protein kinase, Fig. 4a). We tested the inhibitory activity of both 5PP-IP5 and 5PCP-IP5 in a biochemical assay, using purified inactive Akt and activated PDK1. The two kinases were incubated in the presence of varying concentrations of 5PP-IP5 or 5PCP-IP5, and inhibition of Akt phosphorylation at threonine 308 was monitored using a phosphospecific antibody (Fig. 4b). As previously reported, 5PP-IP5 potently inhibited Akt phosphorylation; under our assay conditions, 5PP-IP5 displayed an IC50 of 217 nM.20 5PCP-IP5 closely resembled its natural counterpart with an IC50 of 129 nM. These data illustrate that changing the pyrophosphate moiety to a methylene-bisphosphonate group did not significantly alter the affinity of the small molecule for a known protein binding partner. Consequently, it should be feasible to utilize 5PCP-IP5 as an affinity reagent to identify PP-IP protein binding partners.
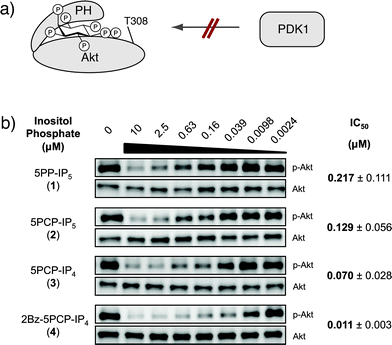 | ||
| Fig. 4 (a) Binding of 5PP-IP5 is proposed to stabilize Akt in an inactive conformation that cannot become phosphorylated by PDK1. (b) Western blots for Akt inhibition experiments. Akt phosphorylation at threonine 308 (p-Akt) was measured using a phosphospecific antibody. A Western blot for total Akt was used as a loading control. IC50 values were determined in three independent experiments, and the errors are indicated. | ||
From a synthetic perspective, the 2-position of 5PCP-IP5 could most easily be derivatized for attachment to a solid support. To determine how important the phosphate group in the 2-position is for the binding interaction with Akt, we evaluated the analogues 5PCP-IP4 (3) and 2Bz-5PCP-IP4 (4), which contain a hydroxyl group or a benzoyl group in the 2-position, respectively. Remarkably, those structural changes did not diminish their affinity for Akt (Fig. 4b).21 Encouraged by these results, we are currently exploring a number of attachment strategies for these molecules.
PP-IP analogues inhibit the Ddp1 phosphohydrolase in vitro
We next sought to validate the non-hydrolysable analogues in a different biochemical assay, but examples of PP-IP binding proteins in the literature are scarce.4,5b,7 Since the enzymes involved in PP-IP metabolism must bind to their substrates, we chose to investigate the binding of our analogues to the yeast polyphosphate phosphatase Ddp1. Ddp1 hydrolyses diadenosine polyphosphates, inorganic polyphosphates, and a subset of PP-IPs.22 A recent report from Saiardi and co-workers demonstrated that Ddp1 exhibited highly varied activity towards different PP-IP isomers: while 1PP-IP5 – a PP-IP family member with the pyrophosphate group in the 1-position – was completely hydrolysed to IP6 after 30 minutes at 37 °C, 5PP-IP5 showed no signs of hydrolysis under the same conditions.22c We therefore reasoned that 5PP-IP5 could be a competitive inhibitor for Ddp1 when used in combination with other Ddp1 substrates, such as diadenosine pentakisphosphate (Ap5A).Ddp1 was expressed and purified using an N-terminal polyhistidine tag (His). When His–Ddp1 was incubated with Ap5A, it displayed robust phosphohydrolase activity, as determined by HPLC analysis of the reaction mixture (Fig. 5a).23 Next, varying concentrations of 5PP-IP5 were added to the Ap5A hydrolysis reaction. As expected, 5PP-IP5 acted as a competitive inhibitor with an IC50 of 2.5 μM (Fig. 5b).24 Testing of 5PCP-IP5 and 5PCP-IP4 revealed very similar inhibition profiles (Fig. 5b and S4†), which corroborates that the bisphosphonate moiety is a suitable mimic for the pyrophosphate group, as it is bound by the protein with similar affinity. Interestingly, 2Bz-5PCP-IP4 displayed the highest potency with an IC50 value in the submicromolar range (0.16 μM). As was also observed for Akt, the benzoyl substituent in the 2-position does not lower the binding affinity; in fact, it made the molecule significantly more potent. Possible explanations for the increased potency include a favourable interaction of the benzoyl group with the protein, and/or changes in solvation of the unbound 2Bz-5PCP-IP4, which may decrease its ground-state stabilization compared to the other analogues.
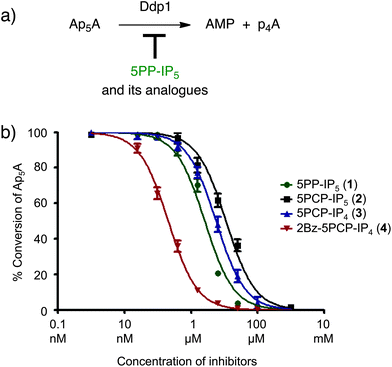 | ||
| Fig. 5 (a) Ddp1 hydrolyzes diadenosine pentakisphosphate (Ap5A) to adenosine monophosphate (AMP) and adenosine tetrakisphosphate (p4A), which is subsequently further degraded. (b) Ddp1 mediated Ap5A hydrolysis is inhibited by 5PP-IP5 and the analogues. IC50 values for 5PP-IP5, 5PCP-IP5, 5PCP-IP4 and 2Bz-5PCP-IP4 were determined in three independent experiments and the inhibition curves are shown (see also Fig. S4†). | ||
Based on its high potency, 2Bz-5PCP-IP4 may become useful to inhibit Ddp1 activity in cell lysates to prevent hydrolysis of Ddp1 substrates, thereby facilitating their identification and analysis. Overall, the Ddp1 inhibition studies paralleled our observations from the Akt assay, further validating that the bisphosphonate analogues are adequate surrogates for the natural molecules.
Conclusions
Even though the PP-IPs were discovered almost 20 years ago, we are just beginning to appreciate their complex functions as central regulators of cell homeostasis. This gap in our understanding is due to the dearth of reagents available to study PP-IP function in vivo and in vitro. The PP-IP analogues described in this paper hold great promise for filling several of these gaps. We have shown that the bisphosphonate group adequately mimics the pyrophosphate group in PP-IPs, while providing easier synthetic accessibility and increased stability. The non-hydrolysable analogues can therefore be developed into affinity reagents for the systematic identification of PP-IP interacting partners. Subsequently, the increased stability of the analogues will be advantageous for co-crystallizations of PP-IPs with their binding partners.The bisphosphonate analogues will also be highly informative with respect to the other PP-IP signalling mechanism, protein pyrophosphorylation. Our compounds are not able to transfer their β-phosphoryl group onto protein substrates, which allow us to distinguish the two signalling mechanisms in biochemical assays. Progress on using the analogues as mechanistic probes will be described in due course.
Until now there has been a reliance on genetic and molecular biology techniques to study PP-IP function. A major focus, however, should be on the molecules themselves. We believe that the non-hydrolysable PP-IPs describe in this paper will help to shift this imbalance, and will provide valuable insight into the biological functions of PP-IP molecules.
Acknowledgements
We would like to thank Dr Adolfo Saiardi for providing the constructs for Ddp1 expression, and the Muir, Doyle, MacMillan, and Sorensen groups for use of their chemicals and instruments. We would also like to thank Dr. Adam Resnick for helpful advice, and Dr. Istvan Pelczer and Dr. Simona Ghizdavu Pellascio for assistance with the NMR titration experiments. Financial support was provided by Princeton University, and the NIH (R00 GM087306).Notes and references
- For recent reviews on diphosphoinositol polyphosphate signalling see: (a) A. Saiardi, Subcell. Biochem., 2012, 59, 413 Search PubMed; (b) A. Chakraborty, S. Kim and S. H. Snyder, Sci. Signaling, 2011, 4, re1 Search PubMed; (c) J. P. Monserrate and J. D. York, Curr. Opin. Cell Biol., 2010, 22, 365 CrossRef CAS; (d) T. Wundenberg and G. W. Mayr, Biol. Chem., 2012, 393, 979 Search PubMed; (e) S. B. Shears, Mol. Pharmacol., 2009, 76, 236 CrossRef CAS. For recent reviews on inositol phosphate chemistry see: (f) S. J. Conway and G. J. Miller, Nat. Prod. Rep., 2007, 24, 687 RSC; (g) M. D. Best, H. Zhang and G. D. Prestwich, Nat. Prod. Rep., 2010, 27, 1403 RSC. While this manuscript was under review, a paper describing the synthesis of phosphonoacetic acid ester analogues of PP-IPs was published: (h) A. M. Riley, H. Wang, J. Weaver, S. Shears and B. V. L. Potter, Chem. Commun., 2012 10.1039/c2cc36044f , in press.
- (a) A. Saiardi, A. C. Resnick, A. M. Snowman, B. Wendland and S. H. Snyder, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 1911 CrossRef CAS; (b) S. J. York, B. N. Armbruster, P. Greenwell, T. D. Petes and J. D. York, J. Biol. Chem., 2005, 280, 4264 Search PubMed.
- S. M. N. Onnebo and A. Saiardi, Biochem. J., 2009, 423, 109 Search PubMed.
- (a) Y.-S. Lee, S. Mulugu, J. D. York and E. K. O'Shea, Science, 2007, 316, 109 CrossRef CAS; (b) Y.-S. Lee, K. Huang, F. A. Quiocho and E. K. O'Shea, Nat. Chem. Biol., 2008, 4, 25 CrossRef CAS.
- (a) R. Bhandari, K. R. Juluri, A. C. Resnick and S. H. Snyder, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 2349 Search PubMed; (b) A. Chakraborty, M. A. Koldobskiy, N. T. Bello, M. Maxwell, J. J. Potter, K. R. Juluri, D. Maag, S. Kim, A. S. Huang, M. J. Dailey, M. Saleh, A. M. Snowman, T. H. Moran, E. Mezey and S. H. Snyder, Cell, 2010, 143, 897 CrossRef CAS.
- C. Illies, J. Gromada, R. Fiume, B. Leibiger, J. Yu, K. Juhl, S.-N. Yang, D. K. Barma, J. R. Falck, A. Saiardi, C. J. Barker and P.-O. Berggren, Science, 2007, 318, 1299 Search PubMed.
- (a) H. B. R. Luo, Y. E. Huang, J. M. C. Chen, A. Saiardi, M. Iijima, K. Q. Ye, Y. F. Huang, E. Nagata, P. Devreotes and S. H. Snyder, Cell, 2003, 114, 559 CrossRef CAS; (b) W. L. Ye, N. Ali, M. E. Bembenek, S. B. Shears and E. M. Lafer, J. Biol. Chem., 1995, 270, 1564 Search PubMed.
- (a) A. Saiardi, R. Bhandari, A. C. Resnick, A. M. Snowman and S. H. Snyder, Science, 2004, 306, 2101 CrossRef CAS; (b) R. Bhandari, A. Saiardi, Y. Ahmadibeni, A. M. Snowman, A. C. Resnick, T. Z. Kristiansen, H. Molina, A. Pandey, J. K. Werner, Jr, K. R. Juluri, Y. Xu, G. D. Prestwich, K. Parang and S. H. Snyder, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 15305 CrossRef CAS.
- (a) C. Azevedo, A. Burton, E. Ruiz-Mateos, M. Marsh and A. Saiardi, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 21161 Search PubMed; (b) Z. Szijgyarto, A. Garedew, C. Azevedo and A. Saiardi, Science, 2011, 334, 802 CrossRef CAS.
- (a) T. S. Elliott, A. Slowey, Y. Ye and S. J. Conway, Med. Chem. Commun., 2012, 3, 735 RSC; (b) G. Mueller, S. Wied, S. Over and W. Frick, Biochemistry, 2008, 47, 1259 Search PubMed; (c) J. D. Verrier, J. L. Exo, T. C. Jackson, J. Ren, D. G. Gillespie, R. K. Dubey, P. M. Kochanek and E. K. Jackson, J. Neurochem., 2011, 118, 979 Search PubMed; (d) I. Mochalkin, J. R. Miller, A. Evdokimov, S. Lightle, C. Yan, C. K. Stover and G. L. Waldrop, Protein Sci., 2008, 17, 1706 Search PubMed; (e) K.-T. Wang, J. Wang, L.-F. Li and X.-D. Su, J. Mol. Biol., 2009, 390, 747 Search PubMed.
- We have also considered the incorporation of a difluoromethylene-bisphosphonate (PCF2P) group, because the PCF2P group more closely resembles the pKa values of the pyrophosphate group.10a However, the PCF2P analogues posed a significant synthetic challenge, and we ultimately focused our efforts on the methylene-bisphosphonates. We acknowledge that the differences in the pKa's of 5PP-IP5versus 5PCP-IP5 could be an issue, but, as our subsequent biochemical studies show, these differences in pKa do not appear to be a significant limitation in the two examples highlighted.
- (a) H. Y. Godage, A. M. Riley, T. J. Woodman and B. V. L. Potter, Chem. Commun., 2006, 2989 RSC; (b) S. K. Chung, Y. T. Chang and Y. U. Kwon, J. Carbohydr. Chem., 1998, 17, 369 CrossRef CAS.
- H. Zhang, J. Thompson and G. D. Prestwich, Org. Lett., 2009, 11, 1551 CrossRef CAS . An alternative synthesis of 5PP-IP5 involved fewer synthetic steps but was carried out on a small scale: .
- (a) R. Engel, Chem. Rev., 1977, 77, 349 CrossRef CAS; (b) A. Flohr, A. Aemissegger and D. Hilvert, J. Med. Chem., 1999, 42, 2633 Search PubMed.
- (a) L. R. Isbrandt and R. P. Oertel, J. Am. Chem. Soc., 1980, 102, 3144 CrossRef CAS; (b) J. C. Lindon, D. J. Baker, R. D. Farrant and J. M. Williams, Biochem. J., 1986, 233, 275 CAS; (c) C. Blum-Held, P. Bernard and B. Spiess, J. Am. Chem. Soc., 2001, 123, 3399 Search PubMed; (d) A. M. Riley, M. Trusselle, P. Kuad, M. Borkovec, J. Cho, J. H. Choi, X. Qian, S. B. Shears, B. Spiess and B. V. L. Potter, ChemBioChem, 2006, 7, 1114 Search PubMed.
- An HPLC purification step before the final deprotection proved to be essential to obtain 5PP-IP5 in the desired purity.
- The assignments of conformations was based on previous reports, coupling constant analysis, 1H–1H COSY, and 1H–31P HSQC (see ESI†).
- Interestingly, there is a noticeable difference in the chemical shifts of H4/6 for 1versus2 at basic pH. We believe that subtle changes in Mg2+ coordination may be responsible for this, as the difference in chemical shift is less pronounced when the NMR spectra were recorded in the absence of Mg2+ (see ESI†).
- Nothing is known about the coordination chemistry of PP-IPs. For studies on the metal-coordination properties of IP5 and IP6 see: (a) N. Veiga, J. Torres, S. Dominguez, A. Mederos, R. F. Irvine, A. Diaz and C. Kremer, J. Inorg. Biochem., 2006, 100, 1800 Search PubMed; (b) J. Torres, S. Dominguez, M. F. Cerda, G. Obal, A. Mederos, R. F. Irvine, A. Diaz and C. Kremer, J. Inorg. Biochem., 2005, 99, 828 CrossRef CAS; (c) N. Veiga, J. Torres, H. Y. Godage, A. M. Riley, S. Domínguez, B. V. Potter, A. Díaz and C. Kremer, J. Biol. Inorg. Chem., 2009, 14, 1001 Search PubMed.
- The published IC50 value is 20 nM. Presumably differences in buffer composition and/or incubation times are responsible for this discrepancy.
- 2Bz-5PCP-IP4 appeared to be the most potent. This trend was paralleled in subsequent experiments using the yeast Ddp1 phosphohydrolase and is discussed in the following section.
- (a) J. L. Cartwright and A. G. McLennan, J. Biol. Chem., 1999, 274, 8604 Search PubMed; (b) S. T. Safrany, S. W. Ingram, J. L. Cartwright, J. R. Falck, A. G. McLennan, L. D. Barnes and S. B. Shears, J. Biol. Chem., 1999, 274, 21735 Search PubMed; (c) A. Lonetti, Z. Szijgyarto, D. Bosch, O. Loss, C. Azevedo and A. Saiardi, J. Biol. Chem., 2011, 286, 31966 Search PubMed.
- We ruled out the possibility that the reaction product IP6 may be responsible for this inhibition, as the IC50 value for IP6 in this reaction is more than ten times higher (data not shown).
- As a control, we confirmed that Ap5A showed no signs of hydrolysis when incubated with His–Ddp1 EE/AQ, a catalytically inactive form of Ddp1 (Fig. S3†).
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures, spectroscopic data, and supporting figures. See DOI: 10.1039/c2sc21553e |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2013 |
