Efficient truxenone-based acceptors for organic photovoltaics†
Christian B.
Nielsen
*a,
Eszter
Voroshazi
b,
Sarah
Holliday
a,
Kjell
Cnops
bc,
Barry P.
Rand
b and
Iain
McCulloch
a
aDepartment of Chemistry and Centre for Plastic Electronics, Imperial College London, London SW7 2AZ, UK. E-mail: c.nielsen@imperial.ac.uk
bIMEC, Kapeldreef 75, B-3001 Heverlee, Belgium
cKatholieke Universiteit Leuven, ESAT, Kasteelpark Arenberg 10, Heverlee, Belgium
First published on 23rd October 2012
Abstract
Two electron-deficient truxenone derivatives are designed and synthesised for organic photovoltaic applications. The promise of this class of compounds as acceptor materials is illustrated by the fabrication of efficient bilayer solar cells with a subphthalocyanine (SubPc) donor, clearly outperforming reference cells with a soluble fullerene derivative as the acceptor.
Faced with the continued improvement of organic photovoltaic (OPV) devices, most materials research has been focused on the development of new polymeric donors.1 These donor materials are routinely tested with phenyl-C61-butyric acid methyl ester (PCBM) or closely related fullerene derivatives and solar cell efficiencies are fast approaching 10%.2 A common theme of this research is the judicious adjustment of donor properties to obtain an advantageous interaction, both energetically and morphologically, with the fullerene acceptor.3–5 Less effort, on the other hand, has been put into the development of new acceptor materials to complement existing or newly developed donor materials, to increase the understanding of the donor–acceptor interactions and to address some of the disadvantages associated with fullerene acceptors such as poor optical absorption and limited solubility especially in non-halogenated solvents.6–10 In order to investigate some of these aspects, there is the possibility to explore novel electron acceptors with curved isotropic electron-accepting motifs. By controlling the molecular curvature and the solubilising substituents, we aim to control the charge transport properties as well as the tendency for these small molecules to crystallise.
Here, we present two new truxenone-based acceptor materials that are highly soluble in many common organic solvents and highly absorptive in most of the ultraviolet-visible region of the spectrum, complementary to the absorption spectra of the majority of low bandgap donor polymers. Our synthetic design allows for easy control of the frontier energy levels and the promise of this new class of acceptors is illustrated with the fabrication of bilayer solar cells with efficiencies around 1% using subphthalocyanine (SubPc) as the donor constituent.
The synthetic route employed for the preparation of the two truxenone-based acceptors is shown in Scheme 1. Starting from 4,9,14-tribromotruxenone,11 the two thienyl-substituted truxenones 1a and 1b were easily obtained in moderate yields through a Suzuki-coupling with 5-hexyl and 3-hexylthiophene-2-boronic acid pinacol ester, respectively. A subsequent Knoevenagel-condensation with malononitrile afforded the dicyanomethylene-derivatives 2a and 2b in moderate yields. Acceptors 2a and 2b are highly soluble in both halogenated and non-halogenated solvents such as tetrahydrofuran, toluene and xylene. Thermogravimetric analysis indicated good thermal stability of both compounds (Table 1), while no clear thermal transitions were observed by differential scanning calorimetry.
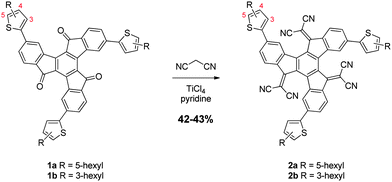 | ||
| Scheme 1 Synthesis of truxenone-based acceptor compounds 2a and 2b. | ||
| Compound | T d (°C)a | λ max (nm) | HOMO (eV) | LUMO (eV) | E g (eV) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Solnb | Filmc | Exp.d | Calc.e | Exp.d | Calc.e | Opt.f | CVd | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| a 5% weight loss by thermogravimetric analysis. b Dichloromethane solution. c Spin-cast from chloroform solution (10 mg ml−1). d Cyclic voltammetry in dichloromethane solution with tetrabutylammonium hexafluorophosphate electrolyte. e Calculated with Gaussian at the B3LYP/6-31G* level. f Determined from the onset of absorption in the solid state. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2a | 338 | 298, 423 | 315, 451, 557, 602 | −5.94 | −6.12 | −4.07 | −4.05 | 1.87 | 1.87 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2b | 340 | 287, 415 | 290, 423 | −6.17 | −6.29 | −4.08 | −4.16 | 2.04 | 2.09 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PCBM | −5.89 | −3.75 | 2.14 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
A computational study of the minimum-energy conformations, as illustrated for compound 2a in Fig. 1, shows that the introduction of the dicyanomethylene moieties forces the truxene core to adapt a bowl-shaped conformation; this finding is in agreement with crystal structures of related compounds.12,13 For 2a, the thienyl group is predicted to be twisted 26° relative to the adjacent phenyl group, whereas the corresponding dihedral angle is 41° for 2b due to the repositioning of the hexyl group. This highlights the fact that the alkylated thienyl moiety can be used effectively to control the degree of conjugation as well as the tendency to crystallise through control of the torsional twist. For both acceptor molecules, the highest occupied molecular orbital (HOMO, Fig. 1 left) is predominantly located on the electron-rich periphery, while the lowest unoccupied molecular orbital (LUMO, Fig. 1 right) is distributed over the electron-poor core of the system.
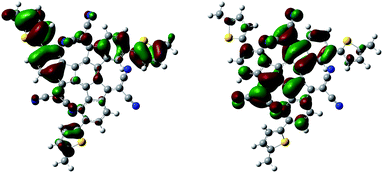 | ||
| Fig. 1 Minimum-energy conformation of 2b (with a methyl group instead of a hexyl group) calculated with Gaussian at the B3LYP/6-31G* level visualising the HOMO (left) and LUMO (right). | ||
Optical absorption spectroscopy supports the increased torsional twist predicted for 2b relative to 2a as illustrated in Fig. 2. While compound 2a shows an absorption maximum at 451 nm in the solid state with additional low-energy peaks at 557 and 602 nm, compound 2b is significantly blue-shifted with a λmax at 423 nm. We moreover find that 2a displays a significantly larger red-shift than 2b upon transition from solution to the solid state as evident from the data in Table 1. The optical band gap is 1.87 eV for 2a and 2.04 eV for 2b, which again is in agreement with the reduced conjugation caused by the twisted thiophene.
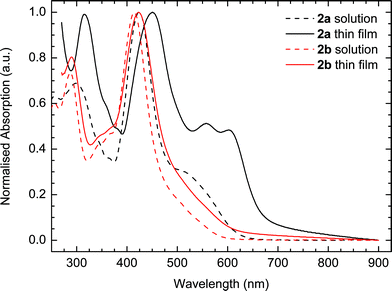 | ||
| Fig. 2 Normalised UV-vis absorption spectra of 2a and 2b in dichloromethane solution and as spin-cast films from chloroform. | ||
In order to assess the frontier energy levels of compounds 2a and 2b, electrochemical characterisation was carried out by means of cyclic voltammetry (CV). The HOMO and LUMO energy levels were estimated from the onsets of oxidation and reduction, respectively (Table 1). The HOMO levels were found to be −5.94 eV for 2a and −6.17 eV for 2b, which is in good agreement with the predicted values from the computational study. The electron-rich 5-hexyl-2-thienyl substituent in compound 2a is less twisted than the 3-hexyl-2-thienyl substituent (2b) and will therefore provide a higher degree of π-conjugation and thus a higher-lying HOMO level. The LUMO levels, on the other hand, are found to be almost identical for the two compounds; −4.07 eV for 2a and −4.08 eV for 2b. These results corroborate nicely the assumption from the computational study that the HOMO level is mainly governed by the peripheral substituents, while the LUMO level is largely controlled by the electron-poor dicyanomethylene-functionalised truxenone core, which is identical for the two compounds. For comparison under identical conditions, we also tested PCBM by CV and found a HOMO level of −5.89 eV and a LUMO level of −3.75 eV indicating that the two truxenone-based acceptors presented herein have slightly larger electron affinities than PCBM.
Acceptor compounds 2a and 2b were tested in inverted bilayer solar cells fabricated with a vacuum-deposited donor material in conjunction with our solution-processed truxenone-based acceptor materials. Devices with inverted architectures were prepared as this allowed for solution-deposition of the truxenone acceptors prior to vacuum-deposition of the donor materials. The bilayer device configuration was chosen over a bulk-heterojunction device architecture in order to eliminate any potential morphology-related issues that could obscure evaluation of the OPV properties of this new class of acceptor compounds. As donor material in the bilayer devices, we investigated both a wide band gap donor, SubPc with HOMO/LUMO levels of −5.5/−3.4 eV and a narrower band gap material, zinc phthalocyanine (ZnPc) with HOMO/LUMO levels of −5.2/−3.5 eV.14,15
Prior to device preparation, the topography of the truxenone thin films was characterised by atomic force microscopy (Fig. S10†). Compound 2a forms ordered features (root mean square (rms) roughness = 5.3 nm) in agreement with the large red-shift in absorption upon transition from solution to the solid state indicative of aggregation and/or crystallisation. In contrast, films of 2b are featureless and smooth (rms roughness = 0.6 nm), suggesting an amorphous film.
Photovoltaic parameters of the best devices are listed in Table 2 and corresponding current–voltage characteristics are shown in Fig. S11 and S12.† Bilayer devices comprising SubPc and 2a or 2b achieve short-circuit current densities (JSC) around 2 mA cm−2 which is comparable to the JSC obtained for reference cells with PCBM. Bilayer devices with ZnPc and 2a or 2b have a JSC of 2.4 mA cm−2 and 1.9 mA cm−2, respectively, whereas the PCBM-based device displays a higher JSC of 3.0 mA cm−2. Complementary and red-shifted absorption (Fig. 3) of ZnPc explain the higher JSC-values obtained for the ZnPc cells in comparison to the SubPc cells. External quantum efficiency (EQE) spectra of the devices as shown in Fig. 3 indicate that both donor (SubPc and ZnPc) and acceptor (2a and 2b) compounds contribute to the photocurrent. This confirms that the energy level alignment at the heterojunction permits an efficient separation of the bound geminate pairs generated both on the truxenone acceptor and SubPc or ZnPc layers. JSC-values extracted from the EQE spectra by integration (Table 2) correlate well with the values obtained from the J–V measurements. The open-circuit voltage (VOC) of the SubPc devices with 2a (947 mV) and 2b (923 mV) is approximately 100 mV lower than the value obtained with PCBM; this observation is in agreement with the lower lying LUMO levels of the truxenones reported in Table 1. Owing to a fill factor (FF) of 52%, the 2a/SubPc device reaches a power conversion efficiency (PCE) of 1.0%, outperforming the PCBM reference device, which has a FF of 34% and a PCE of 0.8%. The 2b/SubPc device performs similarly to the PCBM reference device with a FF of 36% and a PCE of 0.7%.
| Acceptor | Donor | V OC (mV) | J SC (mA cm−2) | J SC (EQE) (mA cm−2) | FF(%) | PCE(%) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Device architecture and thicknesses: ITO/ZnO (10 nm)/2a (65–70 nm) or 2b (50 nm) or PCBM (10 nm)/SubPc (30 nm) or ZnPc (50 nm)/MoO3 (5 nm)/Ag (120 nm). | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2a | SubPc | 947 | 1.9 | 1.8 | 52 | 1.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2b | 923 | 2.0 | 1.7 | 36 | 0.7 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PCBM | 1060 | 2.1 | 1.6 | 34 | 0.8 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2a | ZnPc | 448 | 2.4 | 1.8 | 50 | 0.6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2b | 276 | 1.4 | 1.2 | 28 | 0.1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PCBM | 612 | 3.0 | 2.9 | 61 | 1.1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
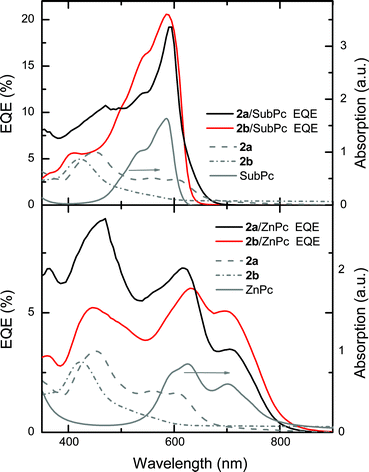 | ||
| Fig. 3 External quantum efficiency (EQE) of 2a or 2b/SubPc (top panel) and 2a or 2b/ZnPc (bottom panel) devices and absorption spectra of the various compounds. | ||
In contrast to the truxenone/SubPc devices, the truxenone/ZnPc bilayer devices display significantly reduced VOC-values in comparison to the PCBM reference device. Although the truxenone-based devices have elevated leakage currents (Fig. S12†), it cannot explain entirely the reduced VOC. Altered growth of the donor molecules on the different acceptor layers might also influence the energy levels of ZnPc and, consequently, reduce the VOC.15 Truxenone derivative 2a affords a VOC of 448 mV, which in conjunction with a FF of 50%, results in a moderate PCE of 0.6%, while a much lower VOC of 276 mV and a lower FF of 28% is responsible for a low PCE-value of 0.1% for 2b. Both truxenone-based acceptors in this case show inferior performance to the PCBM reference device, which has a PCE of 1.1% due to a higher VOC of 612 mV and a FF of 60%. It is notable that, with both donor materials, devices with 2a have higher FFs than devices with 2b. This suggests that 2a, with the nearly coplanar 5-hexyl-2-thienyl substituents, is likely to have a higher charge carrier mobility than 2b, which has the more twisted 3-hexyl-2-thienyl substitution pattern.
In conclusion, we have developed two new truxenone-based acceptor materials (2a and 2b) and shown how chemical modification of the central truxenone core can be used to adjust the LUMO level, while chemical modification of the peripheral substituents can be used to adjust the HOMO level without further affecting the LUMO level. Additionally, the peripheral substituent can very effectively be used to control the degree of torsional twist and hence the degree of intermolecular aggregation.
The two new acceptors were proven to work satisfactorily in bilayer photovoltaic devices with two common donor materials, SubPc and ZnPc. In both cases, acceptor 2a performs significantly better than 2b and with a SubPc donor material compound 2a even outperforms the PCBM acceptor, giving a 25% higher power conversion efficiency. This initial screening clearly suggests that this novel class of acceptors has potential in photovoltaic applications. However, both thin film deposition and growth of the donor materials could be further optimized to reach higher performance. Further device characterisation could also yield insight into the efficiency of the photogeneration process and eventual loss mechanisms limiting the performance of ZnPc/truxenone devices.
This work was in part carried out with financial support from SUPERGEN, EC FP7 Project X10D and EC FP7 Project ONE-P with support from BASF.
Notes and references
- C. L. Chochos and S. A. Choulis, Prog. Polym. Sci., 2011, 36, 1326–1414 CrossRef CAS.
- Z. He, C. Zhong, S. Su, M. Xu, H. Wu and Y. Cao, Nat. Photonics, 2012, 6, 593–597 CrossRef CAS.
- M. C. Scharber, D. Mühlbacher, M. Koppe, P. Denk, C. Waldauf, A. J. Heeger and C. J. Brabec, Adv. Mater., 2006, 18, 789–794 CrossRef CAS.
- J. Kirkpatrick, C. B. Nielsen, W. Zhang, H. Bronstein, R. S. Ashraf, M. Heeney and I. McCulloch, Adv. Energy Mater., 2012, 2, 260–265 CrossRef CAS.
- C. B. Nielsen, R. S. Ashraf, B. C. Schroeder, P. D'Angelo, S. E. Watkins, K. Song, T. D. Anthopoulos and I. McCulloch, Chem. Commun., 2012, 48, 5832–5834 RSC.
- J. E. Anthony, Chem. Mater., 2011, 23, 583–590 CrossRef CAS.
- S. Shoaee, T. M. Clarke, C. Huang, S. Barlow, S. R. Marder, M. Heeney, I. McCulloch and J. R. Durrant, J. Am. Chem. Soc., 2010, 132, 12919–12926 CrossRef CAS.
- J. T. Bloking, X. Han, A. T. Higgs, J. P. Kastrop, L. Pandey, J. E. Norton, C. Risko, C. E. Chen, J.-L. Brédas, M. D. McGehee and A. Sellinger, Chem. Mater., 2011, 23, 5484–5490 CrossRef CAS.
- G. Ren, E. Ahmed and S. A. Jenekhe, Adv. Energy Mater., 2011, 1, 946–953 CrossRef CAS.
- Y. Zhou, L. Ding, K. Shi, Y.-Z. Dai, N. Ai, J. Wang and J. Pei, Adv. Mater., 2012, 24, 957–961 CrossRef CAS.
- C. Lambert, G. Nöll, E. Schmälzlin, K. Meerholz and C. Bräuchle, Chem.–Eur. J., 1998, 4, 2129–2135 CrossRef CAS.
- K. Jacob, J. Y. Becker, A. Ellern and V. Khodorkovsky, Tetrahedron Lett., 1999, 40, 8625–8628 CrossRef CAS.
- E. M. Pérez, M. Sierra, L. Sánchez, M. R. Torres, R. Viruela, P. M. Viruela, E. Ortí and N. Martín, Angew. Chem., Int. Ed., 2007, 46, 1847–1851 CrossRef.
- D. Cheyns, B. P. Rand and P. Heremans, Appl. Phys. Lett., 2010, 97, 033301 CrossRef.
- B. P. Rand, D. Cheyns, K. Vasseur, N. C. Giebink, S. Mothy, Y. Yi, V. Coropceanu, D. Beljonne, J. Cornil, J.-L. Brédas and J. Genoe, Adv. Funct. Mater., 2012, 22, 2987–2995 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, NMR spectra, TGA and DSC traces, cyclic voltammograms, AFM micrographs, J–V curves and photoluminescence spectra. See DOI: 10.1039/c2ta00548d |
| This journal is © The Royal Society of Chemistry 2013 |
