Aluminum-stabilized NASICON-structured Li3V2(PO4)3
Yuhao
Lu
a,
Long
Wang
a,
Jie
Song
a,
Dawei
Zhang
ab,
Maowen
Xu
a and
John B.
Goodenough
*a
aTexas Materials Institute, The University of Texas at Austin, Austin, Texas 78712, USA. E-mail: jgoodenough@mail.utexas.edu; Fax: +1-512-471-7681; Tel: +1-512-471-1646
bSchool of chemical Engineering, Hefei University of Technology, Hefei 230009, China
First published on 21st September 2012
Abstract
The redox couple, V4+/V3+, exhibits a potential of 3.76 V in NASICON-structured Li3Al0.1V1.9(PO4)3, which is suitable for a cathode material of a lithium-ion battery. The rhombohedral NASICON framework provides a large interstitial space for fast lithium transport, but the structure has to be prepared by the lithium ion-exchange method from NASICON-structured Na3V2(PO4)3. We used a LiNO3 aqueous solution to treat Na3V2(PO4)3 for two weeks; a mixture of rhombohedral and monoclinic Li3V2(PO4)3 was obtained in the final product. Therefore, we introduced aluminum into the NASICON framework. Results from phase analysis and electrochemical evaluation have concluded that the aluminum stabilizes the NASICON framework of V2(PO4)3 in the lithium ion-exchange process. The aluminum-stabilized Li3Al0.1V1.9(PO4)3 showed a reversible capacity of 70 mA h g−1 compared to 15 mA h g−1 for the non-aluminum-doped n-LVP at 5C rate.
Introduction
Transition-metal phosphates offer framework structures that can be used as cathode hosts in Li-ion rechargeable batteries (LIBs).1–3 The host M2(PO4)3 framework structure consists of PO43− anions sharing corners with oxygen octahedra containing transition-metal M atoms, forming “lantern” units. The V2(PO4)3, “lantern” units may be oriented parallel to give rhombohedral symmetry or at an angle to one another to give monoclinic symmetry as is shown in Fig. 1. The interstitial space of these hosts can contain up to four Na+ or five Li+ guest ions, and the mobility of the guest ions depends not only on their concentration, but also on the symmetry of the host. Guest-ion mobility is higher where the lantern units are parallel as occurs in the NASICON framework of Na1+xZr2(P1−xSixO4)3.4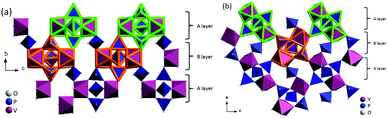 | ||
| Fig. 1 (a) Rhombohedral and (b) monoclinic structures of V2(PO4)3. The highlighted parts are the lantern structures consisting of 2 VO6 and 3 PO4. Repeatable A and B layers connected by PO4 tetrahedra along b-axis constitute the frameworks of V2(PO4)3. | ||
The concentration of Li+ ions in this framework has little influence on the energy of the M(m+1)+/Mm+ redox energies; however, these energies are strongly influenced by the countercation in the tetrahedral polyanion (XO4). The stronger the bonds within the polyanion (XO4), the weaker the M–O bond on the opposite side of the oxygen, which lowers the energy of the M(m+1)+/Mm+ antibonding d-electron configuration of the couple to raise its voltage with respect to lithium. For example, this inductive effect raises by 0.8 eV the voltages with the (SO4)2− anion relative to those with the (PO4)3− anion.1 The effect of the (PO4)3− anion versus a simple O2− anion is illustrated by the Ti4+/Ti3+ couple, which is at 1.5 V versus Li+/Li in the spinel Li4+xTi5O12,5 but is at 2.5 V in Li1+xTi2(PO4)3.6 Moreover, the strong P–O bond in the (PO4)3− anion lowers the energy of the top of O–2p bands, so the cathode has a higher intrinsic voltage limit determined by pinning of the Fermi energy at the top of the O–2p bands.7
The organic liquid-carbonate electrolytes generally used in Li-ion rechargeable batteries decompose above 5.0 V versus Li+/Li, but reaction with an electrode can begin above 4.0 V. Kinetic stability with an oxide cathode may extend the practical highest occupied molecular orbitals (HOMO) to about 4.3 V before a passivating solid electrolyte interface (SEI) layer is needed. The V4+/V3+ couple of rhombohedral Li3−xV2(PO4)3 (n-LVP) is at ca. 3.8 V versus Li+/Li, which places the energy of the couple above the HOMO of the electrolyte. Therefore, safe, fast cycling of this cathode material can be anticipated. However, although Na+ guests stabilize the rhombohedral R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c structure of the V2(PO4)3 framework (n-VP),8 the smaller Li+ ions cannot support the larger interstitial free space and therefore stabilize the monoclinic P21/n V2(PO4)3 framework structure.
c structure of the V2(PO4)3 framework (n-VP),8 the smaller Li+ ions cannot support the larger interstitial free space and therefore stabilize the monoclinic P21/n V2(PO4)3 framework structure.
Monoclinic α-LVP is more stable than rhombohedral n-LVP at room temperature if synthesized by solid state reaction. With increasing temperature, the monoclinic LVP changes from the monoclinic α-phase (room temperature) to an orthorhombic γ-phase (180 °C) via a monoclinic β-phase (120 °C).9 The α-phase LVP (α-LVP) in LVP/lithium batteries demonstrates two main plateaus, one around 3.6 and the other at 4.1 V on charging and 4.0 and 3.5 V on discharging.10–12 Disordering of lithium in the LVP framework at high temperature changes the monoclinic LVP to orthorhombic LVP. The γ-phase stable at high temperate can be stabilized to room temperature by reducing the content of the lithium in LVP.9,13 The γ-phase LVP (γ-LVP) demonstrates a very similar two-plateau voltage-composition plot to that of the α-LVP; but it has a higher mobility of lithium, which improves the C-rate performance of LVP/lithium batteries. In order to increase Li-ion mobility further, Na+-ions were doped into the monoclinic LVP.14,15 It was believed that Na+-ions weakened the Li–O bond to enhance the mobility. Other metal-ions were also doped into the monoclinic LVP to improve its performance. For instance, Al3+-doped LVP demonstrated better cycling stability.16,17 However, it would be better to have a single rather than two voltage plateaus.
The α and γ-LVP have smaller free volumes of the interstitial space than that of the rhombohedral n-LVP. A larger free volume of the interstitial space allows for a faster ionic movement to give a higher specific power. Moreover, unlike α- and γ-LVP, n-LVP has a simple equilibrium V4+/V3+ potential at ∼3.8 V vs. Li/Li+ corresponding to a single two-phase transition.18,19 The single V4+/V3+ plateau of n-LVP simplifies the external circuitry needed for application to electronic devices. Ion exchange in LiNO3 aqueous solution was employed to prepare n-LVP from rhombohedral Na3V2(PO4)3 (n-NVP). However, we observed that a part of the structure changed to the monoclinic phase, which deteriorated significantly the C-rate performance of LVP as the cathode of a Li-ion battery. Therefore, we introduced the smaller Al3+ into the framework of V2(PO4)3 (n-VP) to stabilize the rhombohedral n-LVP phase. Similar results, not reported, were attained with the smaller Cr3+ ion as dopant.
Experimental
The n-LVP was obtained via two steps. First, the n-NVP was synthesized by solid state reaction. V2O5, Na2CO3 (3 at.% in excess) and NH4H2PO4 were stoichiometrically dissolved into distilled water with strong stirring at 80 °C for 2 hours. Citric acid, which forms a gel and provides a carbon coat, was added into the solution with the molar ratio of cations![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) citric acid = 1
citric acid = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. After stirring for 8 hours, the temperature was set at 120 °C to dry the solution. The dried mixture was heated at 350 °C for 6 hours and then ground in an agate mortar for one hour. The reactants were heated at 850 °C for 12 hours under an argon atmosphere containing 10% H2. The carbon-coated n-NVP was washed three times to remove the residual Na2CO3. Second, the n-LVP was obtained in an aqueous solution of LiNO3 with a strong mechanical stirring at room temperature. The amount of LiNO3 was four times that of n-NVP in moles. The lithium ion-exchange step was repeated three times; each step lasted four and a half days. Finally, the n-LVP was washed by distilled-water three times to remove excessive LiNO3. The synthesis of rhombohedral Na3Al0.1V1.9(PO4)3 (n-NAVP) and Li3Al0.1V1.9(PO4)3 (n-LAVP) followed the same procedure except for the addition of a stoichiometric amount of AlNH4(SO4)2·12H2O in the initial solution.
2. After stirring for 8 hours, the temperature was set at 120 °C to dry the solution. The dried mixture was heated at 350 °C for 6 hours and then ground in an agate mortar for one hour. The reactants were heated at 850 °C for 12 hours under an argon atmosphere containing 10% H2. The carbon-coated n-NVP was washed three times to remove the residual Na2CO3. Second, the n-LVP was obtained in an aqueous solution of LiNO3 with a strong mechanical stirring at room temperature. The amount of LiNO3 was four times that of n-NVP in moles. The lithium ion-exchange step was repeated three times; each step lasted four and a half days. Finally, the n-LVP was washed by distilled-water three times to remove excessive LiNO3. The synthesis of rhombohedral Na3Al0.1V1.9(PO4)3 (n-NAVP) and Li3Al0.1V1.9(PO4)3 (n-LAVP) followed the same procedure except for the addition of a stoichiometric amount of AlNH4(SO4)2·12H2O in the initial solution.
Powder X-ray diffraction (XRD) patterns of the samples were obtained with a Philips X-ray diffractometer equipped with Cu Kα radiation (λ = 1.5418 Å). The angular resolution in 2θ scans was 0.02° over a 2θ range of 10–80°. The content of carbon in the samples was obtained by thermogravimetric analysis (TGA) (Mettler-Toledo TGA/DSC 1). Selected-area diffraction (SAD) patterns were obtained with a transmission electron microscope (TEM) (JEOL 2010). X-ray photoelectron spectroscopy (XPS) data were acquired with a Kratos AXIS 165 Multitechnique Electron Spectrometer (Manchester, UK).
A standard CR2032 coin cell was adopted to evaluate the electrochemical behavior of samples. The coin cell included a sandwich structure of the cathode, the electrolyte with a Celgard® polypropylene separator, and a piece of lithium metal as anode. The cathode consisted of 75 wt% active material, 5 wt% polytetrafluoro-ethylene (PTFE) as binder, and 20 wt% acetylene black as conductor. The cathode was rolled into a thin sheet and punched into a circular disk. The typical electrode mass was 3 ± 0.5 mg. The electrolyte used for testing was 1 M LiPF6 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 EC/DEC (v/v). All cells were assembled in an argon-filled glove box. The cells were aged for 5 h before charge/discharge to ensure full absorption of the electrolyte into the electrodes.
1 EC/DEC (v/v). All cells were assembled in an argon-filled glove box. The cells were aged for 5 h before charge/discharge to ensure full absorption of the electrolyte into the electrodes.
Results and discussion
The rhombohedral n-NVP structure provides two interstitial sites, 6b and 18e in space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c, for sodium that are defined as M1 (one site per formula unit) and M2 (three sites per formula unit), respectively. A total of four sodium ions can be inserted into the framework of n-VP. The lithium ions can exchange for sodium ions and re-order in the n-VP in which a total of five lithium ions can be inserted. Fig. 2(a) shows the XRD patterns of n-NVP and the Li-exchanged NVP. Solid-state synthesis gave a high-purity n-NVP. Substitution of Na+ by Li+ shrank the volume, but gave a two-phase (n and α) product. According to Table 1, the lattice parameters of n-VP decrease 5% along the a and b directions, and 0.5% along the c direction in the lithium ion-exchange process. The monoclinic lattice parameters were refined according to the symmetric group P21/n12 and are also listed in Table 1.
c, for sodium that are defined as M1 (one site per formula unit) and M2 (three sites per formula unit), respectively. A total of four sodium ions can be inserted into the framework of n-VP. The lithium ions can exchange for sodium ions and re-order in the n-VP in which a total of five lithium ions can be inserted. Fig. 2(a) shows the XRD patterns of n-NVP and the Li-exchanged NVP. Solid-state synthesis gave a high-purity n-NVP. Substitution of Na+ by Li+ shrank the volume, but gave a two-phase (n and α) product. According to Table 1, the lattice parameters of n-VP decrease 5% along the a and b directions, and 0.5% along the c direction in the lithium ion-exchange process. The monoclinic lattice parameters were refined according to the symmetric group P21/n12 and are also listed in Table 1.
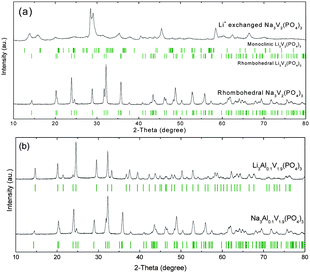 | ||
| Fig. 2 XRD patterns of (a) rhombohedral Na3V2(PO4)3 and Li+-exchanged Na3V2(PO4)3; and (b) Al-stabilized rhombohedral Na3Al0.1V1.9(PO4)3 and Li+-exchanged Na3Al0.1V1.9(PO4)3. | ||
| Compounds | Symmetric group | Lattice parameters |
|---|---|---|
| n-NVP |
R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c c |
a = b = 8.712 Å and c = 21.807 Å |
| n-NAVP |
R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c c |
a = b = 8.708 Å and c = 21.771 Å |
| n-LVP |
R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c c |
a = b = 8.297 Å and c = 21.708 Å |
| P21/n | a = 8.529 Å, b = 8.602 Å, and c = 11.969 Å, α = γ = 90° and β = 90.848° | |
| n-LAVP |
R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c c |
a = b = 8.317 Å and c = 22.452 Å |
The addition of 5 at.% aluminum for vanadium stabilized the rhombohedral structure of V2(PO4)3. Fig. 2(b) shows the XRD patterns of Al-stabilized n-NVP, namely n-NAVP, and the Li ion-exchanged n-NAVP, namely n-LAVP. After lithium-ion exchange over two weeks, all the Al0.1V1.9(PO4)3 framework kept the rhombohedral structure. Comparing with the n-NAVP, the a and b parameters of n-LAVP decreased, but c increased, see Table 1.
The XRD pattern of n-NVP shows that it is consistent with the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c symmetric group. However, the selected area diffraction (SAD) pattern in Fig. 3(a) shows zones of different space groups. These different zones come from structural deformations associated with sodium-ion dis/ordering.20 Doping with aluminum simplified the structure existing in n-NVP as shown in Fig. 3(c). The SAD pattern can be readily indexed with the R
c symmetric group. However, the selected area diffraction (SAD) pattern in Fig. 3(a) shows zones of different space groups. These different zones come from structural deformations associated with sodium-ion dis/ordering.20 Doping with aluminum simplified the structure existing in n-NVP as shown in Fig. 3(c). The SAD pattern can be readily indexed with the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c reflection condition –h + k + l = 3n. Fig. 3(b) shows the SAD pattern of n-LVP from which two sets of reflections could be observed. The two sets of reflections have been identified by the powder XRD patterns to be the rhombohedral and monoclinic phases. In the space group of P21/n, the reflection conditions include k = 2n and l = 2n. The smaller lithium ions reduced the deformation of the n-V2(PO4)3 framework, and only P21/n and R
c reflection condition –h + k + l = 3n. Fig. 3(b) shows the SAD pattern of n-LVP from which two sets of reflections could be observed. The two sets of reflections have been identified by the powder XRD patterns to be the rhombohedral and monoclinic phases. In the space group of P21/n, the reflection conditions include k = 2n and l = 2n. The smaller lithium ions reduced the deformation of the n-V2(PO4)3 framework, and only P21/n and R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c showed on the SAD pattern. Aluminum stabilized the structure of n-VP in the lithium ion-exchange process. The substitution of Li+ for Na+ did not change the main structure of n-NAVP, but it significantly reduced the deformation caused by sodium ordering in the n-NAVP. Fig. 3(d) demonstrates the reflection of n-LAVP in which only one phase could be identified.
c showed on the SAD pattern. Aluminum stabilized the structure of n-VP in the lithium ion-exchange process. The substitution of Li+ for Na+ did not change the main structure of n-NAVP, but it significantly reduced the deformation caused by sodium ordering in the n-NAVP. Fig. 3(d) demonstrates the reflection of n-LAVP in which only one phase could be identified.
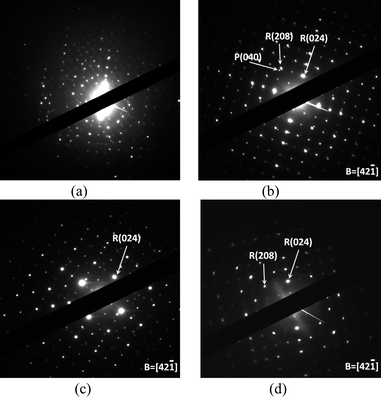 | ||
| Fig. 3 SAD patterns of samples. (a) n-NVP, (b) n-LVP, (c) n-NAVP and (d) n-LAVP. R = rhombohedral phase, P = monoclinic phase, and B = beam direction. | ||
In addition to changing the lattice parameters and phase stability, the addition of Al3+ in n-NAVP changes its chemical environment, which affects the binding energy of different framework elements in NVP. Fig. 4 shows high-resolution XPS spectra of Na(1s), V(2p), P(2p), and O(1s) in n-NVP and n-NAVP. The positions of the main peaks were recorded as the binding energy of elements in our study. The binding energies of Na(1s) in n-NVP and n-NAVP are located at the same position, which implies that the addition of aluminum did not change the chemical bonding of Na. The binding energies of V(2p), P(2p) and O(1s) in n-NVAP were smaller than those in n-NVP. The Al3+ ions reduce the binding energies of their neighboring framework atoms.
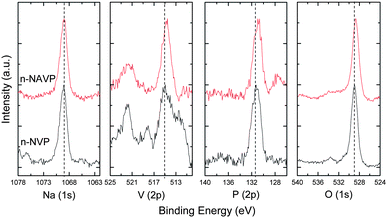 | ||
| Fig. 4 High resolution XPS spectra of Na(1s), V(2p), P(2p) and O(1s) in n-NVP and n-NAVP. | ||
The morphologies of samples obtained by scanning electron microscopy (SEM) are shown in Fig. 5. The n-NVP and n-NAVP samples have similar particle features. After lithium-ion exchange, the particles became fluffy and smaller. The ion-exchange process significantly influenced the morphologies of the particles. The n-LVP consists of small slabs/needles and the n-LAVP consists of small particles. The n-NVP and n-NAVP structures were not stable in water; part of the n-NVP and n-NAVP decomposed and dissolved into the LiNO3 aqueous solution.21,22 The decomposition resulted in the changes of morphologies during ion exchange. Carbon is insoluble in water, so it was chosen as the reference to prove the decomposition and dissolution of n-NVP and n-NAVP in water. The carbon contents were identified by TGA. 2.04 wt% of carbon existed in n-NVP and 2.24 wt% in n-NAVP. After Li+ exchange, n-LVP contains 9.16 wt% of carbon and n-LAVP has 6.88 wt% of carbon. The increase in carbon content indicates the loss of n-NVP and n-NAVP in the aqueous solution.
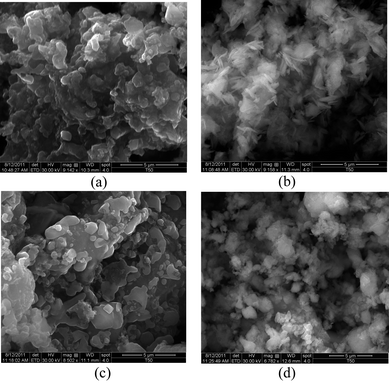 | ||
| Fig. 5 Morphologies of (a) n-NVP, (b) Li-ionic exchanged n-NVP, (c) n-NAVP and (d) Li-ionic exchanged n-NVP. | ||
Fig. 6 compares the electrochemical behavior of n-LVP and n-LAVP. In their voltage range, only the V4+/V3+ couple is accessed. In our study, the charge/discharge voltage was set from 3.2 V to 4.2 V. The charge/discharge profiles of n-LVP in Fig. 6(a) reflect the solid-solution behavior in the lithiation/delithiation process. The chronoamperogram shows that the charge/discharge process includes two two-phase changes. One appeared at 3.79 V in the charge process and 3.73 V in the discharge process, the other at 3.97/3.88 V in the charge/discharge process. The two two-phase changes resulted from the coexistence of the rhombohedral and monoclinic phases. The single plateau in the charge/discharge curves of n-LAVP in Fig. 6(b) indicates that there is only one two-phase change on charge and discharge in the rhombohedral phase. Its chronoamperogram identified the phase-change voltages of 3.79 V and 3.73 V in the processes of charge and discharge, respectively. The addition of aluminum helped to stabilize the rhombohedral V2(PO4)3 framework especially in the aqueous ion-exchange process. Fig. 6(c) displays the discharge capacity of n-LVP and n-LAVP at different discharge currents. Although the Al3+ reduces the capacity of n-LAVP relative to n-LVP in the first three cycles, the n-LAVP exhibited a better C-rate performance than n-LVP. The average capacity of n-LAVP at 5C current is 67 mA h g−1, but that of n-LVP is 14 mA h g−1. The significant difference in C-rate performance is consistent with the larger free volumes of the rhombohedral phase as well as the single-phase character of n-LAVP. Moreover, the electrochemical performance of n-LVP was not stable in the first 30 cycles. For example, the capacity of n-LVP decreases significantly from 66 mA h g−1 at the 11th cycle to 54 mA h g−1 at the 30th cycle at a 0.5C current. The corresponding capacity values for n-LAVP are 98 mA h g−1 and 94 mA h g−1, respectively. The electrochemical results provide clear evidence that the introduction of aluminum to the NASICON-structured V2(PO4)3 stabilizes its rhombohedral phase to provide a better lithium transport and cycle life.
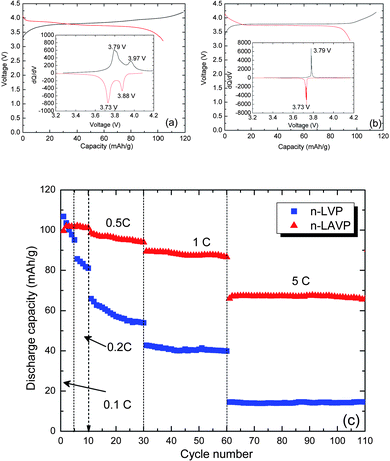 | ||
| Fig. 6 Charge and discharge profiles recorded at the second cycle of (a) n-LVP and (b) n-LAVP. Insets show chronoamperograms of n-LVP and n-LAVP, respectively. (c) C-rate performance of n-LVP and n-LAVP. | ||
Although the stabilized-NASICON framework significantly increases Li+ transport in Li3V2(PO4)3, the performance of the electrode material may be further improved by increasing its electronic conductivity and deceasing the Li+ transport path in it. However, samples in this study have been coated by carbon in the synthesis process, but the carbon film was destroyed in the ion-exchange step. Decreasing the samples' dimensions to nanometers would reduce the path for Li+ transport and improve the C-rate of the NASICON-structured Li3V2(PO4)3.
Conclusions
Room-temperature Na+ to Li+ exchange in aqueous LiNO3 transforms rhombohedral Na3V2(PO4)3 to a mixture of rhombohedral and monoclinic Li3V2(PO4)3. However, a similar exchange with the Al0.1V1.9(PO4)3 formwork retains the rhombohedral structure to give a single two-phase voltage plateau of 3.76 ± 0.03 V for the V4+/V3+ redox couple and a capacity at 5C rate that is about five times greater than that of Li3−xV2(PO4)3. The smaller Al3+ ion was chosen to substitute for V3+ in order to reduce the free volume of the framework to give a better match to the smaller size of the Li+versus Na+ ions.Acknowledgements
This work was supported by the Assistant Secretary for Energy Efficiency and Renewable Energy, Office of Vehicle Technologies, U.S. Department of Energy, under Contract DE-AC02-05CH11231 through the Batteries for Advanced Transportation Technologies (BATT) Program Subcontract 6805919.Notes and references
- A. K. Padhi, K. S. Nanjundaswamy, C. Masquelier and J. B. Goodenough, J. Electrochem. Soc., 1997, 144, 2581 CrossRef CAS.
- A. K. Padhi, K. S. Nanjundaswamy, C. Masquelier and J. B. Goodenough, J. Electrochem. Soc., 1997, 144, 1188 CrossRef CAS.
- J. B. Goodenough and Y. Kim, Chem. Mater., 2010, 22, 587 CrossRef CAS.
- J. B. Goodenough, H. Y.-P. Hong and J. A. Kafalas, Mater. Res. Bull., 1976, 11, 203 CrossRef CAS.
- E. Ferg, R. J. Gummow, A. de Kock and M. M. Thackeray, J. Electrochem. Soc., 1994, 141, L147 CrossRef CAS.
- J. B. Goodenough in Advances in Li-ion Batteries, W. van Schalkwijk and B. Scrosati, ed. Kluwer Academic/Plenum Publishers, New York, 2002, ch. 4 Search PubMed.
- A. Manthiram and J. B. Goodenough, J. Power Sources, 1989, 26, 403 CrossRef CAS.
- J. Gopalakrishnan and K. Rangan, Chem. Mater., 1992, 4, 745 CrossRef CAS.
- M. Sato, H. Ohkawa, K. Yoshida, M. Saito, K. Uematsu and K. Toda, Solid State Ionics, 2000, 135, 137 CrossRef CAS.
- H. Huang, S.-C. Yin, T. Kerr, N. Taylor and L. F. Nazar, Adv. Mater., 2002, 14, 1525 CrossRef CAS.
- S.-C. Yin, H. Grondey, P. Strobel, H. Huang and L. F. Nazar, J. Am. Chem. Soc., 2003, 125, 326 CrossRef CAS.
- S.-C. Yin, H. Grondey, P. Stronbel, M. Anne and L. F. Nazar, J. Am. Chem. Soc., 2003, 125, 10402 CrossRef CAS.
- S.-C. Yin, P. S. Strobel, H. Grondey and L. F. Nazar, Chem. Mater., 2004, 16, 1456 CrossRef CAS.
- Q. Kuang, Y. Zhao and Z. Liang, J. Power Sources, 2011, 196, 10169 CrossRef CAS.
- J. Yan, W. Yuan, H. Xie, Z.-Y. Tang, F.-J. Liu, W.-F. Mao, Q. Xu and X. H. Zhang, J. Solid State Electrochem., 2012, 16, 3201 CrossRef CAS.
- D. Ai, K. Liu, Z. Lu, M. Zou, D. Zeng and J. Ma, Electrochim. Acta, 2011, 56, 2823 CrossRef CAS.
- A. R. Cho, J. N. Son, V. Aravindan, H. Kim, K. S. Kang, W. S. Yoon, W. S. Kim and Y. S. Lee, J. Mater. Chem., 2012, 22, 6556 RSC.
- J. Gaubicher, C. Wurm, G. Goward, C. Masquelier and L. Nazar, Chem. Mater., 2000, 12, 3240 CrossRef CAS.
- B. L. Cushing and J. B. Goodenough, J. Solid State Chem., 2001, 162, 176 CrossRef CAS.
- H. Kabbour, D. Coillot, M. Colmont, C. Masquelier and O. Mentré, J. Am. Chem. Soc., 2011, 133, 11900 CrossRef CAS.
- P. G. Komorowski, S. A. Argyropoulos, R. G. V. Hancock, J. Gulens, P. Taylor, J. D. Canaday, A. K. Kuriakose, T. A. Wheat and A. Ahmad, Solid State Ionics, 1991, 48, 295 CrossRef CAS.
- R. O. Fuentes, F. Figueiredo, F. M. B. Marques and J. I. Franco, Solid State Ionics, 2001, 139, 309 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2013 |
