Development of a surface to enhance the effectiveness of fibroblast growth factor 2 (FGF-2)†
David E.
Robinson
*,
Louise E.
Smith
,
David A.
Steele
,
Robert D.
Short
and
Jason D.
Whittle
*
Mawson Institute, University of South Australia, Mawson Lakes, Adelaide, South Australia 5095, Australia. E-mail: david.robinson3@unisa.edu.au; jason.whittle@unisa.edu.au; Tel: +61 8 8302 3316
First published on 31st January 2014
Abstract
Growth factors (GFs) play an important role in biological processes such as cell proliferation, differentiation and angiogenesis. GFs are known to bind to glycosaminoglycans (GAGs) in the extracellular matrix, aiding projection from degradation and pooling the GFs for quick response to biological stimuli in vivo. GFs are typically expensive and have a relatively short half-life in culture media, requiring regular replenishment. Here the cooperative binding of GF to a plasma polymerised surface decorated with heparin, and the subsequent culture of primary human dermal fibroblasts (HDFs) is investigated. A simple one-step technique suitable for coating a wide range of different substrates was utilised. Substrates such as culture-ware, scaffolds, bandages and devices for implantation could be coated. The modified surface was compared to standard culture techniques of addition of GF to the media. Results demonstrate that surface bound heparin and FGF-2 have a greater effect on cell proliferation especially at reduced serum concentrations. With performance equivalent to supplementing the media achieved at as little as 1% total FGF-2 added. The protective cooperative effect of FGF-2-GAG bound to modified surface at the interface could lead to reduced costs by reduction of FGF-2 required. Furthermore, for applications such as chronic non-healing wounds, bandages can be produced modified by plasma and decorated with GAGs that could utilise and protect important GFs. This would effectively re-introduce important biomolecules which are protected by GAG binding into a harsh environment.
Introduction
Growth factors (GFs) have a wide range of biological functions which affect cell proliferation, migration, angiogenesis, differentiation and apoptosis. GFs are secreted by cells and function by binding to cell surface receptors.1,2 GFs are major regulators of cell growth both in vitro and in vivo. Often multiple growth factors are incorporated into culture media for in vitro cell culture. Growth factors become depleted and degraded more quickly than other media components and therefore require frequent replenishment.3 GFs play an important role in areas such as wound healing, which is a complex multi-cellular process that involves fibroblasts, keratinocytes and endothelial cells.4For example, the wound healing process is regulated by a number of GFs, cytokines and chemokines such as fibroblast growth factor (FGF-2), vascular endothelial growth factor (VEGF), keratinocyte growth factor (KGF) and epidermal growth factor (EGF).5,6 Interactions of GFs with cell surface receptors promote cell infiltration, migration, proliferation and differentiation and culminate in an inflammatory response, formation of new tissue and healing of the wound.7–9 Some wounds (termed chronic wounds) are arrested in the early phases of healing and are unable to heal completely.10 Such wounds are extremely costly to treat, and have a profound effect on patient quality of life, often for many years.11 Non-healing wounds may require grafts in order to promote the healing process. Dermal sheets of cells can be produced by the use of autologous cells or pooled donor cells. Often the patient own serum is used in culture. GFs can be added into the media to aid the dermal cell proliferation.12,13
Another area of particular interest is stem cell research, where GAGs such as heparan sulfate and GF modified surfaces have been applied.9,14 It has been shown that GFs affect human embryonic stem cell (hES) differentiation. Schuldiner et al. investigated eight different growth factors including FGF-2 and EGF on hES cells. They showed that different growth factors produced different cell lineages, which were analyzed by polymerase chain reactions (PCR) for cell specific molecular markers. The GF effects fell into 3 main groups; those that produce mesodermal, ectodermal or endodermal cells.15
GAGs such as heparan sulfate (which is a structural mimetic of heparin) are known to bind FGF-2, VEGF and other important GFs.16 GAGs have been shown to enhance GF interactions and protect GFs such as FGF-2 from degradation in the ECM.16,17 They also exhibit cytokine and chemokine binding, which is important in cell migration, inflammation and immune response. For example the GAG dermatan sulfate is the most abundant GAG in the skin and has been shown to bind KGF, increasing keratinocyte production in the wound environment.4 GAGs are also known to bind fibronectin, vitronectin and laminin which are important in aiding cell attachment to scaffolds or other cell devices.13,18
Therefore, a modified surface consisting of GAGs that can bind functional GFs, cytokines, chemokines and other important biomolecules could be used to develop an artificial extracellular matrix (ECM).17 Surfaces could be produced which incorporate different growth factors to induce cell proliferation and differentiation in a spatially controlled fashion. Here a plasma polymer surface and the cooperative effects of FGF-2 bound to the heparin decorated surface on HDFs proliferation are investigated.
Previously it has been shown that plasma polymer films of allylamine (ppAA) deposited onto microtitre plates can be used to non-covalently bind a range of GAGs, via positive charges derived from the primary amine groups present in the allylamine monomer.19 Plasma polymerisation is generally achieved under reduced pressure, with the basic requirements being a vessel containing the vapour of an organic compound at low pressure and some means of coupling energy into the system (typically radio frequency radiation at 13.56 MHz). Unlike conventional polymers, plasma polymers do not contain regular repeat units and there is a degree of fragmentation of the monomer precursor during deposition, which leads to a relative loss of functionality.20–25 The density of functional groups retained can be optimised by altering monomer flow rates and pressures during plasma treatment, surfaces for GAG binding have previously been optimised.19
Modified surfaces have previously been used to screen the GAG-binding profiles and specificities of a number of important biologically relevant proteins.19 Proteins investigated included tumour necrosis factor-stimulated gene-6 (TSG-6), complement factor H, fibrillin-1, versican, osteoprotegerin and tissue inhibitor of metalloproteinase 3, and their relative binding specificities for GAGs has been studied.26
Mahoney et al. showed that heparin was ionically bound to plasma polymerised allylamine surfaces and probed its functionality using TSG-6. This research showed that the GAG was functionally bound and that non-modified multi-well plates gave no signal indicating heparin did not bind.27 It was subsequently shown by Salim et al. using zeta potential measurements that plasma polymerised allylamine surfaces maintain a positive charge across a broad range, from pH 3 to pH 9.28 More recently Meade et al. showed that allylamine surfaces with passively adsorbed heparan sulfate (HS) (a structural mimetic of heparin) labelled with H3 groups, retained 51% HS after 10 days in PBS under cell culture conditions of 5% CO2, 37 °C and 95% humidity.29–31
FGF-2 promotes proliferation of fibroblast cells in the dermis. The effect of binding FGF-2 to a heparin decorated plasma polymer surface on fibroblast proliferation has been compared with the addition of FGF-2 to the culture media. If FGF-2 can be bound and protected on the modified surface the half-life could be extended compared with the addition of GF directly into the media this strategy might increase the effectiveness of primary or stem cell culture.6,7
Modified surfaces may also be transferred to 3D synthetic scaffolds to aid the production of skin models containing both dermis and epidermis. Plasma polymerisation has previously been shown by Barry et al. to penetrate 3D scaffolds.32 The scaffolds used in that work were 3 mm thick, with pore sizes between 300 and 160 microns in size. Scaffolds for the creation of a synthetic skin model would be much thinner (in the range of 400 to 500 microns) and therefore it would be reasonable to expect an even coating throughout. Such scaffolds could be used to investigate non-healing wounds in a lab environment without the need for animal models and avoiding the need to gather skin from patients, which is a feature of current human skin equivalent models.33–38 The ultimate goal of this work is a more complete and more ethical skin model for lab based experiments on non-healing wounds.13,39,40
Experimental
Materials and methods
1000 HDFs in FCM were added to wells containing either: ppAA, ppAA + heparin and FGF-2 (HFB), ppAA and FGF-2 (FB). Extra ppAA wells were also seeded so that heparin + FGF-2 (HFM) and FGF-2 alone (FM) alone could be added as a media supplement after seeding. A TCP plate was also seeded as a control. The cells were left for 30 minutes post seeding to allow attachment before the media was removed and the cells washed 3 times with PBS. 200 μl of DMEM (including antibiotics and amphotericin B) containing a serial dilution of serum concentrations (10%, 5%, 2.5%, 1.25%, 0.625% and 0%) was then added to the wells. Some wells contained heparin and FGF-2 as a supplement to the media for comparison to those where it was pre-bound.
Cells were incubated at 37 °C, 95% humidity and 5% CO2. The culture media was refreshed after 4 days and 8 days with no heparin or FGF2 supplements added to any wells, but with serum concentrations maintained. Cells were counted at 5 fields of view/well for all conditions at 1, 2, 4, 5 and 9 days. The field of view equated to 0.3 mm2 the average of the 5 fields were calculated and the average of 3 replicate experiments were plotted against time to investigate differences on HDF cell proliferation.
In a subsequent experiment, FGF-2 dilutions between 10 ng ml−1 and 0.00001 ng ml−1 (200 μl) were incubated with wells pre-incubated with heparin at 10 μg ml−1 (200 μl) in order to investigate if the effect of reducing the concentration of GF during the initial binding step. This experiment was carried out at 2.5% serum containing media and compared to addition of heparin and FGF-2 to the media in a 1 off (equivalent to the previous experiment) and 2 times addition. The effect of heparin alone was also investigated. Cells were again counted at 5 fields of view/well for all conditions at 1, 2, 4, 5 and 9 days.
Statistical analysis
ANOVA with Tukey multiple comparison test was performed to analyse the statistical difference between various conditions in which HDFs were cultured (mean of n = 3 ± 1 SD) and to determine whether the average cell viability was affected by bio-molecules bound or in the media. The number of cells at each condition was compared with TCP as a control within the same time period at the same serum concentration. The heparin + FGF-2 both bound and in the culture media were also compared at different serum concentrations. GraphPad Prism 6 was used to perform the ANOVA and post multivariant Tukey tests and the statistical difference was marked as: significant *(P < 0.05), **(P < 0.01); very significant ***(P < 0.001) or extremely significant ****(P < 0.0001). (Significant differences were calculated by taking averages from 5 fields of view for each well for each condition investigated in triplicates for each. These experiments were also repeated 3 times).Ethics
All patients gave informed consent for skin to be used for research through a protocol approved by the Ethical Committee at the Queen Elizabeth Hospital, Adelaide and the University of South Australia Human Ethics Committee.Results and discussion
The purpose of this study was to investigate the cooperative interactions between growth factor and adsorbed GAG to a PP film on HDF cell culture, with the objective of developing an artificial extracellular matrix which is capable of protecting GFs in cell culture and enhancing their performance. An enhancement may be either an increased yield of cells for the same amount of GF, or alternatively a reduced amount of growth factor required for achieving the same effect.Plasma polymerisation of allylamine
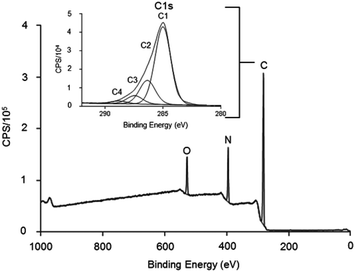
Low pressure cold plasma polymerisation was used to modify TCP plates with allylamine to add amine functionality to the surface and likely a positive charge at physiological pH. The results in Fig. 1 show the XPS survey spectra and C 1s narrow scan for the deposited allylamine surface. It shows the chemical composition of the film is carbon 76.6%, nitrogen 15.7% and oxygen 7.7% which gives an N/C ratio of 0.20. Surfaces with this chemical composition have previously been shown to have functional GAG binding abilities and have been used in ELISA to characterise GAG-binding proteins.27 Inset is a typical C 1s spectra for the allylamine plasma polymer, fitted for binding environments, C1 (C–R) at 285 eV, C2 (C–N, C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) at 286.4 eV, C3 (C
N) at 286.4 eV, C3 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) at 287.5 eV and C4 (RC(
O) at 287.5 eV and C4 (RC(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)NR and C(
O)NR and C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)OH/R) at 288.9 eV.
O)OH/R) at 288.9 eV.
Curve fitting of the C 1s in Fig. 1, clearly showed a strong binding peak (labelled C2) at 286.4 eV that indicated the presence of amines in the deposited films. It is not possible to differentiate between primary, secondary or tertiary amines by XPS alone. However, this spectra is consistent with the many literature reports, which show ppAA films contain positive charges in solution.26–30,36–38
Determining optimal FGF-2 and HDF concentrations
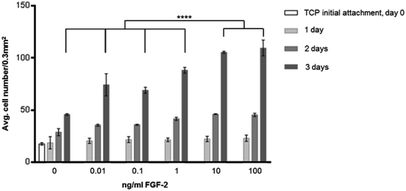
Fig. 2 shows the effect of FGF-2 on proliferation with increasing concentration in the media. It can be seen that HDF cell proliferation is increased with increasing concentration of FGF-2 up to 100 ng ml−1. 10 ng ml−1 and 100 ng ml−1 have the greatest proliferative effect and are significantly different (P ≤ 0.0001) to the other concentrations investigated. This experiment also showed that 5000 cells used in initial attachment were confluent by 4 days, so the later experiments were seeded at 1000 HDFs per well.In Fig. 2, the effect of including FGF-2 at various concentrations in culture media was explored. At all concentrations, there was an enhancement in cell proliferation resulting in significantly higher cell counts at 4 days when compared to cell culture without GF. On the basis of this experiment, a concentration of 10 ng ml−1 was chosen for subsequent investigations. Subsequently, the surface was modified by the addition of a plasma polymer interlayer, followed by the provision of heparin (as a model GAG) following a well-established protocol.26
Effects of binding heparin and FGF-2 to ppAA treated cell culture plates compared with direct addition to the media
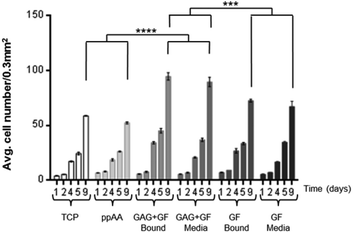
Initial studies were carried out on the effect of binding heparin (GAG) (10 μg ml−1) and GF (10 ng ml−1) together or individually to an allylamine modified surface, or adding them as supplements to the culture media (containing 10% FCS). The culture was used to investigate if the combination of GAG and GF bound to the surface would have the same proliferative effect on HDFs as if added as media supplements. The results of this experiment are shown in Fig. 3. There are several important observations to be made from this data. Firstly, there is no difference in the proliferation of HDFs on the allylamine plasma polymer (ppAA) compared to TCP. Secondly, the addition of growth factor leads to an increase in cell number, but there is no apparent difference between addition of the GF by pre-adsorbing it to the surface, or addition to the media as a supplement. Thirdly, the addition of heparin and GF together gave the highest cell numbers. Similar to the observation for GF alone, there was no significant difference between the pre-adsorption of heparin and GF and their addition as part of the culture media. The significance values on the graph are calculated after 9 days in culture.It was also shown that at 1 and 2 days there is no significant difference seen between the numbers of cells counted per 0.3 mm2 which suggests that the observations at later times points are due to differences in proliferation and not initial attachment.
Since FCS is likely to contain many other proteins and growth factors that could mask the effects of any GF added, a subsequent experiment was carried out at reduced serum concentration. Culture of HDFs was repeated with a serial dilution of 5%, 2.5%, 1.25%, 0.625% and 0% (v/v) serum added to the media. The key results are summarised in Fig. 5. GAG and GF, either bound or in the media, at different serum concentrations are compared after 9 days culture. Statistical differences are calculated at 9 days. The full data which includes all serum conditions and time periods can be found in ESI (Fig. S1A–E†).
The data in Fig. 3 indicated that differences in the observed response is not due to differences in initial attachment, because the numbers of cells counted per well at day 1 are similar across all conditions. From the results obtained with 10% serum (typical for many cell culture applications), three clear observations can be made. Firstly, that the allylamine plasma polymer surface does not increase the proliferation of fibroblasts in comparison to TCP. Secondly, that either adding growth factor to the media (10 ng ml−1), or pre-adsorbing it to the surface from a 10 ng ml−1 solution results in a significant increase in the cell number after 9 days in culture. Thirdly, that the addition of FGF in combination with heparin, either as a media supplement (10 ng ml−1 FGF-2 and 10 μg ml−1 heparin), or pre-adsorbed to the surface, significantly increases the proliferation of HDFs in comparison to FGF-2 alone.
At this serum concentration, there is no measurable difference between the addition of these components to the culture media, or binding them to the surface. This is also true for the addition of GF alone. The increased proliferative effect may be due to the co-receptor binding effect of heparin (a mimetic of heparin sulfate) like GAG molecules for FGF-2 tyrosine-kinase trans-membrane receptor.45
It is also clear that at 5% serum the cells also proliferate well, with the overall cell number being higher than at 10% serum. It is therefore likely that at higher serum concentrations other proteins present in serum interfere with the GF activity thereby reducing the response. By lowering the serum concentration the effect of GF both in solution and bound to the surface can be seen.
Effect of GAG and GF both bound and unbound at reduced serum conditions
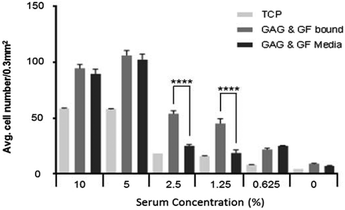
The data for heparin and GF bound to the surface in Fig. 4 at different serum concentration indicates a significant reduction in cell number at all reduced serum conditions, with the exception of 5%, where there is an increase in cell number compared with 10% serum. The same trend is observed when GAG + GF are added into the media (data in Fig. 3). This suggests that other important bio-molecules are present at high enough concentrations at 5% serum concentration to act effectively, but the concentration was low enough not to mask the effect of FGF-2 on cell proliferation. The reduction in cell number is significantly less when GAG and GF are bound to ppAA, at 2.5% and 1.25% serum, compared to their addition to the media.As can be seen in Fig. 4 there is a significant difference (P ≤ 0.0001) between wells which have GAG and GF bound to the surface over those which have biomolecules added to the media at serum concentrations of 2.5% and 1.25%. At higher and lower serum conditions no significant difference is seen between the cell numbers at 9 days. The data also showed that GF alone gave a significant increase in HDF cell proliferation compared to TCP and ppAA control wells at all serum conditions except in the absence of serum (see S1A–E and S2A–B†). However, the proliferation was significantly lower than the combination of both GAG and GF together bound or in the media.
As the serum concentration is reduced further (to 2.5% and 1.25%) it is observed that binding the GF to the surface via a GAG, in a way that mimics the ECM, is advantageous. In the ECM, reservoirs of GF are stored for quick response through binding to heparan sulfate molecules.41–44 This binding has been shown to reduce GF degradation and therefore could increase its half-life and its effectiveness in biological processes,17 in areas such as wound healing. At 2.5% and 1.25% serum the wells with surface-bound bio-molecules appear to significantly outperform those wells where GAG and GF are added to the media. It would be anticipated that GF added to media would have a shorter half-life because there are many other proteins in serum which are capable of binding to GAGs, and therefore available binding sites may quickly become blocked. This may limit the binding and therefore the protective effect of heparin on the GF, which would then be susceptible to degradation by enzymes present in the serum or produced by cells.
There is also a period at the start of the experiment (days 1 and 2) where cells appear not to be growing. This is believed to be the result of damage caused to cell membrane proteins and receptors following trypsinisation of the cells prior to seeding. If GF in the media is depleted during this time then, by the time damaged receptors are replaced on the surface of the cells, the activity of the GF would be lower. This might be overcome if GF was added after 3 days, (when seeded cells have had time to recover) or if different agent was used for cell removal (such as EDTA).
These results may translate directly to a reduction in the cost of cell culture, since supplementing with GF would be required less frequently. The data for 1.25% and 2.5% serum also suggest that there are other bio-molecules in the media which are important for cell viability and this is not a surprising finding.3 This is shown by the reduced numbers of cells/field of view at 9 days compared with 10% and 5% serum shown in Fig. 4. Therefore FGF-2 on its own is not suggested to be a total replacement for serum. Other suitable bio-molecules could either be bound alongside FGF-2 to combat this or added via a defined media.
Effect on HDF proliferation at lower GF concentrations incubation with heparin coated ppAA plates
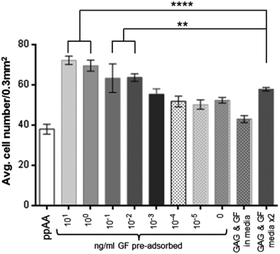
The data in Fig. 5 show the results obtained by incubating lower concentrations of FGF-2 with the heparin bound plates at 2.5% serum. These data indicate that significantly increased cell numbers are still achievable at incubation concentrations of 0.1 to 0.01 ng ml−1 GF. The response is not only an improvement on a single dose of heparin and FGF-2 added to the media but is also superior in the case where fresh heparin and FGF-2 is added to the culture during the media change at day 4.A significant difference (P ≤ 0.0001) is also observed after 9 days in culture between GF bound to heparin from a 0.01 ng ml−1 solution, and the addition of heparin and GF (10 ng ml−1 each) as a media supplement at day 0 and day 4. Below these concentrations there is no significant effect on cell proliferation above that of heparin alone. The binding of heparin to the surface does increase cell proliferation over ppAA and a single dose of heparin and GF added to the media.
The cell numbers in Fig. 5 at day five are similar for both bound and unbound heparin and growth factor but they increase significantly by 9 days for the bound over the unbound. This is not simply due to the change of media at 4 days without further addition of heparin and GF, since, if the media is supplemented again the cell numbers are still not as high as biomolecules are pre-bound. It is possible that there is some release of GF and GAG molecules from the surface over time as shown by Meade.29 However, the results still confirm that pre-adsorbing the biomolecules to the surface is advantageous over media supplementation.
It is clear that heparin alone has some effect of cell proliferation and this can be seen in Fig. 5. Heparin alone gives similar cell numbers even when combined with upto 0.001 ng ml−1 FGF-2. FGF-2 concentrations above 0.001 ng ml−1 resulted in significantly higher cell numbers. It is clear from Fig. 5 that the long term effect of the biomolecules on HDF cultures are improved when pre-adsorbed compared to addition as media supplement. By 9 days heparin and GF pre-bound confer a significant advantage over media supplement or heparin alone (whether bound or in the media).
From the data presented in Fig. 5, it has been shown that the adsorption of GF to a surface-bound GAG (heparin) can be carried out at much lower concentrations than would be used as a media supplement. A comparison with addition of GF to the media indicates that the pre-adsorption step could use around 1/100 to possibly 1/1000 of the amount of FGF-2 without any observable difference in cell proliferation. This observation has the potential to have a significant impact on the costs of cell culture for many different cell types which have therapeutic applications.
The data in this paper supports the other findings in the literature which show that GAGs protect GFs from degradation.17 It is also shown that modified surfaces can be produced that, under standard 10% serum conditions, compare favourably with the conventional approach of adding GF to the media. The 2.5% and 1.25% data also indicates that bound GF is active for longer than when used as a media supplement. This effect is not seen at higher serum concentrations because it is masked by the presence of other bio-molecules in the media. The data also suggests that the amount of FGF-2 added to the media in traditional culture is vastly in excess and much is wasted. The use of the modified surfaces in this research could vastly reduce cost of culture by reducing amount of GF required.
Conclusions
Plasma polymerised allylamine surfaces have been used to bind heparin in a way which does not interfere with the binding of FGF-2 to form an artificial extracellular matrix. By exposing the heparin-decorated surface to a solution of FGF-2, sufficient growth factor is captured to significantly increase the proliferation of human dermal fibroblasts at low serum concentrations. The protective effect of the surface modification on FGF-2 once bound significantly improves the GFs effective half-life. It is suggested that this approach could be used for multiple growth factors and other signalling molecules to either (i) increase the yield of cells in culture, or (ii) dramatically reduce the usage of these expensive culture components.Acknowledgements
The authors would like to acknowledge Professor Michael Roberts from the Division of Health Sciences, School of Pharmacy and Medical Sciences, University of South Australia for the provision of skin from which the HDFs were isolated. DER would also like to acknowledge the WMICRC for funding.Notes and references
- J. M. Trowbridge and R. L. Gallo, Dermatan sulphate new functions from an old glycosaminoglycan, Glycobiology, 2002, 12, 117–125 CrossRef PubMed.
- A. S. Eriksson and D. Spillmann, The Mutual Impact of Syndecan-1 and Its Glycosaminoglycan Chains – A Multivariable Puzzle, J. Histochem. Cytochem., 2012, 60(12), 936–942 CrossRef PubMed.
- A. S. Goustin, E. B. Leof, G. D. Shipley and H. L. Moses, Growth Factors and Cancer, Cancer Res., 1986, 46(3), 1015–1029 CAS.
- J. M. Trowbridge, J. A. Rudisill, D. Ron and R. L. Gallo, Dermatan Sulfate Binds and Potentiates Activity of Keratinocyte Growth Factor (FGF-7), J. Biol. Chem., 2002, 277(45), 42815–42820 CrossRef CAS PubMed.
- J. Imanishi, K. Kamiyama, I. Iguchi, M. Kita, C. Sotozono and S. Kinoshita, Growth factors: importance in wound healing and maintenance of transparency of the cornea, Prog. Retinal Eye Res., 2000, 19(1), 113–129 CrossRef CAS.
- J. M. Trowbridge, J. A. Rudisill, D. Ron and R. L. Gallo, Dermatan sulfate binds and potentiates activity of keratinocyte growth factor (FGF-7)*, J. Biol. Chem., 2002, 277(45), 42815–42820 CrossRef CAS PubMed.
- N. T. Bennett and G. S. Schultz, Growth factors and wound healing: Biochemical properties of growth factors and their receptors, Am. J. Surg., 1993, 165(6), 728–737 CrossRef CAS.
- K. G. Cornwell and G. D. Pins, Enhanced Proliferation and Migration of Fibroblasts on the Surface of Fibroblast Growth Factor-2-Loaded Fibrin Microthreads, Tissue Eng., Part A, 2010, 16(12), 3669–3677 CrossRef CAS PubMed.
- S. Y. Song, H. M. Chung and J. H. Sung, The pivotal role of VEGF in adipose-derived-stem-cell-mediated regeneration, Expert Opin. Biol. Ther., 2010, 10(11), 1529–1537 CrossRef CAS PubMed.
- N. J. Trengove, H. Bielefeldt-Ohmann and M. C. Stacey, Mitogenic activity and cytokine levels in non-healing and healing chronic leg ulcers, Wound Repair Regen., 2000, 8(1), 13–25 CrossRef CAS.
- S. MacNeil, Progress and opportunities for tissue-engineered skin, Nature, 2007, 445(7130), 874–880 CrossRef CAS PubMed.
- J. F. Hansbrough, M. L. Cooper, R. Cohen, R. Spielvogel, G. Greenleaf and R. L. Bartel, et al., Evaluation of a biodegradable matrix containing cultured human fibroblasts as a dermal replacement beneath meshed skin grafts on athymic mice, Surgery, 1992, 111(4), 438–446 CAS.
- T. Sun, S. M. Mai, D. Norton, J. W. Haycock, A. J. Ryan and S. MacNeil, Self-organization of skin cells in three-dimensional electrospun polystyrene scaffolds, Tissue Eng., 2005, 11(7–8), 1023–1033 CrossRef CAS PubMed.
- Y. K. Jeon, Y. H. Jang, D. R. Yoo, S. N. Kim, S. K. Lee and M. J. Nam, Mesenchymal stem cells’ interaction with skin: Wound-healing effect on fibroblast cells and skin tissue, Wound Repair Regener., 2010, 18(6), 655–661 CrossRef PubMed.
- M. Schuldiner, O. Yanuka, J. Itskovitz-Eldor, D. A. Melton and N. Benvenisty, Effects of eight growth factors on the differentiation of cells derived from human embryonic stem cells, Proc. Natl. Acad. Sci. U. S. A., 2000, 97(21), 11307–11312 CrossRef CAS PubMed.
- R. Raman, V. Sasisekharan and R. Sasisekharan, Structural Insights into Biological Roles of Protein-Glycosaminoglycan Interactions, Chem. Biol., 2005, 12(3), 267–277 CrossRef CAS PubMed.
- B. J. Zern, H. H. Chu and Y. D. Wang, Control Growth Factor Release Using a Self-Assembled polycation:heparin Complex, PLoS One, 2010, 5(6), e11017 Search PubMed.
- R. E. Hileman, J. R. Fromm, J. M. Weiler and R. J. Linhardt, Glycosaminoglycan-protein interactions: definition of consensus sites in glycosaminoglycan binding proteins, BioEssays, 1998, 20(2), 156–167 CrossRef CAS.
- A. Marson, D. E. Robinson, P. N. Brookes, B. Mulloy, M. Wiles and S. J. Clark, et al., Development of a microtiter plate-based glycosaminoglycan array for the investigation of glycosaminoglycan “protein interactions”, Glycobiology, 2009, 19(12), 1537–1546 CrossRef CAS PubMed.
- H. Aizawa, S. Kurosawa, K. Kobayashi, K. Kashima, T. Hirokawa and Y. Yoshimi, et al., Tuning of contact angle on glass plates coated with plasma-polymerized styrene, allylamine and acrylic acid, Mater. Sci. Eng., C, 2000, 12(1–2), 49–54 CrossRef.
- J. G. Calderon, A. Harsch, G. W. Gross and R. B. Timmons, Stability of plasma-polymerized allylamine films with sterilization by autoclaving, J. Biomed. Mater. Res., 1998, 42(4), 597–603 CrossRef CAS.
- T. R. Gengenbach, R. C. Chatelier and H. J. Griesser, Characterization of the ageing of plasma-deposited polymer films: Global analysis of x-ray photoelectron spectroscopy data, Surf. Interface Anal., 1996, 24(4), 271–281 CrossRef CAS.
- J. D. Whittle, R. D. Short, C. W. I. Douglas and J. Davies, Differences in the aging of allyl alcohol, acrylic acid, allylamine, and octa-1,7-diene plasma polymers as studied by X-ray photoelectron spectroscopy, Chem. Mater., 2000, 12(9), 2664–2671 CrossRef CAS.
- Z. Zhang, Q. Chen, W. Knoll and R. Forch, Effect of aqueous solution on functional plasma polymerized films, Surf. Coat. Technol., 2003, 174, 588–590 CrossRef.
- Z. Zhang, W. Knoll and R. Forch, Amino-functionalized plasma polymer films for DNA immobilization and hybridization, Surf. Coat. Technol., 2005, 200(1–4), 993–995 CrossRef CAS PubMed.
- D. E. Robinson, D. J. Buttle, R. D. Short, S. L. McArthur, D. A. Steele and J. D. Whittle, Glycosaminoglycan (GAG) binding surfaces for characterizing GAG-protein interactions, Biomaterials, 2012, 33(4), 1007–1016 CrossRef CAS PubMed.
- D. J. Mahoney, J. D. Whittle, C. M. Milner, S. J. Clark, B. Mulloy and D. J. Buttle, et al., A method for the non-covalent immobilization of heparin to surfaces, Anal. Biochem., 2004, 330(1), 123–129 CrossRef CAS PubMed.
- M. Salim, P. C. Wright and S. L. McArthur, Studies of electroosmotic flow and the effects of protein adsorption in plasma-polymerized microchannel surfaces, Electrophoresis, 2009, 30, 1877–1887 CrossRef CAS PubMed.
- K. A. Meade, K. J. White, C. E. Pickford, R. J. Holley, A. Marson and D. Tillotson, et al., Immobilization of Heparan Sulfate on Electrospun Meshes to Support Embryonic Stem Cell Culture and Differentiation, J. Biol. Chem., 2013, 288, 5530–5538 CrossRef CAS PubMed.
- A. G. Shard, J. D. Whittle, A. J. Beck, P. N. Brokes, N. A. Bullet, R. A. Talib, A. Mistry, D. Barton and S. L. McArthur, A NEXAFS examination of unsaturation in plasma polymers of allylamine and propylamine, J. Phys. Chem. B, 2004, 108, 12472–12480 CrossRef CAS.
- H. A. Franch, S. Sooparb, J. Du and N. S. Brown, A Mechanism Regulating Proteolysis of Specific Proteins during Renal Tubular Cell Growth, J. Biol. Chem., 2001, 276(22), 19126–19131 CrossRef CAS PubMed.
- J. J. A. Barry, M. Silva, K. M. Shakesheff, S. M. Howdle and M. R. Alexander, Using plasma deposits to promote cell population of the porous interior of three-dimensional poly(D,L-lactic acid) tissue-engineering scaffolds, Adv. Funct. Mater., 2005, 15, 1134–1140 CrossRef CAS.
- Y. Xie, S. C. Rizzi, R. Dawson, E. Lynam, S. Richards and D. Leavesley, et al., Development of a 3-dimensional human skin equivalent wound model for investigating novel wound healing therapies, Wound Repair Regener., 2010, 18(4), 66 Search PubMed.
- S. Commandeur, V. van Drongelen, F. R. de Gruijl and A. El Ghalbzouri, Epidermal growth factor receptor activation and inhibition in 3D in vitro models of normal skin and human cutaneous squamous cell carcinoma, Cancer Sci., 2012, 103(12), 2120–2126 CrossRef CAS PubMed.
- K. Moharamzadeh, H. Colley, C. Murdoch, V. Hearnden, W. L. Chai and I. M. Brook, et al., Tissue-engineered Oral Mucosa, J. Dent. Res., 2012, 91(7), 642–650 CrossRef CAS PubMed.
- A. Michelmore, C. Charles, R. W. Boswell, R. D. Short and J. D. Whittle, Defining plasma polymerization: new insight into what we should be measuring, ACS Appl. Mater. Interfaces, 2013, 5(12), 5387–5391 CAS.
- L. Denis, D. Cossement, T. Godfroid, F. Renaux, C. Bittencourt, R. Snyders and M. Hecq, Synthesis of Allylamine Plasma Polymer Films: Correlation Plasma Diagnostic and Film Characteristics, Plasma Processes Polym., 2009, 3(6), 199–208 CrossRef.
- D. R. Ralston, C. Layton, A. J. Dalley, S. G. Boyce, E. Freedlander and S. Mac Neil, The requirement for basement membrane antigens in the production of human epidermal/dermal composites in vitro, Br. J. Dermatol., 1999, 140(4), 605–615 CrossRef CAS.
- D. R. Ralston, C. Layton, A. J. Dalley, S. G. Boyce, E. Freedlander and S. MacNeil, Keratinocytes contract human dermal extracellular matrix and reduce soluble fibronectin production by fibroblasts in a skin composite model, Br. J. Plast. Surg., 1997, 50(6), 408–415 CrossRef CAS.
- S. MacNeil and J. Shepherd, et al., Production of Tissue-Engineered Skin and Oral Mucosa for Clinical and Experimental Use. 3D Cell Culture, Humana Press, 2011, pp. 129–153 Search PubMed.
- M. Rusnati, E. Tanghetti, C. Urbinati, G. Tulipano, S. Marchesini and M. Ziche, et al., Interaction of Fibroblast Growth Factor-2 (FGF-2) with Free Gangliosides: Biochemical Characterization and Biological Consequences in Endothelial Cell Cultures, Mol. Biol. Cell, 1999, 10(2), 313–327 CrossRef CAS.
- H. Y. Zhu, L. Duchesne, P. S. Rudland and D. G. Fernig, The heparan sulfate co-receptor and the concentration of fibroblast growth factor-2 independently elicit different signalling patterns from the fibroblast growth factor receptor, Cell Commun. Signaling, 2010, 8, 14 CrossRef PubMed.
- D. E. Robinson, A. Marson, R. D. Short, D. J. Buttle, A. J. Day, K. L. Parry, M. Wiles, P. Highfield, A. Mistry and J. D. Whittle, Surface Gradient of Functional Heparin, Adv. Mater., 2008, 1166–1169 CrossRef CAS.
- S. Ashikari-Hada, H. Habuchi, N. Sugaya, T. Kobayashi and K. Kimata, Specific inhibition of FGF-2 signaling with 2-O-sulfated octasaccharides of heparan sulfate, Glycobiology, 2009, 19(6), 644–654 CrossRef CAS PubMed.
- N. Mejdoubi-Charef, J. Courty, F. Sineriz, D. Papy-Garcia and S. Charef, Heparin Affin Regulatory Peptide Modulates the Endogenous Anticoagulant Activity of Heparin and Heparan Sulphate Mimetics, Basic Clin. Pharmacol. Toxicol., 2012, 111(5), 296–302 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: S1 to S3 show cell counts at all time periods investigated 1, 2, 4, 5 and 9 days. For all condition investigated. See DOI: 10.1039/c4bm00018h |
| This journal is © The Royal Society of Chemistry 2014 |