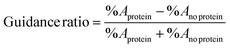Light-reactive dextran gels with immobilized guidance cues for directed neurite growth in 3D models
Elaine L.
Horn-Ranney
,
Parastoo
Khoshakhlagh
,
Julie W.
Kaiga
and
Michael J.
Moore
*
Department of Biomedical Engineering, Tulane University, New Orleans, LA, USA. E-mail: mooremj@tulane.edu
First published on 10th July 2014
Abstract
We present here a novel light-reactive dextran gel for immobilizing guidance cues in neural growth models. The dextran gel is functionalized with glycidyl methacrylate to afford crosslinking abilities, and is combined with polyethylene glycol (PEG) acrylate grafted with thiol groups caged by a UV-light sensitive moiety. The gel is chemically crosslinked within a cell-restrictive PEG micromold with two channels, and then irradiated with UV light to liberate the thiol groups in a spatially defined manner. Maleimide-conjugated NeutrAvidin (NA), neurotrophin-3 (NT-3) and semaphorin 3A (Sema3A) are then bound to the free thiols, resulting in regions of immobilized guidance cues. Dorsal root ganglia explants were cultured in these dual hydrogel constructs, and the neurite response was quantified by comparing the neurite growth in the channel with the immobilized cue to the channel without any protein. We found that immobilized NT-3 elicited a moderate attractive response, while bound Sema3A elicited a strong repulsive response from neurites. This work establishes a model for investigating growth cone responses to immobilized cues in three dimensions.
1. Introduction
Neural navigation during embryonic development depends on chemotactic and haptotactic cues to synapse neurites with their appropriate targets. Axon growth cones from dorsal root ganglia (DRG) interpret these cues through metabolic pathways associated with specific receptor–ligand interactions, resulting in dynamic responses to changes in the in vivo environment.1 Some of these cues have been characterized and translated to an in vitro setting in order to recreate these in vivo phenomena in cell culture models for wound healing and disease studies.2,3Chemoattractive cues like nerve growth factor (NGF) and neurotrophin-3 (NT-3) are secreted by target cells to form soluble concentration gradients or immobilized in the extracellular matrix via affinity interactions.4–6 Both NGF and NT-3 have been utilized in in vitro studies to elicit attractive responses from neural cultures. One study fabricated 3D growth models using poly(2-hydroxyethyl-methacrylate) and poly(L-lysine) with immobilized gradients of NGF and NT-3 and observed a graded attractive response by DRG neurites to the immobilized cues.7 Neurites have also been shown to navigate toward sources of soluble NGF and NT-3 gradients in 2D models for controlled growth.8 Other studies have focused on the interaction of NT-3 with muscle fibers, as NT-3 plays a supportive role in maintaining proprioceptive axons after they have synapsed with their targets.9–11 As a wound-healing model, NT-3 delivered to the site of spinal cord injury in rats via NT-3-loaded gels12 or NT-3-expressing lentiviral vectors13 significantly improved functional recovery of axons. Though the effectiveness of soluble NT-3 was limited by the distance, the NT-3 gradient produced by the vectors was enough to overcome the glial scar at the site of the spinal cord injury. As a chemoattractant for proprioceptive neurites, NT-3 has also been observed to be upregulated at the site of cutaneous nerve injury.14 Conversely, overexpression of NT-3 has been shown to result in limb proprioceptive deficits in spite of no apparent nerve loss, further supporting the importance of NT-3 as a maintenance factor for neurites.15
Semaphorin 3A (Sema3A) is a chemorepulsive factor that may be expressed as soluble gradients or immobilized transmembrane proteins that bind to the plexin/neuropilin receptor complex in axons to initiate the depolymerization of actin filaments.16,17 This response induces growth cone collapse in the presence of Sema3A, causing the axon to turn away from the source of the repulsive cue. For pyramidal neurons, Sema3A repels axons but acts as a chemoattractant for apical dendrites in order to orient the neurons appropriately.18 As a repulsive cue, Sema3A prevents neurites from navigating away from their targets, and is essential in the pruning of errant axons during nerve mapping. Two dimensional in vitro models have observed strong graded responses by DRG neurites to both immobilized and soluble Sema3A, highlighting the sensitivity of axons to chemorepellant cues.19,20 This sensitivity persists in fully developed nervous systems, as Sema3A is an inhibitory cue found at nerve injury sites that severely hampers axon regeneration.21,22 To eliminate the effects of Sema3A in spinal cord injury, one study administered a Sema3A inhibitor to adult rats at the lesion site.23 This inhibitor effectively prevented Sema3A from interacting with regenerating axons and allowed axon navigation through the lesion site.
Light-sensitive gels have been used previously for the immobilization of guidance cues within hydrogel cell culture models to provide spatial control over the location of proteins within a growth substrate. Thiol-based chemistries have been utilized extensively for introducing patterned guidance cues.24–26 Gels that crosslink in the presence of light can physically entrap proteins within the mesh of the hydrogel network, and some studies have fabricated gels with protein gradients with this method.7,8 Another method incorporates photolabile nitrobenzyl-based caging moieties into gels to allow proteins to be covalently bound to the hydrogel matrix in very spatially defined regions after the gel has formed.27–29 These methods have been utilized in the development of nerve growth models for eliciting physiological responses from in vitro cultured neurites.
Nerve growth models have been critical for discovering specific receptor–ligand interactions for directing neurite growth in a controlled manner. Our goal is to develop a neurite culture platform that can present physiological cues in a 3D, in vitro setting for investigation of growth cone responses. We have previously utilized a dynamic mask photolithography device to develop 3D dual hydrogel constructs with a cell-permissive agarose gel and a cell-restrictive PEG boundary that incorporated both immobilized and soluble cues.28,30 Here we present a photoreactive dextran gel as a cell-permissive substrate for immobilizing guidance cues within a dual hydrogel construct. To this dextran gel, we introduced chemoattractive NT-3 and chemorepulsive Sema3A and observed the response of neurites from DRGs to these cues. Dextran has not yet been explored as a substrate for neural growth, but its ability to be functionalized and limited interfering side groups make it an attractive candidate for nerve growth models. This study demonstrates how 3D presentation of immobilized cues influences growth cone responses in an in vitro model.
2. Methods and materials
All chemicals were purchased and used as received from Sigma Aldrich unless otherwise noted.2.1 Synthesis of photoreactive reagents
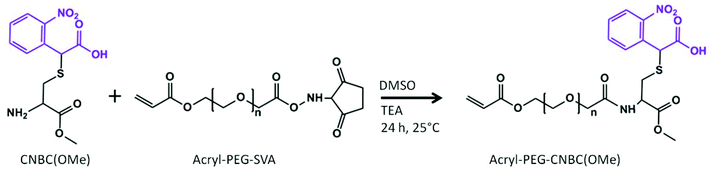
The photolabile compound S-(α-carboxy-2-nitrobenzyl)-L-cysteine methyl ester (CNBC(OMe)) was synthesized according to the detailed protocol published by Horn-Ranney et al.28 The CNBC(OMe) compound was then conjugated to a polyethylene glycol (PEG) spacer. Briefly, 0.1 g acryl-PEG-succinimidyl valerate (A-PEG-SVA, MW = 3400 kDa; Laysan Bio, Arab, AL) was dissolved in 1.5 ml dimethyl sulfoxide (DMSO). Next, 4.1 μl triethylamine (1 molar eq. to A-PEG-SVA) was added to the solution dropwise. A 3 molar equivalent of CNBC(OMe) to Acryl-PEG-SVA (0.027 g) was dissolved in 0.5 ml DMSO, and then added to the Acryl-PEG-SVA solution dropwise. The reaction was stirred at room temperature overnight. The solution was then added to a dialysis cassette (MWCO = 2000 kDa) and dialyzed in water for 2 d, followed by lyophilization for 3 d to yield Acryl-PEG-CNBC(OMe) (>99%; 1H NMR (DMSO-d6): δ 8.0–7.5 (m, 4H, Ar), δ 6.5 (d, 1H, CH2), δ 6.2 (m, 1H, CH), δ 6.0 (d, 1H, CH2), δ 3.5 (t, 3H, OCH3), δ 3.1–2.9 (m, 2H, CH2)). The structures of the reagents and product are shown in Fig. 1.Glycidyl methacrylate (GMA) was grafted onto dextran (MW = 70 kDa) according to a published procedure.31 First, 0.5 g dextran was dissolved in 4.5 ml DMSO under nitrogen. Next, 0.1 g 4-dimethylaminopyridine was dissolved in 0.5 ml DMSO and added to the reaction dropwise, followed by 116 μl GMA. The solution was degassed with nitrogen for 10 min, and then stirred for 48 h at room temperature. The reaction was quenched by adding 140 μl 37% HCl, and then dialyzed in water for 3 d. The solution was lyophilized to yield glycidyl methacrylate-dextran (Me-Dex; 1H NMR (D2O): δ 6.1–5.7 (m, 2H, CH2), δ 5.2 (m, 1H, CH), δ 4.9 (m, 1H, CH), δ 1.9 (s, 3H, CH3)). The degree of substitution was determined by adding the integrals from protons of the double bond of the methacrylate group (δ 6.1–5.7 ppm) and dividing this sum by the integral from the anomeric proton of the dextran ring (δ 4.9 ppm). The final value from this calculation was multiplied by 100 to give a 42% degree of substitution of Me-Dex.
2.2 Fabrication of dual hydrogel constructs
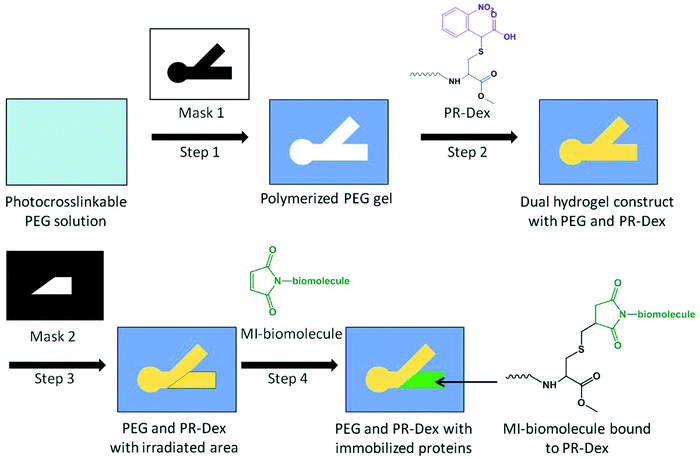
Dynamic mask projection photolithography apparatus consisting of a UV light source (OmniCure 1000 with 320–500 nm filter, EXFO, Quebec, Canada) with a collimating adapter (EXFO), a DMD as a dynamic photomask (Discovery™ 3000, Texas Instruments, Dallas, TX), and a 2× objective lens (Plan Fluor, Nikon Instruments, Tokyo, Japan) was used for hydrogel patterning, as previously described.28,30 The UV light was focused on the surface of the cell culture insert membrane to allow bulk irradiation through the depth of the photoreactive material at an intensity of 181 mW cm−2.The dual hydrogel system consisted of a photoreactive dextran (PR-Dex) gel in a PEG mold on a permeable cell culture insert. The walls of collagen-coated, 6-well PTFE cell culture inserts with a diameter of 24 mm and a pore size of 0.4 μm (Transwell®, Corning Inc., Corning, NY) were treated with Rain-X (SOPUS Products, Houston, TX) to minimize meniscus formation. A solution of 10% w/v PEG-diacrylate (MW = 1000; Polysciences Inc., Warrington, PA) and 0.5% Irgacure 2959 (Ciba Specialty Chemicals, Basel, Switzerland) in water was added to the treated inserts (500 μl per insert), followed by irradiation with UV light from the photolithography apparatus for 55 s per construct to yield photocrosslinked PEG molds on the cell culture insert membrane. The PEG molds have two channels to represent a choice point for neurites. Inserts were washed with PBS to remove the uncrosslinked PEG solution.
The cell-permissive PR-Dex gel was chemically crosslinked using a standard ammonium persulfate (AP)/tetramethylethylenediamine (TEMED) technique. The acrylate groups from the Acryl-PEG-CNBC(OMe) integrated with the methacrylate network of Me-Dex to afford photocaged sites for biomolecule immobilization. Murine laminin-1 (Gibco-Invitrogen, Carlsbad, CA) was added to the PR-Dex gel solution to provide sites for cell attachment. The gel was prepared by combining 29.4 μl 10% w/v Me-Dex in water, 9.0 μl 20% w/v Acryl-PEG-CNBC(OMe) in water, 6.0 μl 2 M ammonium persulfate, 12.6 μl PBS, 0.3 μl laminin (1 mg ml−1) and 3 μl 2 M TEMED. The PEG molds were filled with approximately 5 μl of this PR-Dex solution, and the PR-Dex gels were fully formed after 30 min. Between 4 and 6 dual hydrogel constructs were fabricated on each 6-well cell culture insert. Since all PR-Dex gels tested in this study contained laminin, the abbreviation “PR-Dex” refers to PR-Dex gels with laminin, henceforth.
2.3 Rheological evaluation of gels
Dynamic mechanical analysis of the PR-Dex gels using an AR2000 rheometer (Texas Instruments) was performed in order to determine the modulus. An oscillating 1° steel cone applied a constant strain of 4% across a frequency range of 0.100 to 100 Hz, and the storage (G′) and loss (G′′) moduli were obtained. Mean storage moduli of gels were analyzed at 10 Hz by one-way ANOVA.2.4 Immobilization of proteins in gels
Once the dual hydrogel constructs were complete, specified regions of PR-Dex were irradiated with UV light for 90 s to liberate the carboxy-nitrobenzyl (CNB) moiety from Acryl-PEG-CNBC(OMe) and subsequently present free thiol groups for biomolecule immobilization. The inserts were soaked in 5% w/v bovine serum albumin (BSA) in PBS for 1 h to block non-specific binding. The chemoattractive molecule neurotrophin-3 (NT-3; R&D Systems, Minneapolis, MN) and chemorepulsive molecule semaphorin 3A (Sema3A; R&D Systems) were preconjugated with fluorescent secondary antibodies (Alexa Fluor 488 Goat anti-Human IgG (H + L), AF488-Ab; Jackson Immuno, West Grove, PA) by anti-human IgG affinity with the recombinant human NT-3 and Sema3A. Briefly, 15 μl of 0.5 nM NT-3 and 23 μl of 0.2 nM Sema3A were each mixed with 1 μl of 1 mg ml−1 AF488-Ab solution (134 nmol antibody per nmol protein), and allowed to react overnight at 4 °C. Antibody preconjugation was used for observation and quantification of protein binding, and was not used when neurite outgrowth experiments were performed. To afford reactivity to thiol groups, the proteins were conjugated with a maleimide-containing crosslinker (sulfosuccinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate (Sulfo-SMCC; Thermo Scientific, Rockford, IL)). To the NT-3 solution, 4 μl of 1 mg ml−1 Sulfo-SMCC in water was added, and 10 μl Sulfo-SMCC was added to the Sema3A solution for a final molar ratio of 146 nmol Sulfo-SMCC per nmol protein. The reaction was carried out for 15 min at 4 °C, the volume of each solution was increased to 70 μl by adding water, and then filtered over spin columns (MWCO = 7 kDa; Thermo Scientific). Each protein solution was added to 900 μl PBS, and then subsequently added to the well of a 6-well plate containing the cell culture inserts. As a control, 1 ml of 20 nM maleimide-activated NeutrAvidin (NA; Thermo Scientific) in PBS was added to a well with a cell culture insert. The inserts were soaked in their respective protein solutions for 48 h at 4 °C, and then washed thoroughly with 2% w/v BSA in PBS to remove unbound proteins. For the NA-inserts, a 1.5 ml solution of 10 μM Alexa Fluor 488 biocytin salt (AF488-biocytin; Molecular Probes, Eugene, OR) was added to the well after washing out excess NA. The inserts were soaked in the AF488-biocytin overnight at 4 °C, and then washed thoroughly with 2% w/v BSA in PBS. The fabrication scheme for preparing dual hydrogel constructs with immobilized proteins is presented in Fig. 2.Bound proteins were quantified by comparing the relative fluorescence with known standards. First, standard curves of known concentrations AF488-Ab/AF488-biocytin were assembled to measure the concentration of the fluorescent tag in the dual hydrogel constructs. Then, solutions of known protein concentration and unknown fluorescent tag concentration were compared against the standard curve to derive a relationship between the protein concentration and the associated fluorescent tag. This relationship was used to convert the concentration of AF488-Ab/AF488-biocytin to the protein concentration, and subsequently quantify the bound protein in each gel. Because of the autofluorescence of PR-Dex gels, calculations were normalized by subtracting this background fluorescence from the measured fluorescence values of bound proteins in gels.
2.5 Varying irradiation time and photoreactive reagents in gels
Both the irradiation time and the concentration of Acryl-PEG-CNBC(OMe) in PR-Dex were varied in order to determine their effects on biomolecule immobilization. To investigate how the irradiation time affects biomolecule immobilization, PR-Dex gels were prepared as described in section 2.2. These gels were then irradiated with UV light for 10, 30, 60, or 90 seconds. Following irradiation, the gels were blocked with 5% w/v BSA for one hour. The gels were soaked in a solution of 1 μg ml−1 of maleimide-conjugated Texas Red (MI-TR; Invitrogen) in PBS overnight at 4 °C, and then washed thoroughly with 2% w/v BSA.To determine the effect of the CNBC(OMe) concentration on biomolecule immobilization, PR-Dex gels were prepared as described in section 2.2, but with fractions of Acryl-PEG-CNBC(OMe) substituted with Acryl-PEG-SVA in order to decrease the overall concentration of CNBC(OMe) present in the gels. Gels with final concentrations of 8.08, 6.06, 4.04, and 2.02 mM Acryl-PEG-CNBC(OMe) in PR-Dex were prepared, irradiated with UV light for 90 s and then blocked with 5% w/v BSA. These gels were soaked in 1 μg ml−1 MI-TR in PBS overnight at 4 °C, and then washed with 2% w/v BSA.
Immobilized MI-TR molecules in all gels were quantified by comparing the relative fluorescence of MI-TR in PR-Dex gels with that of a standard curve prepared with known concentrations, as described in section 2.4.
2.6 Culturing dorsal root ganglia in dual hydrogel constructs
All procedures involving vertebrate animals were approved by the Institutional Animal Care and Use Committee. Inserts prepared with immobilized proteins in dual hydrogel constructs were soaked overnight at 37 °C in 1.5 ml adhesion media (neurobasal medium supplemented with L-glutamine (L-Glu), nerve growth factor (NGF), 10% fetal bovine serum, and penicillin/streptomycin; Invitrogen). Cervical dorsal root ganglia (DRGs) isolated from embryonic day 15 Long-Evans rat pups (Charles River, Wilmington, MA) were inserted into each gel by making a small slit in the gel and gently pushing the DRG explant through the gel using forceps. The explants were maintained in growth medium (Neurobasal medium supplemented with B-27, L-glu, NGF, and penicillin/streptomycin) in an incubator (37 °C, 5% CO2) for 5 d, and the medium was changed every 48 h.After 5 d, the DRGs were fixed in 4% paraformaldehyde for 2 h at 37 °C, and then washed with 0.1% w/v saponin in PBS. Neurites were tagged with mouse monoclonal [2G10] neuron-specific βIII tubulin primary antibody (AbCam, Cambridge, MA), followed by fluorescent tagging with Cy 3.5-conjugated goat anti-mouse IgG (H + L) secondary antibody (Jackson Immuno). The glial cell marker S100 was tagged with rabbit polyclonal S100 antibody (AbCam), followed by fluorescent tagging with Alexa Fluor 488-conjugated goat anti-rabbit IgG (H + L) secondary antibody (Jackson Immuno). The staining and tagging steps were carried out in 2% BSA/0.1% saponin in PBS, followed by 0.1% saponin washes. Fluorescence imaging was carried out using a Nikon AZ100 stereo zoom microscope. Confocal imaging was carried out using a Nikon A1 confocal laser microscope system. Z-stack projection images were depth-coded to visualize 3D neurite growth.
2.7 Quantification of the neurite response
Fluorescence images were processed with the Image J software (National Institutes of Health, Bethesda, MD). Images were thresholded to produce binary representations of fluorescent-tagged neurites, in which the pixels comprising of neurites have a value of 1 (black) and non-fluorescent regions have pixels with a value of 0 (white). Regions of interest (ROI) were analyzed for each channel of the construct. For the channel with the immobilized protein, the ROI taken was of trapezoidal shape, with one side along the immobilized cue boundary, and the remaining 3 sides square with the channel boundaries. For the channel without the immobilized protein, a rectangular ROI was taken. Both ROI had equal areas of 2.50 mm2. These ROI of unequal shape and equal area were taken to best represent the opportunity for neurites to grow in one channel versus the other, such that the area of neurite growth in either channel was equal without the presence of guidance cues. From these ROI, the area fraction (non-zero area, %A) was measured. To compare the neurite growth in channels with (%Aprotein) and without (%Ano protein) immobilized protein, a guidance ratio was calculated to be the difference in area fractions between the channels divided by the total area fraction:19This guidance ratio is normalized by the total neurite outgrowth in the individual dual hydrogel constructs, rendering it independent of the total neurite growth across all gels. A positive value for the guidance ratio indicated a chemoattractive response, while a negative value indicated a repulsive response. Gels with no neurite growth in either channel, likely due to damage sustained by the DRG explant during dissection and/or insertion, were excluded from the study. Statistical evaluation of neurite responses to immobilized proteins was determined by one-way ANOVA, with post-hoc analysis performed with the Tukey method.
3. Results
3.1 Concentration of immobilized guidance cues
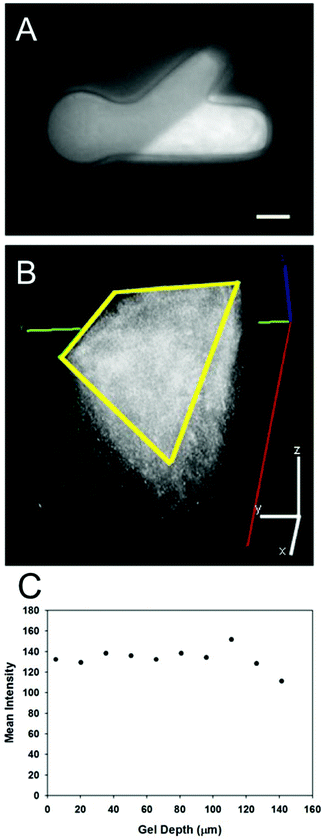
The photolithography apparatus allowed for both structural and molecular micropatterning of light-sensitive gels in a reproducible manner. Both the PEG molds and PR-Dex gels maintained their structural integrity throughout all fabrication steps. Rheological analysis determined the storage modulus of PR-Dex to be 91.7 ± 1.09 Pa (n = 3). This magnitude of stiffness is on the low end of the range typically used for neurite growth (∼100–1000 Pa),32 and comparable to 0.15% Puramatrix (∼100 Pa),33 which we had used previously in these neurite growth model systems.30Fig. 3 shows a representative image of the immobilized protein (NA tagged with AF488-biocytin) in the dual hydrogel constructs. Protein immobilization occurred only in the regions of PR-Dex irradiated by UV light, and was not present in either PEG or unirradiated sections of PR-Dex. The discrepancy in brightness between the PEG mold and the unirradiated PR-Dex was due to the autofluorescence of the PR-Dex gel itself. Confocal imaging of the bound NA (Fig. 3) shows homogeneous binding of proteins throughout the depth of the gel (227 μm) with some fluorescent biocytin trapped in the cell culture insert membrane (bottom plane of image). Because the protein was present in relatively equal concentration throughout the PR-Dex gel, neurites cultured in the dual hydrogel constructs encountered the same concentration of protein regardless of the plane on which the neurite extended. Irradiation of PR-Dex using the photolithography apparatus afforded sharp edges to immobilized protein regions, demonstrating fine control of the spatial distribution of proteins.
The concentration of immobilized cues was quantified in constructs using standard curves of the relative fluorescence. From Table 1, the concentration of the immobilized NA was 122 ± 2.50 nM (n = 6), that of immobilized NT-3 was 151 ± 15.7 nM (n = 4), and that of immobilized Sema3A was 87.6 ± 6.74 nM (n = 4). The immobilization of proteins was consistent across constructs with the same protein. The molecular weights of these are also listed in Table 1, with NT-3 having a low molecular weight (13.6 kDa), NA having a medium molecular weight (60.0 kDa), and Sema3A having a high molecular weight (114 kDa). Though the molecular weights of these proteins vary widely, they bound to the gel in similar quantities, indicating that the concentration of CNBC in the PR-Dex gel is a more important factor than the size of the target cue in controlling the immobilized cue concentration.
| Protein | Molecular weight (kDa) | Concentration (nM) |
|---|---|---|
| NeutrAvidin | 60.0 | 122 ± 2.50 |
| Neurotrophin-3 | 13.6 | 151 ± 15.7 |
| Semaphorin 3A | 114 | 87.6 ± 6.74 |
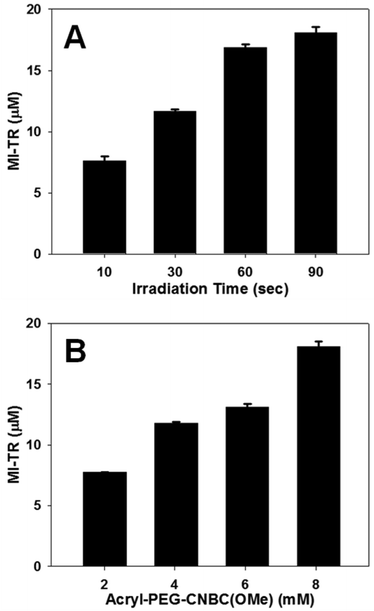
To highlight the controllability of the immobilized cue concentration in this model, PR-Dex gels were either irradiated for variable time lengths (Fig. 4A) or incorporated at variable Acryl-PEG-CNBC(OMe) concentrations (Fig. 4B) prior to introduction of MI-TR (729 Da). In Fig. 4A, the concentration of immobilized MI-TR in PR-Dex irradiated for 10 s was 7.60 ± 0.365 μM (n = 3) and 11.7 ± 0.161 μM for 30 s (n = 3). Gels irradiated for 60 s and 90 s bonded to MI-TR at concentrations of 16.9 ± 0.230 μM (n = 3) and 18.1 ± 0.452 μM (n = 3), respectively. All of these concentrations were statistically significant from each other, thereby confirming the irradiation time as a mechanism of controlling the concentration of the presented cue. A similar trend in cue immobilization was observed in PR-Dex gels of varying Acryl-PEG-CNBC(OMe) concentrations. Gels with 2.02 mM of the photoreactive molecule bonded to MI-TR at a concentration of 7.76 ± 0.0132 μM (n = 3), and gels with 4.04 mM of Acryl-PEG-CNBC(OMe) immobilized 11.8 ± 0.0882 μM MI-TR (n = 3). Bound MI-TR was present at concentrations of 13.1 ± 0.243 μM (n = 3) and 18.1 ± 0.452 μM (n = 3) in PR-Dex gels with 6.06 mM and 8.08 mM Acryl-PEG-CNBC(OMe), respectively. Thus, increasing the concentration of Acryl-PEG-CNBC(OMe) increases the concentration of immobilized MI-TR. These results support the assumptions that both the irradiation time and CNBC concentration can be used to control the quantity of guidance cues present in PR-Dex.
3.2 Neurite growth in photoreactive dextran
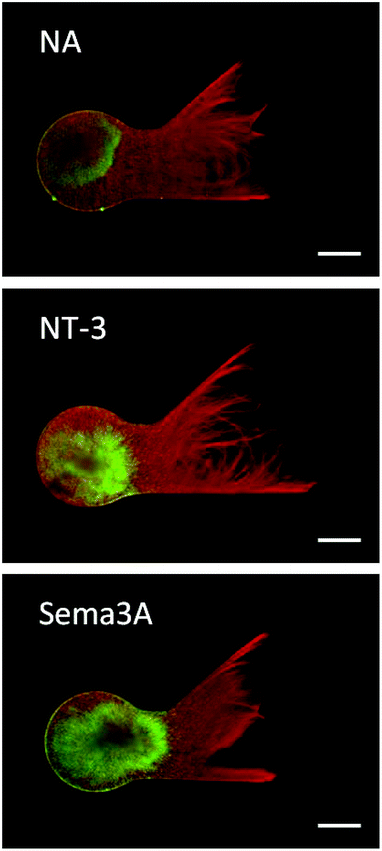
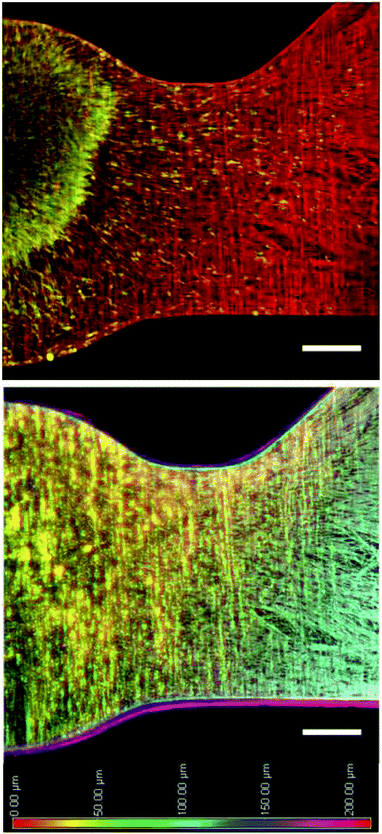
The photoreactive dextran developed in this study provided an amenable scaffold for supporting neurite growth in 3D. Neurites extending from DRG explants demonstrated robust growth in PR-Dex after 5 days, as seen in Fig. 5. Neurite growth (red) is restricted to the PR-Dex region by the PEG mold boundary, and is of homogeneous density across the primary channel. Glial cells (green) were contained within the PEG boundary and mostly concentrated in the DRG explant, with some migration along the neurites into the primary channel of PR-Dex. After 5 days, the glial cells had not yet migrated to the channel bifurcation for any condition. From the images of Fig. 5, it was observed that neurites tended to first grow along the boundary between PEG and PR-Dex before navigating into the bulk of PR-Dex. Because of this behavior, neurite growth was able to extend into both channels equally, despite the difference in the angle of the bifurcated channels compared to the primary PR-Dex channel. Confocal imaging (Fig. 6) of neurites cultured in PR-Dex gels with immobilized NA confirmed that neurites extended throughout the entire depth of the PR-Dex gel (213 μm), rather than a single plane. According to the depth-coded image in Fig. 6, most of the growth was concentrated to the center plane of the gel between depths of about 40 μm to 125 μm, with height 0 μm being the cell culture membrane. This finding was notable because it confirmed that the neurites were not growing strictly along the cell culture membrane in 2D.3.3 Neurite response to immobilized guidance cues
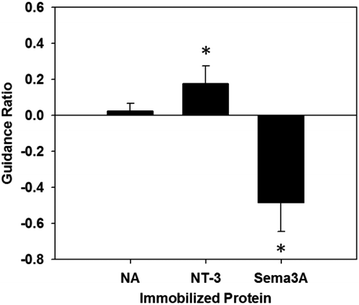
The response of neurites was quantified according to the previously stated guidance ratio, and presented in Fig. 7 (n = 7 for all conditions). Using this ratio, positive numbers indicate chemoattraction, negative numbers indicate chemorepulsion, and zero indicates no response. Gels with immobilized NA did not elicit an attractive or repulsive response, with a guidance ratio of 0.024 ± 0.043, confirming NA as an appropriate control protein. Additionally, this supports the assumption that the process of immobilizing proteins onto PR-Dex does not in itself elicit a response from neurites.For PR-Dex gels with the chemoattractive cue NT-3, a moderate attractive response was observed, with these gels having an overall guidance ratio of 0.18 ± 0.097. Conversely, gels with the chemorepulsive cue Sema3A elicited a strong repulsive response from neurites, with an average guidance ratio of −0.48 ± 0.16. These responses indicate that the neurites respond predictably to NT-3 and Sema3A when presented as immobilized cues. Statistical evaluation indicated that responses from both NT-3 and Sema3A were significant compared to the response to NA (p < 0.005), while a stronger difference in response was observed between NT-3 and Sema3A (p < 0.001). Neurites fully avoided the regions of bound Sema3A, as seen in Fig. 5. This behavior confirms that unbound proteins were completely washed out from the dual hydrogel constructs, as the neurites grew robustly in regions without immobilized Sema3A. Neurites that grew in the Sema3A channel tended to extend along the boundary between the PEG mold and the PR-Dex channel, without fully crossing into the region of immobilized Sema3A. The neurite responses to both NT-3 and Sema3A indicate that neither the commercially available kit used to conjugate maleimide to the proteins nor the fluorescent secondary antibody affected the proteins’ reactivity with cervical DRG neurites.
4. Discussion
The dual hydrogel constructs fabricated by the dynamic mask photolithography apparatus accommodated both structural and molecular control over 3D neurite outgrowth from DRGs in a simple and reproducible manner. One particularly useful aspect of this methodology was that the constructs could be fully assembled directly onto the cell culture insert membranes prior to the addition of live tissue. This meant that fabrication steps that would be otherwise harmful in the presence of neurites, such as chemically crosslinking PR-Dex with AP/TEMED, could still be performed, as cytotoxic reagents are washed out prior to the addition of DRG explants. Additionally, multiple constructs could be fabricated on each cell culture insert, promoting reproducibility, material conservation, and potentially high-throughput means for developing a 3D nerve growth assay.The photoreactive dextran described in this study promoted robust growth of neurites and afforded a means to incorporate molecular cues without altering the integrity of the scaffold. The CNBC moiety of Acryl-PEG-CNBC provided a photocaged site for maleimide-conjugated biomolecules to bind. Using UV light to irradiate PR-Dex in selected regions, proteins could be incorporated into the scaffold in a spatially controlled manner, as UV light liberated the caging moiety of CNBC, thus exposing free thiols for maleimide-protein binding. Confocal imaging confirmed the homogeneous immobilization of protein throughout the depth of PR-Dex, promoting equal exposure of proteins to the extending neurites. Because the biomolecules were covalently bound to the scaffold, the proteins remained in place for the duration of the experiment. This controlled spatial resolution was reinforced by the robust structural integrity of the dual hydrogel construct. Immobilizing cues of varying size onto PR-Dex resulted in protein concentrations in a similar range, indicating that the concentration of free thiols, and thus CNBC moieties, was the governing factor for the immobilized protein concentration. This is an easily exploitable benefit, as the concentration of Acryl-PEG-CNBC in the PR-Dex formula can be altered to change the final immobilized protein concentration. Additionally, because the dynamic photolithography apparatus irradiates substrates with a 3D extrusion of a 2D mask, the UV-light irradiation time may also be adjusted to affect the number of free thiols, thereby affecting the maximum possible concentration of maleimide-proteins. Thus, the photoreactive dextran presented in this study allows for two different methods for controlling the spatial distribution of immobilized proteins, promoting fine control over molecular guidance of cells in this model. The stiffness of PR-Dex may also be adjusted by altering the degree of methacrylation and the weight percent of dextran in the gel in order to accommodate different cell types.
To ensure that the process of immobilizing proteins in the PR-Dex gel did not affect neurite growth, the medium-weight protein NeutrAvidin (60.0 kDa) was chosen as the control, as NA does not elicit any response from neurites. The neurite outgrowth experiments performed in this study resulted in a non-specific response to NA by the neurites. Because neurites are sensitive to structural differences in their in vivo environment, an implicit guidance response would have been observed if the PR-Dex had undergone mechanical changes after UV irradiation or protein immobilization. Since the neurites did not exhibit any preference to either channel, this expected outcome to the control protein suggests that no significant structural changes occur in the PR-Dex gel during protein immobilization.
Both neurotrophin-3 and semaphorin 3A, with respective molecular weights of 13.6 kDa and 114 kDa, were conjugated with maleimide moieties using a commercially available kit without losing their reactivity. Though the relative bioactivity for either NT-3 or Sema3A after maleimide conjugation is not known, the proteins exhibited enough bioactivity to induce responses from the neurites. The response to the maleimide-conjugated cues supports the assumption that any protein with a free amine for maleimide conjugation can be immobilized onto the PR-Dex scaffold, rendering the dual hydrogel construct non-specific to the cell type for molecular guidance by immobilized cues.
Neurite growth from DRG explants extended throughout the depth of the PR-Dex gel and was contained within the PEG boundary. For our model to be an appropriate platform for investigating in vivo processes, it was essential to demonstrate the 3D nature of neurite growth in the dual hydrogel construct. Neurites primarily extended through the bulk of PR-Dex, without concentrating along the interface of the two gels, or along the cell culture membrane. Growth was robust through all planes of PR-Dex, indicating that dextran may be a useful polymer for developing nerve growth assays.
The incorporation of NT-3 and Sema3A into our model elicited predicable responses from DRG neurites. Cervical DRGs were used for this study due to their documented reactivity to NT-3 and Sema3A.10,11,15 In the developing embryo, NT-3 accumulates at the limb buds to direct proprioceptive neurites to their motor neuron targets.15 While NT-3 is traditionally associated with the maintenance of sensory neurons during nervous system mapping, recent studies have observed a chemoattractive response to NT-3 in DRG neurites.9,11 Like most neurotrophins, NT-3 is a soluble factor that can become affinity-immobilized in the extracellular matrix. In our model, we observed a moderate attractive response of DRG neurites to a uniform presentation of covalently-immobilized NT-3. Since DRGs consist of both proprioceptive and nociceptive fibers, it is expected that only a portion of neurites from the DRG explant would possess TrkC receptors to bind NT-3.8,12 Additionally, the response to NT-3 may be more pronounced if NT-3 were presented as a gradient, as other in vitro studies have observed a graded neurite response to changes in the NT-3 concentration from 0 to 500 ng ml−1 (0–36.8 nM).7,11,15,16 Because our model is able to bind over four times the quantity of NT-3 used in other studies, establishing a significant gradient of immobilized protein is very feasible, and has been demonstrated in an earlier iteration of this model.28
Neurites exhibited a strong repulsive response to immobilized Sema3A in our model. The protein Sema3A induces growth cone collapse in DRG axons through actin cytoskeleton depolymerization, and is presented as a transmembrane protein or a secreted guidance cue.1,16,34,35 Some in vitro studies have utilized the repulsive nature of Sema3A as an immobilized cue in concentrations up to 50 nM, with DRG neurite responses to Sema3A increasing as the protein concentration increases.16,20 Here we have presented immobilized Sema3A in a substantial quantity and observed a strong response by cervical DRG neurites, as seen in previous in vitro studies. Neurites extending through all planes of PR-Dex navigated the boundary of the immobilized Sema3A region and redirected toward the channel without Sema3A. This very distinct boundary between the neurite growth and the immobilized cues indicated that the concentration of Sema3A was too high for neurites to overcome, as neurites were unable to grow on the surface of the gel or the cell culture membrane in the Sema3A region. Since the concentration of Sema3A directly affects the neurite response to the protein, neurites were observed to navigate the interface between PR-Dex and PEG where the concentration of Sema3A was assumed to be lower compared to the bulk PR-Dex since Sema3A did not bind to PEG. This behavior also indicates that any unbound and soluble protein is not accumulating at the interface between the two gels, thereby reaffirming that the protein is only present in the regions irradiated by UV light.
The ability to manipulate the structural and molecular presentation of guidance cues independently in this dual hydrogel system broadens the potential applications for cell growth assays. In our model, we observed 3D growth of neurites and elicited in vivo responses in an in vitro environment. Further investigation of the electrophysiological responses of the neurite growth in these substrates will help validate our model as an appropriate platform for neural cell culture assays. Future studies will expand the model for use with other neural cell types and guidance cues.
5. Conclusions
We have developed a 3D model for neurite growth using a novel photoreactive dextran gel and observed predictable responses to relevant immobilized guidance cues. The utility of the photolithography apparatus allowed for control over structural and molecular components of the dual hydrogel construct, as well as for promoting reproducibility of results. The 3D model for neurite growth incorporated a choice point for cervical DRG neurites, and the immobilized chemoattractive and chemorepulsive cues elicited predictable and quantifiable responses. This model system can incorporate a wide range of maleimide-conjugated biomolecules, enhancing the utility of the system with a variety of cell types.Acknowledgements
This research was funded in part by an NSF CAREER Award to MJM (CBET-1055990).References
- M. Tessier-Lavigne and C. S. Goodman, Science, 1996, 274, 1123–1133 CrossRef CAS.
- P. Lotfi, K. Garde, A. K. Chouhan, E. Bengali and M. I. Romero-Ortega, Front Neuroeng, 2011, 4, 11 CAS.
- J. Roy, T. E. Kennedy and S. Costantino, Lab. Chip, 2013, 13, 498–508 RSC.
- L. Aloe, M. L. Rocco, P. Bianchi and L. Manni, J. Transl. Med., 2012, 10, 239 CrossRef CAS PubMed.
- E. J. Huang and L. F. Reichardt, Annu. Rev. Neurosci., 2001, 24, 677–736 CrossRef CAS PubMed.
- M. V. Chao, Nat. Rev. Neurosci., 2003, 4, 299–309 CrossRef CAS PubMed.
- K. Moore, M. MacSween and M. Shoichet, Tissue Eng., 2006, 12, 267–278 CrossRef CAS PubMed.
- X. Cao and M. S. Shoichet, Neuroscience, 2003, 122, 381–389 CrossRef CAS PubMed.
- N. Usui, K. Watanabe, K. Ono, K. Tomita, N. Tamamaki, K. Ikenaka and H. Takebayashi, Development, 2012, 139, 1125–1132 CrossRef CAS PubMed.
- F. Hory-Lee, M. Russell, R. M. Lindsay and E. Frank, Proc. Natl. Acad. Sci. U. S. A., 1993, 90, 2613–2617 CrossRef CAS.
- B. Genc, P. H. Ozdinler, A. E. Mendoza and R. S. Erzurumlu, PLoS Biol., 2004, 2, e403 Search PubMed.
- D. A. Houweling, A. J. Lankhorst, W. H. Gispen, P. R. Bar and E. A. J. Joosten, Exp. Neurol., 1998, 153, 49–59 CrossRef CAS PubMed.
- L. Taylor, L. Jones, M. H. Tuszynski and A. Blesch, J. Neurosci., 2006, 26, 9713–9721 CrossRef CAS PubMed.
- S. Beggs, D. Alvares, A. Moss, G. Currie, J. Middleton, M. W. Salter and M. Fitzgerald, Pain, 2012, 153, 2133–2139 CrossRef CAS PubMed.
- T. Ringstedt, J. Kucera, U. Lendahl, P. Ernfors and C. F. Ibanez, Development, 1997, 124, 2603–2613 CAS.
- E. K. Messersmith, E. D. Leonardo, C. J. Shatz, M. Tessierlavigne, C. S. Goodman and A. L. Kolodkin, Neuron, 1995, 14, 949–959 CrossRef CAS.
- B. Rohm, A. Ottemeyer, M. Lohrum and A. W. Puschel, Mech. Dev., 2000, 93, 95–104 CrossRef CAS.
- F. Polleux, T. Morrow and A. Ghosh, Nature, 2000, 404, 567–573 CrossRef CAS PubMed.
- W. J. Rosoff, J. S. Urbach, M. A. Esrick, R. G. McAllister, L. J. Richards and G. J. Goodhill, Nat. Neurosci., 2004, 7, 678–682 CrossRef CAS PubMed.
- B. Joddar, A. T. Guy, H. Kamiguchi and Y. Ito, Biomaterials, 2013, 34, 9593–9601 CrossRef CAS PubMed.
- R. J. Pasterkamp, R. J. Giger, M. J. Ruitenberg, A. J. G. D. Holtmaat, J. De Wit, F. De Winter and J. Verhaagen, Mol. Cell. Neurosci., 1999, 13, 143–166 CrossRef CAS PubMed.
- R. J. Pasterkamp, P. N. Anderson and J. Verhaagen, Eur. J. Neurosci., 2001, 13, 457–471 CrossRef CAS.
- S. Kaneko, A. Iwanami, M. Nakamura, A. Kishino, K. Kikuchi, S. Shibata, H. J. Okano, T. Ikegami, A. Moriya, O. Konishi, C. Nakayama, K. Kumagai, T. Kimura, Y. Sato, Y. Goshima, M. Taniguchi, M. Ito, Z. G. He, Y. Toyama and H. Okano, Nat. Med., 2006, 12, 1380–1389 CrossRef CAS PubMed.
- C. A. DeForest and K. S. Anseth, Angew. Chem. Int. Ed. Engl., 2012, 51, 1816–1819 CrossRef CAS PubMed.
- K. A. Mosiewicz, L. Kolb, A. J. van der Vlies, M. M. Martino, P. S. Lienemann, J. A. Hubbell, M. Ehrbar and M. P. Lutolf, Nat. Mater., 2013, 12, 1072–1078 CrossRef CAS PubMed.
- R. G. Wylie, S. Ahsan, Y. Aizawa, K. L. Maxwell, C. M. Morshead and M. S. Shoichet, Nat. Mater., 2011, 10, 799–806 CrossRef CAS PubMed.
- Y. Luo and M. S. Shoichet, Biomacromolecules, 2004, 5, 2315–2323 CrossRef CAS PubMed.
- E. L. Horn-Ranney, J. L. Curley, G. C. Catig, R. M. Huval and M. J. Moore, Biomed. Microdevices, 2013, 15, 49–61 CrossRef CAS PubMed.
- Y. Luo and M. S. Shoichet, Nat. Mater., 2004, 3, 249–253 CrossRef CAS PubMed.
- J. L. Curley and M. J. Moore, J. Biomed. Mater. Res., Part A, 2011, 99A, 532–543 CrossRef CAS PubMed.
- S. Z. Zhou, A. Bismarck and J. H. G. Steinke, J. Mater. Chem., 2012, 22, 18824–18829 RSC.
- X. W. Li, E. Katsanevakis, X. Y. Liu, N. Zhang and X. J. Wen, Prog. Polym. Sci., 2012, 37, 1105–1129 CrossRef CAS PubMed.
- K. Shroff, E. L. Rexeisen, M. A. Arunagirinathan and E. Kokkoli, Soft Matter, 2010, 6, 5064–5072 RSC.
- H. Aizawa, S. Wakatsuki, A. Ishii, K. Moriyama, Y. Sasaki, K. Ohashi, Y. Sekine-Aizawa, A. Sehara-Fujisawa, K. Mizuno, Y. Goshima and I. Yahara, Nat. Neurosci., 2001, 4, 367–373 CrossRef CAS PubMed.
- C. X. Li, Y. Sasaki, K. Takei, H. Yamamoto, M. Shouji, Y. Sugiyama, T. Kawakami, F. Nakamura, T. Yagi, T. Ohshima and Y. Goshima, J. Neurosci., 2004, 24, 6161–6170 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |