Stem cell culture using cell-derived substrates
Binata
Joddar
a,
Takashi
Hoshiba
bc,
Guoping
Chen
c and
Yoshihiro
Ito
*ad
aNano Medical Engineering Laboratory, RIKEN, 2-1 Hirosawa, Wako, Saitama 351-0198, Japan. E-mail: y-ito@riken.jp; Fax: +81-48-467-9300; Tel: +81-48-467-5809
bDepartment of Biochemical Engineering, Graduate School of Science and Engineering, Yamagata University, 4-3-16 Jonan, Yonezawa, Yamagata 992-8510, Japan
cTissue Regeneration Materials Unit, International Center for Materials Nanoarchitectonics, National Institute for Materials Science, 1-1 Namiki, Tsukuba, Ibaraki 305-0044, Japan
dEmergent Bioengineering Materials Research Team, RIKEN Center for Emergent Matter Science, 2-1 Hirosawa, Wako, Saitama 351-0198, Japan
First published on 8th August 2014
Abstract
There have been great efforts to develop cell culture systems to regulate stem cell functions. Development of cell culture substrates is one of the important approaches for stem cell culture because substrates influence stem cell functions such as attachment, proliferation, self-renewal, and induction of differentiation. Stem cells are surrounded by their specific microenvironments in vivo, composed of cells, cytokines, and an extracellular matrix (ECM), which may dynamically change and affect cellular activities accordingly. To mimic such microenvironments, cell culture substrates can be prepared by coating bioactive proteins such as ECM proteins and signaling molecules as ligands for cell surface receptors. Compared with protein-coated substrates, cell- and cell-formed ECM-derived substrates have shown great progress and attracted significant attention as functional and prospective biomaterials for stem cell culture and regenerative medicine. In this review, we summarize the latest progress of these new substrates derived from cells and cell-formed ECMs.
1. Introduction
Stem cells are important cell sources for regenerative medicine.1 There have been great efforts to develop cell culture systems to regulate stem cell functions. Development of cell culture substrates is an important approach for stem cell culture because substrates influence stem cell functions such as attachment, proliferation, self-renewal, and induction of differentiation.2,3 Although there are many reports of culture substrates composed of synthetic polymers and proteins, it is difficult to fully control the functions of stem cells.One attractive strategy for the development of substrates to control stem cell functions is mimicking cellular microenvironments, because stem cells are surrounded by their specific microenvironments in vivo. Microenvironments are composed of cells, cytokines, and an extracellular matrix (ECM). As shown in Fig. 1, stem cells interact with their microenvironments, triggering activation of various signals for proliferation, self-renewal, and differentiation. For example, hematopoietic stem cells interact with several cells in their stem cell niche to maintain their stemness.4 Neural stem cells are surrounded by ECM structures called “fractones” to maintain their stemness.5 When stem cells differentiate, the composition of the ECM surrounding the cells is changed to provide appropriate differentiation signals to differentiating cells according to their developmental states.6,7
 | ||
| Fig. 1 The three main interactions between cells and their microenvironments for regulation of cell functions (reproduced with permission from ref. 37). | ||
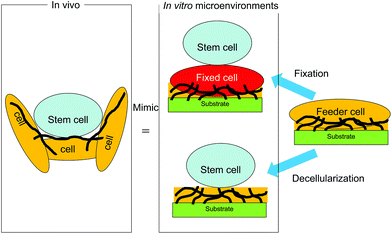
To provide such biomimetic microenvironments to maintain the undifferentiated state of stem cells, cell- and cell-formed ECM-derived substrates have been developed for stem cell culture (Fig. 2). Substrates with a feeder cell layer presenting signaling molecules on the feeder cell surface have been used to culture embryonic stem (ES) cells and induced pluripotent stem (iPS) cells.8,9 In addition to the use of feeder cells, coating of bioactive proteins such as ECM proteins and certain signaling molecules on substrates has been widely applied to construct biomimetic microenvironments for stem cell culture.10–16 The coating method is very convenient and the composition of coated bioactive proteins is controllable. However, it is difficult to reconstitute in vivo microenvironments with synthetic polymers and single/multiple proteins by conventional chemical and physical methods because of the complexity of the ECM composition and the ligands presented by cells. Therefore, cell-formed ECM-derived substrates have been developed to more closely mimic cellular microenvironments in vivo. In this review, the progress of these new substrates derived from cells and cell-formed ECMs for stem cell culture is summarized together with the future prospects.
2. Cell-derived substrates
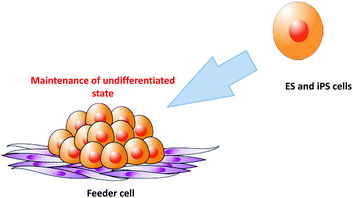
Although numerous approaches have been evaluated for feeder-free culture of stem cells,17–20 co-culture is sometimes used for stem cell culture because it significantly improves the understanding of what actually constitutes a stem cell niche.21 As shown in Fig. 3, ES and iPS cells are generally maintained on a feeder cell layer in vitro.8,9 Mouse-derived cells are the most frequently used feeders to maintain the pluripotency of human ES and iPS cells. Human-derived feeder cells are also used for human ES/iPS culture. However, in some cases, these cells have proven to be unsuitable for stem cell maintenance.22,23 Feeder cells typically need to be growth inactivated prior to use as feeders to prevent their proliferation and contamination of the stem cell culture.24 Growth inactivation is commonly performed by treatment with mitomycin-C or X-ray irradiation.20 These steps for feeder cell preparation require significant time and effort.25 To reduce these steps, chemically cross-linked feeder cells have been used as culture substrates for stem cell culture. Such chemical fixation techniques are gradually being recognized as convenient methods by researchers.26–282.1. Chemically cross-linked cells
Chemically cross-linked cells have been used in fundamental cell biology to reveal the mechanisms by which membrane-associated factors or proteins affect cells.29–32 Higashiyama et al. employed chemically cross-linked donor cells to evaluate the biological effects of the membrane-anchored epidermal growth factor (EGF) on acceptor cells.29 By co-culturing donor cells expressing the pro-heparin-binding epidermal growth factor (pro HB-EGF/DTR) with acceptor cells expressing the EGF receptor, they demonstrated that pro-HB-EGF/DTR stimulated cell growth in a juxtacrine manner.29 The mechanism of cell stimulation by immobilized growth factors has been delineated in studies of intercellular regulation by chemically cross-linked cells that express growth factors or cytokines. For example, Stein et al. demonstrated the biological activity of immobilized colony-stimulating factor-1 (CSF-1) expressed on the surface of fibroblasts.30 After chemical fixation of the fibroblast monolayer, the soluble form of CSF-1 was undetectable in the culture medium. Furthermore, the inability of CSF-1-dependent cells to form colonies on physically scraped areas of tissue culture coverslips suggested that direct cell–cell contact was required for cell stimulation.23 Similarly, Yaeger et al. investigated the keratinocyte growth-promoting activity of chemically cross-linked fibroblast feeder cells on normal human keratinocytes, and found that glutaraldehyde-fixed fibroblast feeder cells promoted keratinocyte growth.31 This growth-promoting effect required contact between fixed fibroblasts and keratinocytes. Moreover, the feeder activity was highly enriched in the plasma membrane fraction of fibroblasts. Therefore, at least some of the fibroblast feeder activity involved a keratinocyte growth-promoting factor that was immobilized on the fibroblast surface. In contrast, direct contact with chemically cross-linked stroma inhibits both the proliferation and survival of long-term culture-initiating cells.32Collectively, these studies demonstrate that chemically cross-linked cells present membrane-associated factors or proteins that affect cells in a juxtacrine manner. Thereafter, this method has been applied to stem cell culture.
2.2. Maintenance of the undifferentiated state of stem cells
Chemically cross-linked feeder cells have been used to maintain the undifferentiated state of stem cells. Lai and Ma demonstrated the feasibility of a glutaraldehyde cross-linked amniotic membrane as a niche to expand and transplant limbal epithelial progenitor cells.33 Roy and Verfaillie used fixed stromal cells as feeders to culture cord blood-derived stem cells. The fixed feeder cells presented immobilized growth factors that affected cellular functions.34 Meissner and colleagues immobilized human stromal cells in porous glass carriers in a fixed-bed reactor and co-cultured human hematopoietic progenitor cells for several weeks.35 After inoculating mononuclear cells derived from umbilical cord blood or peripheral blood stem cells into this fixed-bed reactor, the expansion of early progenitor cells increased by up to four-fold and that of later progenitor cells increased by up to seven-fold.35Ito et al. applied chemically cross-linked human stromal cells to support ex vivo expansion of human cord blood hematopoietic progenitor cells.36,37 In addition, they demonstrated the utility of chemically cross-linked nurse cells as feeders to maintain ES cells in an undifferentiated state.38 In the previous study, mouse and monkey ES cells were grown on chemically fixed mouse embryonic fibroblasts (MEFs) and human amniotic epithelial (HAE) cells, respectively. MEFs were fixed by incubation in glutaraldehyde or formaldehyde solutions. HAE cells were immortalized by transfection with human telomerase reverse transcriptase and chemical fixation with the same reagents. When mouse and monkey ES cells were cultured on these chemically fixed cells, the mouse ES cells proliferated and expressed pluripotency markers including alkaline phosphatase, stage-specific embryonic antigen (SSEA)-1 and -4, and octamer binding protein 3/4 (Oct-3/4). Furthermore, freeze-drying HAE cells and MEFs did not change their ability to support undifferentiated growth of ES cells. Conveniently, the chemically fixed cells could be used repeatedly to culture ES cells.38
As shown in Fig. 4, mouse embryonic fibroblasts (MEFs) cross-linked with formaldehyde (FA-MEFs) or glutaraldehyde (GA-MEFs) have also been used to maintain the pluripotency of mouse iPS cells.39 Chemically fixed MEF feeders maintain both the pluripotency and undifferentiated state of mouse iPS cells and can be re-used several times without a change in their function.39 Currently, efforts are underway to culture human iPS cells on chemically fixed autologous feeder cells (unpublished).
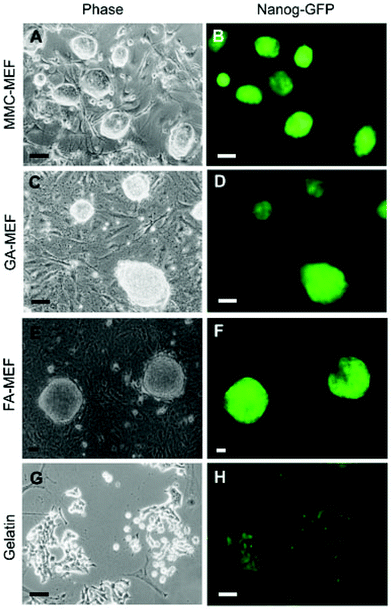 | ||
| Fig. 4 Morphology and Nanog-green fluorescent protein (GFP) expression in mouse-induced pluripotent stem cells cultured on mitomycin C-treated mouse embryonic fibroblasts (MEFs), chemically fixed MEFs, and gelatin-coated substrates (reproduced from ref. 39). MMC-MEFs, GA-MEFs, and FA-MEFs refer to mitomycin C-, glutaraldehyde-, and formaldehyde-treated mouse embryonic fibroblasts, respectively. | ||
2.3. Regulation of stem cell differentiation
Feeder cells can also be used to induce differentiation of ES and iPS cells.40 Chemically cross-linked cells can also induce differentiation of stem cells. The effect of cross-linked cells on differentiation of human ES cells was investigated by Vazin et al.40 Dopaminergic neurons can be differentiated from human ES cells by co-culture with the mouse PA6 stromal cell line. This type of feeder cell-induced differentiation is termed stromal-derived inducing activity (SDIA). Because the exact mechanism of SDIA is unknown, various studies have suggested that SDIA involves either a fixation-resistant component located on the PA6 cell surface or factors secreted into the medium by PA6 cells. To further explore the exact mechanism, the number of differentiated tyrosine hydroxylase (TH)+ cells was reduced by only three-fold by fixation or irradiation of PA6 feeder cells, whereas mitomycin C treatment of PA6 feeder cells reduced the number of TH+ cells by 32%. The neural-inducing effect of PA6 cells, as monitored by β-III-tubulin expression, was minimally affected by mitomycin C treatment or fixation but was reduced to 50% by irradiation. The medium conditioned by PA6 cells was ineffective for inducing differentiation of TH+ cells when used alone. However, such a conditioned medium combined with heparin and/or fixed PA6 cells induced TH+ cell differentiation, although less effectively than that of PA6 cell co-culture. Therefore, it was concluded that PA6 cell surface activity is required for neural differentiation of human ES cells, while secreted factors are required for the specific dopaminergic neuron-inducing effect.40Recently, Lee et al. reported a technique to transfer the complex membrane surface of glutaraldehyde-fixed human blood mononucleated cells to a polydimethylsiloxane (PDMS) substrate using an intermediate poly(vinyl alcohol) film as a transporter system, and demonstrated the bioactivity of the transferred membranes.41 PDMS display of cell membranes is indistinguishable from fixed mononuclear blood cells in terms of morphology and biological activity to support human leukemic cell adhesion.41 This observation further implies that, instead of live feeders, chemically cross-linked cells with their quintessential membrane proteins and growth factors immobilized on their surfaces can be used to culture stem cells in vitro.
3. Cell-formed ECM-derived substrates
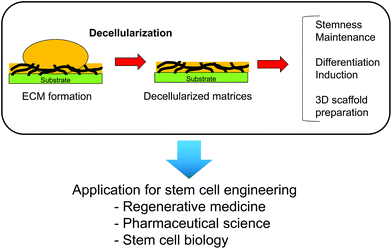
Similar to chemically cross-linked, cell-derived substrates, cell-formed ECM is used as a substrate after removal of the cellular components or decellularization treatment. Such decellularized ECMs are important for tissue engineering because they retain the complex ECM composition to present essential information to the cells. There have been many efforts to apply decellularized ECMs for the reconstruction of tissues such as the heart,42 lung,43,44 bladder,45,46 tendons,47 and liver.48,49 Other applications of decellularized ECMs are well reviewed elsewhere.50,51 Generally, a decellularized ECM is obtained from tissues by removal of the cellular components. Tissue-derived decellularized ECMs are used in many formats such as three-dimensional architectures,43,44,46,49 patch-type,47 coating-type,52 and injectable gels.45,53 Thus, tissue-derived decellularized ECMs are useful tools for tissue engineering and regenerative medicine. However, it remains difficult to apply a tissue-derived ECM in large-scale cell culture because of the limited sources of tissue-derived ECMs. Moreover, it is difficult to obtain the correct ECM surrounding stem cells by decellularization of whole or minced tissues because the ECM surrounding stem cells is located in a limited space. Cells cultured in vitro secrete ECM proteins and deposit them beneath the cells. Similar to decellularized ECMs derived from tissues, these deposited ECM proteins can be used as cell culture substrates after decellularization.54 In this section, we reviewed cell-derived ECMs to both maintain the undifferentiated state and enhance the differentiation induction of stem cells.3.1. Preparation methods for decellularized ECMs
There are review articles that summarize the preparation methods for a decellularized ECM and highlight the dependence of ECM components and structures on such methods.55,56 Therefore, the preparation methods should be carefully chosen to meet specific purposes. Moreover, a decellularized ECM is often treated with chemical cross-linking agents to stabilize its structure and composition. For chemical cross-linking of the ECM, it is commonly treated with glutaraldehyde, carbodiimide, or photo-oxidizing agents. As an alternative to chemical cross-linking reagents, natural products have been used to cross-link decellularized ECMs. Jiang et al. employed genipin, a naturally occurring cross-linker derived from the fruit of the gardenia plant (Gardenia jasminoides).57 They prepared decellularized fresh rat spinal cords by cross-linking them with genipin. Compared with a decellularized spinal cord ECM cross-linked by glutaraldehyde, the genipin-cross-linked ECM demonstrated good biocompatibility while maintaining similar structural stability and mechanical properties to those of glutaraldehyde-cross-linked ECM.Quercetin, a naturally occurring polyphenolic flavonol, has also been used as a natural cross-linker. Zhai et al. applied quercetin to cross-link porcine heart valve ECM and evaluated its mechanical properties, stability, anti-calcification effect, and cytocompatibility.58 Their results showed that the tensile strength of a quercetin-cross-linked ECM was higher than that of glutaraldehyde-cross-linked ECM. After cross-linking with quercetin, there was also a clear increase in the thermal denaturation temperature of the ECM. The quercetin-cross-linked ECM could be stored in a buffer solution for at least 30 days without any loss of tensile strength and elasticity. Cell culture experiments showed no inhibition of the proliferation of vascular endothelial cells. Furthermore, in vitro anti-calcification experiments showed that quercetin cross-linking could protect the ECM from deposition of minerals in a simulated body fluid.
In contrast to the improvement of the stability and mechanical properties of cross-linked decellularized ECMs, cross-linking may induce a host immune response and decrease the biocompatibility of the cross-linked ECM. Cross-linking may also influence the function of cross-linked ECM. Therefore, to prepare a decellularized ECM, the need for cross-linking and selection of an appropriate cross-linker should be considered in addition to the decellularization method.
3.2. Maintenance of the undifferentiated state of stem cells
Because the ECM has been reported to play important roles in maintenance of the undifferentiated state of stem cells,59,60 decellularized ECMs have been used for feeder cell-free culture of stem cells. Klimanskaya et al. prepared a decellularized ECM derived from MEFs to maintain human ES cells in serum-free medium.61 The human ES cells cultured on the decellularized ECM maintained a normal karyotype and expression of stem cell markers such as OCT-4, SSEA-3, SSEA-4, tumor-rejection antigen (TRA)-1-60, TRA-1-81, and alkaline phosphatase. The ES cells retained the potential to differentiate into all three embryonic germ layers in both in vitro culture and in vivo teratoma formation, even after more than 6 months of culture. Moreover, this decellularized ECM can be used to generate new human ES cell lines, which is similar to the conventional feeder cell-based method.In addition to the maintenance of ES cells, decellularized ECMs have been reported as substrates to maintain other stem cells. Prewitz et al. prepared a decellularized ECM derived from mesenchymal stem cells (MSCs) undergoing osteogenesis and applied the ECM to in vitro hematopoietic stem and progenitor cell (HSPC) culture.62 The cultured human peripheral blood CD34+ HSPCs showed clustering and attachment to the decellularized ECM. In addition, the HSPCs showed high proliferation on the decellularized ECM compared with that on conventional and fibronectin-coated culture plates. Transplantation of the HSPCs into mice demonstrated that the HSPCs engrafted and differentiated into CD3+ T cells, CD19+ B cells, and CD33+ myeloid hematopoietic cells. These results indicated that the decellularized ECM was applicable to in vitro expansion of HSPCs while maintaining their undifferentiated states.
MSCs are a promising cell source for regenerative medicine of cartilage, bone, and adipose tissues because they can differentiate into chondrocytes, osteoblasts, and adipocytes. However, these differentiation properties decrease during culture.63 This limitation of cultured MSCs is a major barrier for their application to tissue regeneration.64 Chen and colleagues attempted to prevent the decline in the differentiation abilities of MSCs by culturing them on a decellularized ECM derived from MSCs. The MSCs proliferated on this decellularized ECM and maintained their differentiation properties, even up to passage 10 in culture. It is also generally accepted that MSCs gradually lose their differentiation properties during aging. The differentiation properties of aged MSCs can be recovered by culturing them on a decellularized ECM derived from MSCs isolated from young MSCs.64
Spontaneous differentiation of MSCs can be prevented on a decellularized ECM derived from MSCs by suppression of bone morphogenetic protein (BMP) signaling.65 In addition, the osteogenesis and adipogenesis of MSCs are suppressed on such decellularized ECMs, even in differentiation induction media.66 On a decellularized ECM derived from MSCs, Wnt/β-catenin signaling, which inhibits osteogenesis and adipogenesis, is strongly activated by Wnt ligands bound to the chondroitin sulfate chains in the decellularized ECM.67 It is likely that the decellularized ECM derived from MSCs possesses the ability to maintain the undifferentiated state of MSCs. Therefore, such decellularized ECMs may be useful substrates to culture stem cells in an undifferentiated state.
3.3. Regulation of stem cell differentiation
ECM components also play important roles in stem cell differentiation.2,3 Decellularized ECMs can be readily adapted to study ECM environments with complex compositions by culturing different cell types. Therefore, decellularized ECMs have been studied as substrates for stem cell differentiation.An artificial basement membrane prepared by decellularization has been applied to differentiate ES and iPS cells into pancreatic β cells.68 The artificial basement membrane was prepared from a culture of HEK293 cells that stably expressed laminin-511. The HEK293 cells were cultured for deposition of basement membrane components, and then the cells were removed from the substrate to prepare the artificial basement membrane. Subsequently, mouse ES or iPS cells were seeded on the basement membrane, and the cells were sequentially differentiated into definitive endoderm, pancreatic progenitor cells, and then insulin-secreting pancreatic β cells.
Similar to ES/iPS cell differentiation culture, a decellularized ECM can be applied to differentiation of MSCs. Cheng et al. prepared a decellularized ECM derived from chondrocytes cultured in collagen microspheres. The chondrogenesis of MSCs was well supported in these decellularized microspheres.69 Choi et al. prepared a decellularized ECM derived from chondrocytes in pellet culture.70 MSCs were cultured in this three-dimensional scaffold that facilitated chondrogenesis compared with that in polyglycolic acid scaffolds.
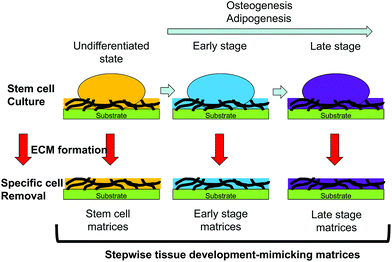
Decellularized ECMs can be prepared by culture of developmentally mature cells. However, the composition of the ECM surrounding differentiating cells is altered according to their developmental stages.6,7 ECMs surrounding cells that are developmentally immature may facilitate stem cell differentiation. Substrates mimicking the ECM surrounding developmentally immature cells can be prepared by culture of differentiating cells in vitro and decellularization techniques. Hoshiba et al. reported a decellularized ECM mimicking the in vivo ECM surrounding MSCs undergoing osteogenesis or adipogenesis at each developmental stage (Fig. 5).71–73 The MSCs were cultured in vitro under osteogenic or adipogenic conditions to obtain cells at different developmental stages. After obtaining cells expressing marker genes of the expected developmental stages, the cellular components were removed from the substrates to obtain decellularized ECMs. The ECMs derived from cells undergoing osteogenesis or adipogenesis are called “stepwise osteogenesis-mimicking matrices” and “stepwise adipogenesis-mimicking matrices”, respectively.
Such matrices support the attachment and proliferation of MSCs. The osteogenesis of MSCs is promoted on a substrate mimicking the ECM surrounding cells at the early stage of osteogenesis (osteogenic early stage matrices).66 Conversely, the adipogenesis of MSCs is promoted on a substrate mimicking the ECM surrounding the cells at the early stage of adipogenesis (adipogenic early stage matrices).72 This regulation of MSC differentiation is mediated by regulation of transcription factor expression. When MSCs undergo osteogenesis, osteogenic early-stage matrices activate BMP signaling to express RUNX2, a bone-related transcription factor.66 Moreover, osteogenic early-stage matrices activate Wnt/β-catenin signaling through Wnt ligands bound to chondroitin sulfate chains.67 The Wnt/β-catenin signaling suppresses PPARG expression, which induces osteogenesis of MSCs on osteogenic early-stage matrices. Transcription factor regulation also promotes adipogenesis on adipogenic early stage matrices. The expression of RUNX2, a transcription factor that facilitates osteogenesis and inhibits adipogenesis, is suppressed by adipogenic early-stage matrices. In addition to RUNX2 suppression, TAZ and MSX2, which act as suppressors of adipogenesis, are suppressed on adipogenic early-stage matrices. These results suggest that tissue- and stage-specific ECMs can control the differentiation of MSCs into specific cell types.71 Decellularized ECMs prepared by culture of differentiating cells is an effective approach to present tissue- and stage-specific ECMs to stem cells to regulate their differentiation. Moreover, stepwise tissue development-mimicking matrices may be useful as in vitro models to investigate ECM–cell interactions and elucidate the roles of the ECM in tissue development.
Decellularized ECMs can be easily applied as three-dimensional scaffolds. Lu et al. prepared decellularized ECM scaffolds from MSCs, chondrocytes, and fibroblasts by culturing the cells in a poly (lactic-co-glycolic acid) (PLGA) scaffold.73 After formation of the cell-derived ECMs, the PLGA scaffold and cellular components were removed from the constructs to obtain three-dimensional decellularized ECMs that can be applied to MSC culture. Similar to these decellularized ECMs, a decellularized ECM derived from MSC-derived osteoblasts has been prepared on a titanium mesh.74 Three-dimensional scaffolds can be prepared by cell culture in certain scaffolds as molds and subsequently applied to tissue regeneration. Therefore, cell-derived ECMs and scaffolds can mimic the ECM microenvironment in vivo and are useful to control stem cell functions for applications in regenerative medicine (Fig. 6).
4. Conclusions and outlook
Regulation of stem cell functions is an important issue in regenerative medicine. Mimicking the in vivo extracellular microenvironments surrounding stem cells is an effective approach to regulate stem cell functions by preparation of functional substrates. Although protein coating and immobilization are useful tools to create such microenvironments,75,76 cell- and ECM-derived substrates show significant advantages. On substrates derived from chemically cross-linked cells or cell-formed ECMs, stem cells can either maintain their undifferentiated state or differentiate into a specific lineage. These methods can be further expanded to prepare any desirable ECM and scaffold by co-culturing two or more cell types. Therefore, the preparation of substrates using cultured cells to mimic in vivo extracellular microenvironments surrounding stem cells is beneficial for stem cell research and tissue regeneration.Acknowledgements
This review was supported by JSPS KAKENHI (grant number: 22220009).References
- S. M. Wu and K. Hochedlinger, Harnessing the potential of induced pluripotent stem cells for regenerative medicine, Nat. Cell Biol., 2011, 13(5), 497–505 CrossRef CAS PubMed.
- M. P. Lutolf, P. M. Gilbert and H. M. Blau, Designing materials to direct stem-cell fate, Nature, 2009, 462(7272), 433–441 CrossRef CAS PubMed.
- D. E. Discher, D. J. Mooney and P. W. Zandstra, Growth factors, matrices, and forces combine and control stem cells, Science, 2009, 324(5935), 1673–1677 CrossRef CAS PubMed.
- K. A. Moore and I. R. Lemischka, Stem cells and their niches, Science, 2006, 311(5769), 1880–1885 CrossRef CAS PubMed.
- A. Kerever, J. Schnack, D. Vellinga, N. Ichikawa, C. Moon, E. Arikawa-Hirasawa, J. T. Efird and F. Mercier, Novel extracellular matrix structures in the neural stem cell niche capture the neurogenic factor fibroblast growth factor 2 from the extracellular milieu, Stem Cells, 2007, 25(9), 2146–2157 CrossRef CAS PubMed.
- M. Nakamura, S. Sone, I. Takahashi, I. Mizoguchi, S. Echigo and Y. Sasano, Expression of versican and ADAMTS1, 4, and 5 during bone development in the rat mandible and hind limb, J. Histochem. Cytochem., 2005, 53(12), 1553–1562 CrossRef CAS PubMed.
- Y. Sasano, H. C. Li, J. X. Zhu, K. Imanaka-Yoshida, I. Mizoguchi and M. Kagayama, Immunohistochemical localization of type I collagen, fibronectin and tenascin C during embryonic osteogenesis in the dentary of mandibles and tibias in rats, Histochem. J., 2000, 32(10), 591–598 CrossRef CAS.
- K. Takahashi, K. Tanabe, M. Ohnuki, M. Narita, T. Ichisaka, K. Tomoda and S. Yamanaka, Induction of pluripotent stem cells from adult human fibroblasts by defined factors, Cell, 2007, 131(5), 861–872 CrossRef CAS PubMed.
- J. A. Thomson, J. Itskovitz-Eldor, S. S. Shapiro, M. A. Waknitz, J. J. Swiergiel, V. S. Marshall and J. M. Jones, Embryonic stem cell lines derived from human blastocysts, Science, 1998, 282(5391), 1145–1147 CrossRef CAS.
- A. M. Moursi, C. H. Damsky, J. Lull, D. Zimmerman, S. B. Doty, S. Aota and R. K. Globus, Fibronectin regulates calvarial osteoblast differentiation, J. Cell Sci., 1996, 109(6), 1369–1380 CAS.
- X.-S. Yue, Y. Murakami, T. Tamai, M. Nagaoka, C. S. Cho, Y. Ito and T. Akaike, A fusion protein N-cadherin-Fc as an artificial extracellular matrix surface for maintenance of stem cell features, Biomaterials, 2010, 31(20), 5287–5296 CrossRef CAS PubMed.
- M. Nagaoka, Y. Hagiwara, K. Takemura, Y. Murakami, J. Li, S. A. Duncan and T. Akaike, Design of the artificial acellular feeder layer for the efficient propagation of mouse embryonic stem cells, J. Biol. Chem., 2008, 283(39), 26468–26476 CrossRef CAS PubMed.
- P. C. Dingal and D. E. Discher, Combining insoluble and soluble factors to steer stem cell fate, Nat. Mater., 2014, 13, 532–537 CrossRef CAS PubMed.
- W. L. Murphy, T. C. McDevitt and A. J. Engler, Materials as stem cell regulators, Nat. Mater., 2014, 13, 547–557 CrossRef CAS PubMed.
- A. D. Celiz, J. G. W. Smith, R. Langer, D. G. Anderson, D. A. Winkler, D. A. Barrett, M. C. Davies, L. E. Young, C. Denning and M. R. Alexander, Materials for stem cell factories of the future, Nat. Mater., 2014, 13, 570–579 CrossRef CAS PubMed.
- A. D. Celiz, J. G. W. Smith, A. K. Patel, R. Langer, D. G. Anderson, D. A. Barrett, L. E. Young, M. C. Davies, C. Denning and M. R. Alexander, Chemically diverse polymer microarrays and high throughput surface characterisation: a method for discovery of materials for stem cell culture, Biomater. Sci., 2014 10.1039/C4BM00054D.
- Y. Hasegawa, D. Tang, N. Takahashi, Y. Hayashizaki, A. R. R. Forrest, the FANTOM consortium and H. Suzuki, CCL2 enhances pluripotency of human induced pluripotent stem cells by activating hypoxia related genes, Sci. Rep., 2014, 4, 5228 Search PubMed.
- J. Lin, I. Fernandez and K. Roy, Development of feeder-free culture systems for generation of ckit+sca1+ progenitors from mouse iPS cells, Stem Cell Rev., 2011, 7(3), 736–747 CrossRef CAS PubMed.
- H. Fukusumi, T. Shofuda, D. Kanematsu, A. Yamamoto, H. Suemizu, M. Nakamura, M. Yamasaki, M. Ohgushi, Y. Sasai and Y. Kanemura, Feeder-free generation and long-term culture of human induced pluripotent stem cells using pericellular matrix of decidua derived mesenchymal cells, PLoS One, 2013, 8(1), e55226 CAS.
- C. Tamm, S. P. Galitó and C. Annerén, A comparative study of protocols for mouse embryonic stem cell culturing, PLoS One, 2013, 8(12), e81156 Search PubMed.
- Y. Sun, C. S. Chen and J. Fu, Forcing stem cells to behave: A biophysical perspective of the cellular microenvironment, Ann. Rev. Biophys., 2012, 41, 519–542 CrossRef PubMed.
- K. Takahashi, K. Takahashi, M. Narita, M. Yokura, T. Ichisaka and S. Yamanaka, Human induced pluripotent stem cells on autologous feeders, PLoS One, 2009, 4(12), e8067 Search PubMed.
- I. Rodríguez-Pizà, Y. Richaud-Patin, R. Vassena, F. González, M. J. Barrero, A. Veiga, A. Raya and J. C. Izpisúa Belmonte, Reprogramming of Human Fibroblasts to Induced Pluripotent Stem Cells under Xeno-free Conditions, Stem Cells, 2010, 28(1), 36–44 Search PubMed.
- M. Richards, C. Y. Fong, W. K. Chan, P. C. Wong and A. Bongso, Human feeders support prolonged undifferentiated growth of human inner cell masses and embryonic stem cells, Nat. Biotechnol., 2002, 20(9), 933–936 CrossRef CAS PubMed.
- C. Xu, M. S. Inokuma, J. Denham, K. Golds, P. Kundu, J. D. Gold and M. K. Carpenter, Feeder-free growth of undifferentiated human embryonic stem cells, Nat. Biotechnol., 2001, 19(10), 971–974 CrossRef CAS PubMed.
- M. R. Zonca Jr. and Y. Xie, Chemically modified micro- and nanostructured systems for pluripotent stem cell culture, BioNanoSci., 2012, 2, 287–304 CrossRef.
- J. W. Lambshead, L. Meagher, C. O'Brien and A. L. Laslett, Defining synthetic surfaces for human pluripotent stem cell culture, Cell Regen., 2013, 2, 7 CrossRef.
- A. Perestrelo, A. Grenha, A. Rosa da Costa and J. Belo, Locust bean gum as an alternative polymeric coating for embryonic stem cell culture, Mater. Sci. Eng., C, 2014, 40, 336, DOI:10.1016/j.msec.2014.04.022.
- S. Higashiyama, R. Iwamoto, K. Goishi, G. Raab, N. Taniguchi, M. Klagsbrun and E. Mekada, The membrane protein CD9/DRAP 27 potentiates the juxtacrine growth factor activity of the membrane-anchored heparin-binding EGF-like growth factor, J. Cell Biol., 1995, 128(5), 929–938 CrossRef CAS.
- J. Stein, G. V. Borzillo and C. W. Rettenmier, Direct stimulation of cells expressing receptors for macrophage colony-stimulating factor (CSF-1) by a plasma membrane-bound precursor of human CSF-1, Blood, 1990, 76(7), 1308–1314 CAS.
- C. Verfaillie and P. Catanzaro, Direct contact with stroma inhibits proliferation of human long-term culture initiating cells, Leukemia, 1996, 10(3), 498 CAS.
- P. C. Yaeger, C. D. Stiles and B. J. Rollins, Human keratinocyte growth promoting activity on the surface of fibroblasts, J. Cell. Physiol., 1991, 149(1), 110–116 CrossRef CAS PubMed.
- J. Lai and D. Ma, Glutaraldehyde cross-linking of amniotic membranes affects their nanofibrous structures and limbal epithelial cell culture characteristics, Int. J. Nanomed., 2013, 8(1), 4157–4168 CrossRef PubMed.
- V. Roy and C. M. Verfaillie, Soluble factor (s) produced by adult bone marrow stroma inhibit in vitro proliferation and differentiation of fetal liver BFU-E by inducing apoptosis, J. Clin. Invest., 1997, 100(4), 912 CrossRef CAS PubMed.
- P. Meissner, B. Schröder, C. Herfurth and M. Biselli, Development of a fixed bed bioreactor for the expansion of human hematopoietic progenitor cells, Cytotechnology, 1999, 30(1–3), 227–234 CrossRef CAS.
- Y. Ito, H. Hasuda, T. Kitajima and T. Kiyono, Ex vivo expansion of human cord blood hematopoietic progenitor cells using glutaraldehyde-fixed human bone marrow stromal cells, J. Biosci. Bioeng., 2006, 102(5), 467–469 CrossRef CAS PubMed.
- B. Joddar and Y. Ito, Artificial niche substrates for embryonic and induced pluripotent stem cell cultures, J. Biotechnol., 2013, 168, 218–228 CrossRef CAS PubMed.
- Y. Ito, M. Kawamorita, T. Yamabe, T. Kiyono and K. Miyamoto, Chemically fixed nurse cells for culturing murine or primate embryonic stem cells, J. Biosci. Bioeng., 2007, 103(2), 113–121 CrossRef CAS PubMed.
- X.-S. Yue, M. Fujishiro, C. Nishioka, T. Arai, E. Takahashi, J. S. Gong, T. Akaike and Y. Ito, Feeder cells support the culture of induced pluripotent stem cells even after chemical fixation, PLoS One, 2012, 7(3), e32707 CAS.
- T. Vazin, J. Chen, C. T. Lee, R. Amable and W. J. Freed, Assessment of stromal derived inducing activity in the generation of dopaminergic neurons from human embryonic stem cells, Stem Cells, 2008, 26(6), 1517–1525 CrossRef PubMed.
- J. Lee, J. B. Wang, F. Bersani and B. Parekkadan, Capture and printing of fixed stromal cell membranes for bioactive display on PDMS surfaces, Langmuir, 2013, 29(34), 10611–10616 CrossRef CAS PubMed.
- H. C. Ott, T. S. Matthiesen, S.-K. Goh, L. D. Black, S. M. Kren, T. I. Netoff and D. A. Taylor, Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart, Nat. Med., 2008, 14(2), 213–221 CrossRef CAS PubMed.
- H. C. Ott, B. Clippinger, C. Conrad, C. Schuetz, I. Pomerantseva, L. Ikonomou, D. Kotton and J. P. Vacanti, Regeneration and orthotopic transplantation of a bioartificial lung, Nat. Med., 2010, 16(8), 927–933 CrossRef CAS PubMed.
- A. P. Price, K. A. England, A. M. Matson, B. R. Blazar and A. Panoskaltsis-Mortari, Development of a decellularized lung bioreactor system for bioengineering the lung: The matrix reloaded, Tissue Eng., Part A, 2010, 16(8), 2581–2591 CrossRef CAS PubMed.
- D. O. Freytes, J. Martin, S. S. Velankar, A. S. Lee and S. F. Badylak, Preparation and rheological characterization of a gel form of the porcine urinary bladder matrix, Biomaterials, 2008, 29, 1630–1637 CrossRef CAS PubMed.
- L. Song, Bladder acellular matrix and its application in bladder augmentation, Tissue Eng., Part B, 2014, 20(2), 163–172 CrossRef CAS PubMed.
- G. Yang, B. B. Rothrauffa, H. Lina, R. Gottardia, P. G. Alexandera and R. S. Tuan, Enhancement of tenogenic differentiation of human adipose stem cells by tendon-derived extracellular matrix, Biomaterials, 2013, 34, 9295–9306 CrossRef CAS PubMed.
- P. Lin, Assessing porcine liver-derived biomatrix for hepatic tissue engineering, Tissue Eng., 2004, 10(7–8), 1046–1053 CrossRef CAS PubMed.
- B. E. Uygun, A. Soto-Gutierrez, H. Yagi, M. L. Izamis, M. A. Guzzardi, C. Shulman, J. Milwid, N. Kobayashi, A. Tilles, F. Berthiaume, M. Hertl, Y. Nahmias, M. L. Yarmush and K. Uygun, Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix, Nat. Med., 2010, 16(7), 814–820 CrossRef CAS PubMed.
- T. Hoshiba, H. Lu, N. Kawazoe and G. Chen, Decellularized matrices for tissue engineering, Expert Opin. Biol. Ther., 2010, 10(12), 1717–1728 CrossRef CAS PubMed.
- S. F. Badylak, The extracellular matrix as a biologic scaffold materials, Biomaterials, 2007, 28, 3587–3593 CrossRef CAS PubMed.
- M. Rojkind, Z. Gatmaitan, S. Mackensen, M. A. Giambrone, P. Ponce and L. M. Reid, Connective tissue biomatrix: Its isolation and utilization for long-term cultures of normal rat hepatocytes, J. Cell Biol., 1980, 87, 255–263 CrossRef CAS.
- J. M. Singelyn and K. L. Christman, Injectable materials for the treatment of myocardial infarction and heart failure: The promise of decellularized matrices, J. Cardiovasc. Trans. Res., 2010, 3, 478–486 CrossRef PubMed.
- M. L. Kutys, et al., Regulation of cell adhesion and migration by cell-derived matrices, Exp. Cell Res., 2013, 319, 2434–2439 CrossRef CAS PubMed.
- T. W. Gilbert, T. L. Sellaro and S. F. Badylak, Decellularization of tissues and organs, Biomaterials, 2006, 27(19), 3675–3683 CAS.
- H. Lu, T. Hoshiba, N. Kawazoe and G. Chen, Comparison of decellularization techniques for preparation of extracellular matrix scaffolds derived from three – dimensional cell culture, J. Biomed. Mater. Res., Part A, 2012, 100(9), 2507–2516 Search PubMed.
- T. Jiang, X. J. Ren, J. L. Tang, H. Yin, K. J. Wang and C. L. Zhou, Preparation and characterization of genipin-crosslinked rat acellular spinal cord scaffolds, Mater. Sci. Eng., C, 2013, 33(6), 3514–3521 CrossRef CAS PubMed.
- W. Zhai, X. Lü, J. Chang, Y. Zhou and H. Zhang, Quercetin-crosslinked porcine heart valve matrix: mechanical properties, stability, anticalcification and cytocompatibility, Acta Biomater., 2010, 6(2), 389–395 CrossRef CAS PubMed.
- D. T. Scadden, The stem-cell niche as an entity of action, Nature, 2006, 441(7097), 1075–1079 CrossRef CAS PubMed.
- Y. Hayashi, M. K. Furue, T. Okamoto, K. Ohnuma, Y. Myoishi, Y. Fukuhara, T. Abe, J. D. Sato, R. Hata and M. Asashima, Integrins regulate mouse embryonic stem cell self – renewal, Stem Cells, 2007, 25(12), 3005–3015 CrossRef CAS PubMed.
- I. Klimanskaya, Y. Chung, L. Meisner, J. Johnson, M. D. West and R. Lanza, Human embryonic stem cells derived without feeder cells, Lancet, 2005, 365(9471), 1636–1641 CrossRef CAS.
- M. C. Prewitz, F. P. Seib, M. von Bonin, J. Friedrichs, A. Stißel, C. Niehage, K. Müller, K. Anastassiadis, C. Waskow, B. Hoflack, M. Bornhäuser and C. Werner, Tightly anchored tissue-mimetic matrices as instructive stem cell microenvironments, Nat. Methods, 2013, 10, 788–794 CrossRef CAS PubMed.
- Y. Lai, Y. Sun, C. M. Skinner, E. L. Son, Z. Lu, R. S. Tuan, R. L. Jilka, J. Ling and X. D. Chen, Reconstitution of marrow-derived extracellular matrix ex vivo: a robust culture system for expanding large-scale highly functional human mesenchymal stem cells, Stem Cells Dev., 2010, 19(7), 1095–1107 CrossRef CAS PubMed.
- Y. Sun, W. Li, Z. Lu, R. Chen, J. Ling, Q. Ran, R. L. Jilka and X. D. Chen, Rescuing replication and osteogenesis of aged mesenchymal stem cells by exposure to a young extracellular matrix, FASEB J., 2011, 25(5), 1474–1485 CrossRef CAS PubMed.
- X.-D. Chen, V. Dusevich, J. Q. Feng, S. C. Manolagas and R. L. Jilka, Extracellular matrix made by bone marrow cells facilitates expansion of marrow-derived mesenchymal progenitor cells and prevents their differentiation into osteoblasts, J. Bone Miner. Res., 2007, 22(12), 1943–1956 CrossRef CAS PubMed.
- T. Hoshiba, N. Kawazoe, T. Tateishi and G. Chen, Development of stepwise osteogenesis-mimicking matrices for the regulation of mesenchymal stem cell functions, J. Biol. Chem., 2009, 284(45), 31164–31173 CrossRef CAS PubMed.
- T. Hoshiba, N. Kawazoe and G. Chen, Mechanism of regulation of PPARG expression of mesenchymal stem cells by osteogenesis-mimicking extracellular matrices, Biosci., Biotechnol., Biochem., 2010, 75(11), 2099–2104 CrossRef PubMed.
- Y. Higuchi, N. Shiraki, K. Yamane, Z. Qin, K. Mochitate, K. Araki, T. Senokuchi, K. Yamagata, M. Hara, K. Kume and S. Kume, Synthesized basement membranes direct the differentiation of mouse embryonic stem cells into pancreatic lineages, J. Cell Sci., 2010, 123(16), 2733–2742 CrossRef CAS PubMed.
- H.-W. Cheng, Y. K. Tsui, K. M. Cheung, D. Chan and B. P. Chan, Decellularization of chondrocyte-encapsulated collagen microspheres: a three-dimensional model to study the effects of acellular matrix on stem cell fate, Tissue Eng. Pt C-Meth., 2009, 15(4), 697–706 CrossRef CAS PubMed.
- K.-H. Choi, B. H. Choi, S. R. Park, B. J. Kim and B. H. Min, The chondrogenic differentiation of mesenchymal stem cells on an extracellular matrix scaffold derived from porcine chondrocytes, Biomaterials, 2010, 31(20), 5355–5365 CrossRef CAS PubMed.
- T. Hoshiba, N. Kawazoe and G. Chen, The balance of osteogenic and adipogenic differentiation in human mesenchymal stem cells by matrices that mimic stepwise tissue development, Biomaterials, 2012, 33(7), 2025–2031 CrossRef CAS PubMed.
- T. Hoshiba, N. Kawazoe, T. Tateishi and G. Chen, Development of extracellular matrices mimicking stepwise adipogenesis of mesenchymal stem cells, Adv. Mater., 2010, 22(28), 3042–3047 CrossRef CAS PubMed.
- H. Lu, T. Hoshiba, N. Kawazoe, I. Koda, M. Song and G. Chen, Cultured cell-derived extracellular matrix scaffolds for tissue engineering, Biomaterials, 2011, 32(36), 9658–9666 CrossRef CAS PubMed.
- N. Datta, H. L. Holtorf, V. I. Sikavitsas, J. A. Jansen and A. G. Mikos, Effect of bone extracellular matrix synthesized in vitro on the osteoblastic differentiation of marrow stromal cells, Biomaterials, 2005, 26(9), 971–977 CrossRef CAS PubMed.
- Y. Ito, Covalently immobilized biosignal molecule materials for tissue engineering, Soft Matter, 2008, 4(1), 46–56 RSC.
- B. Joddar and Y. Ito, Biological modifications of materials surfaces with proteins for regenerative medicine, J. Mater. Chem., 2011, 21(36), 13737–13755 RSC.
| This journal is © The Royal Society of Chemistry 2014 |