The development, characterization, and cellular response of a novel electroactive nanostructured composite for electrical stimulation of neural cells
D.
Depan
and
R. D. K.
Misra
*
Biomaterials and Biomedical Engineering Research Laboratory, Center for Structural and Functional Materials, University of Louisiana at Lafayette, P.O. Box 44130, Lafayette, LA 70504, USA. E-mail: dmisra@louisiana.edu; Fax: +1-337-482-1220; Tel: +1-337-482-6430
First published on 22nd July 2014
Abstract
Electrical stimulation is a primary method to repair or restore neurological functions. In this regard, we describe the underlying strategy, chemical/structural design, and gifted attributes of a novel nanostructured electroactive composite electrode comprising organic (highly conducting polymer) and inorganic (carbon nanotubes) components. The electrode was characterized in terms of electrical properties, structure and neural cell response. First, the electrical probe is robust and characterized by low impedance, high charge injection capacity, and is biocompatible to facilitate cellular interactions. Second, the combination of in vitro experiments, fluorescence, and electron microscopy of electrically stimulated cells (NB-39-Nu human neuroblastoma cells) at different applied electric field strengths demonstrated that the electrochemically synthesized hybrid nanostructured coating is conducive to neurite growth. Third, the study on the effects of electrical field strength on protein synthesis and mechanism implied cell-to-cell communication based on the change in the regulation of endogenous cytoskeletal proteins, actin, vinculin, and fibronection involving cell mobility and cell adhesion. The cell-to-cell communication is of significant relevance to functional neuronal circuits in cell translational therapy. Taken together, the results also point to the potential of nanostructured composites for use as substrates to modulate cellular activity via electrical stimulation.
1. Introduction and design considerations in the synthesis of an electroactive neural electrode
1.1 Introduction
Electrical stimulation of nerve cells is widely employed in neural prostheses (for hearing, vision, and limb movement restoration),1–4 and in clinical therapy to cure Parkinson's disease.5 However, functional stimulation especially in the central nervous system requires the use of small electrodes with high current density, which conflicts with the electrochemical safety requirements. Undesirable electrochemical change damages the electrode and may cause abnormalities in neural functions and the cell structure,6 primarily because of the contact problems at the electrode/tissue interface.Neural devices used to repair or restore damaged neurological functions by electrical stimulation or to record the neural activity from the peripheral or central nervous system (CNS) involve the use of microelectrodes, referred to as neural electrodes.7 The neural interface facilitates smooth exchange of information between the central nervous system and the device. These two tasks require transduction of electrical charge via an electrode–electrolyte interface, where the interface is characterized by electrical impedance and the charge injection limit of the interfacial double-layer capacitance.8 Capacitive charge injection is an ideal mechanism for neural stimulation because no chemical change is induced to either the electrode or the tissue.
In the nervous system, there are neurons and non-neuronal cells, referred to as glial cells. A neuron communicates with other neurons by carrying signals over its axon, a projection that is several orders of magnitude longer than the neuron diameter, and transmits the signal to the target neuron using transmitting synapses. Glial cells support neurons with nutrients, protection, neurotransmission, and maintenance of their environment.9
The neural device consists of a number of microelectrodes arrayed on a substrate with small geometrical size to facilitate excitation of a group of neurons. This aspect is a challenge because microelectrodes have to sustain high current density pulses through the electrode–tissue interface without causing irreversible Faradaic reactions and tissue damage.10 A viable approach to improve the electrochemical characteristics of the microelectrode is to employ a reversible charge injection process either via a double layer capacitive mechanism or through a reversible Faradaic mechanism.7 However, the functioning of neural microelectrodes is associated with the inconsistent performance caused by the tissue response.11 It has been previously noted that the electrodes implanted in the central nervous system get covered with a highly resistive astroglial encapsulation because of the inflammatory response, leading to the electrical isolation of the electrodes from the target neurons.12 This astroglial encapsulation results in the loss of neurons near the electrodes and elevates the electrode impedance and power consumption of the implantable device.13,14 Thus, current efforts are devoted towards superior electrochemical performance and biocompatibility. For electrical stimulation, majority of the neural electrodes are made from stable and corrosion resistant metals, such as platinum, iridium, gold, platinum–iridium alloy, titanium, and stainless steel.
The charge injection capacity (CIC) of an electrode is important in the selection of an electrode material. It is defined as the amount of charge that can be transferred through a specific area (C cm−2) before the irreversible corrosion mechanism (electrode dissolution) is initiated.15
The most widely used electrode is Pt, for instance, in cochlear implant electrodes, because of excellent biostability and corrosion resistance.16 But, an inherent drawback of Pt is the maximum safe charge injection (Qinj) limit of only ∼0.15 mC cm−2 (ref. 17), which limits its application in neural microelectrodes. The bare electrodes are also known to exhibit poor performance during long-term stimulation and recording because of the poor contact with the tissue or due to scar formation. Thus, they are surface modified to improve the electrode functionality. A thin layer of rough and porous coating of conducting material (e.g. carbon) on the surface of neural electrodes increases the effective surface area, improves the efficiency of charge transfer, and favorably modulates the electrode biocompatibility.18
Neural electrodes are coated with iridium oxide (IrO2) because of its unique properties that favor neural stimulation. IrO2-coated electrodes are non-cytotoxic19 and have very low impedance and a high charge injection limit. These characteristics enable high levels of charge injection capacity without electrode dissolution or water electrolysis.20 However, IrO2 has poor adhesion to the underlying substrate and may degrade under chronic aggressive stimulation because of inferior structural and chemical stability. Iridium oxide (IrO2) by itself is widely acceptable for microelectrodes, because of its superior characteristics including good biostability, low impedance at 1 kHz, and high Qinj limit of ∼4.0 mC cm−2. While IrO2 electrodes are acceptable, they are generally used as recording electrodes because when used as stimulating electrodes, delamination of IrO2 film takes place at high stimulation densities, leading to tissue damage and inflammatory response.21,22
Based on the above discussion, it is expected that the neural electrodes are characterized by the following desired attributes, notably, physical or structural biocompatibility, chemical and biological inertness, electrochemical stability, and high charge injection limit.
In recent years, the general assumption has been that the cell body (soma) of a neuron, which contains the nucleus, is primarily responsible for the synthesis of macromolecules and has a limited role in cell-to-cell communication.23 It is reported that depolarization in neurons could trigger capacitance increase, indicating somatic exocytosis.24 A group of researchers25,26 noted the release of pre-loaded transmitters or dyes and pain-related peptides including the calcitonin-gene related peptide (CGRP) from the neuron somata in response to the action potential. The somatic secretion provided a method to excite the neuron in an autocrine or paracrine fashion via extrasynaptic mechanisms for modulating the activity of neurons and thereby influence the transmission of sensing input to the central nervous system (CNS) and the spinal cord.25,26
1.2 Design of the novel electroactive electrode
In the attempt to design a chemically and structurally stable and robust electrode for chronic neural stimulation, we have considered Langer's pioneering studies on rat nerve proliferation on polymer polypyrrole substrates.27 Conducting polymers have been used as components for in vivo nerve guides and as coatings to lower the impedance of neural electrodes. The considerations of organic electronic materials are limited to simple polypyrroles and polythiophenes, and furthermore, their covalent modification to enhance biofunction continues to be a challenging task.27Hybrids of conducting polymers and hydrogels have recently been explored as soft electroactive coatings for improving the mechanical and electrical performance of metallic implant electrodes. Since, hydrogel fabrication methods generally tend to produce bulky implants, which displace a large volume of tissue, the fabrication of a thin film is a challenging task. To alleviate this aspect, poly(2-hydroethyl methacrylate) (p-HEMA) polymer brushes were grafted onto gold electrodes followed by poly(3,4-ethylenedioxythiophene) (PEDOT) coating on brushes.28
There are also reports on organic transistor for stimulation and recording of primary neurons.29 These organic ultra-thin film transistors were observed to be suitable for good cellular adhesion and efficient coupling of the ionic currents at the neural interface.30 The studies also indicated that astroglial cells play an active role in information processing than previously anticipated. Elucidating the role of glial cells is expected to identify new targets, thereby enabling the development of effective therapy.31,32 Organic semiconducting thin films also allow interaction between astrocytes and biomaterials to be studied from the viewpoint of control and minimization of reactive astrogliosis. Thus, organic transistors provide a new perspective for selective manipulation of astrocyte bioelectrical activity by non-invasive, label-free organic-based, photostimulation approach.33
In regard to short term effects of electrode insertion on surrounding tissue, Polikov and Bellamkonda13 studied the effects in terms of cell death (both neuronal and glial), severed neuronal processes and blood vessels, mechanical tissue compression, and collection of debris resulting from cell death. Current theories suggest that glial encapsulation, i.e. gliosis, insulate the electrode from nearby neurons, hinder diffusion, increase impedance, and extend the distance between the electrode and its nearest target neuron.14
Based on the above discussion, the design of robust bioelectronic materials that integrate cell adhesive cues and favorable electrical transport incorporating nanostructures (e.g. CNTs, carbon nanohorns, graphene) are envisaged to take the next step toward binding the biotic–abiotic interface. This will eliminate the need to modify conductive polymer assemblies with interactive biological functionalities. Given the practical importance of fabricating an ideal electrode that reliably performs for a long period and the need for the electrodes to sense signals emanating from individual neurons, our first focus was to design/fabricate robust electrical probes with the desired attributes outlined above that can be reliably used to stimulate neurons.
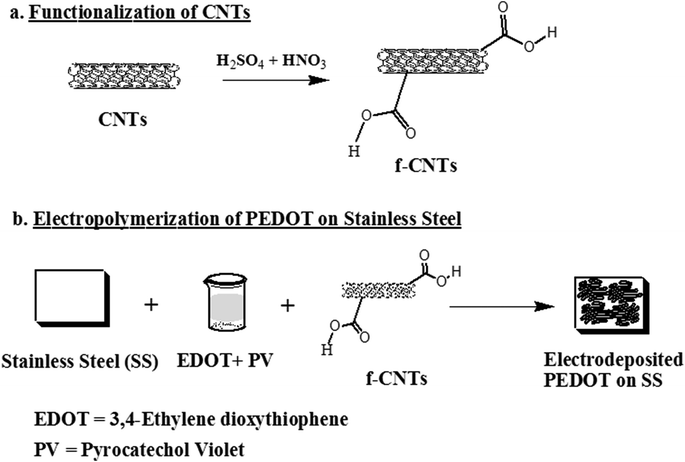
In this study, we exploited the attributes of conducting polymer (poly(3,4-ethylenedioxythiophene): PEDOT) and carbon nanotubes (CNTs) for neural interfacing. PEDOT is a promising conducting polymer because it is characterized by an ordered and a well-defined chemical structure.34 On the other hand, purified CNTs have extraordinary electrical and mechanical strength and are biocompatible. Thus, by incorporating CNTs at small concentrations (0.1 wt%) into PEDOT, we can accomplish the task of fulfilling biocompatibility and stability requirements of PEDOT as a coating material. This is important because PEDOT coatings delaminate during electrical stimulation. CNTs provide mechanical stability to the electrode during electrical stimulation. Furthermore, CNTs appear to promote neuron differentiation,35,36 stimulate neurite growth,37 enhance neuronal performance and recording,38 and promote neuronal electrical signaling.37 Thus, they can be advantageously incorporated into conducting polymers.39 But bare CNT electrodes are not promising because of their relatively low charge injection (Qinj) limit of 1–1.6 mC cm−2 (ref. 40), when neurons are excited with a capacitive charge-injection mechanism. Thus, it was important for us to synergistically utilize the benefits of both PEDOT and CNTs via co-electrodeposition of CNT-PEDOT on stainless steel electrodes. The synthesis of PEDOT-CNT hybrid nanostructured composite coating on the stainless steel electrode culminated in interfacial adhesion, coming from electrostatic and van der Waals interaction in the hybrid polymer-CNT nanostructure (see section 2).
CNT-PEDOT combination provides electronic cues to influence cell growth and guide repair following severe damage to the central nervous system, which offers innovative therapeutic interventions. The design of the electrode material studied here provides an innovative platform technology with several distinct advantages over other existing electroactive materials. Electrically conductive polymers when doped (in our case CNTs) draw analogies to the neural and cardiac biosystem that rely on electrical conduction.
The objective of the study is to first demonstrate the potential of the proposed design of a robust neural probe based on the required attributes of the electrode, study its characteristics, and prove its efficacy for electrical stimulation. This was accomplished through combination of the conducting polymer and CNTs. Given that the mechanical stability of a neural electrode is of prime concern in neural tissue engineering, CNT-PEDOT combination provides the following advantages over other conventional neural probes: (a) chemical and mechanical stability, (b) biocompatibility, (c) promotion of neuronal adhesion and neuronal outgrowth by positively charged polymer, and (d) high charge injection capacity. Furthermore, to understand the cell-to-cell interaction under weak electric field stimulation and regulation of the cytoskeletal proteins is another aspect, which is not well understood and is a part of our objective.
2. Materials and experimental methods
2.1 Materials
Single-walled carbon nanotubes (CNTs) of length ∼30 μm and diameter ∼2 nm were obtained from Global Nanotech, Mumbai. The monomer, 3,4-ethylenedioxythiophene (EDOT) and phosphate buffered saline (PBS, pH 7.4, 10 mM sodium phosphate and 0.9% NaCl) was purchased from Sigma-Aldrich, USA. All chemicals used were of analytical grade. Sulphuric acid, nitric acid, 1-ethyl-3 (3-dimethyl aminopropyl) carbodiimide (EDC), N-hydroxysuccinimide (NHS), and ethanol were obtained from Sigma-Aldrich, USA.2.2 Fabrication of poly(3,4 ethylenedioxythiophene) (PEDOT) CNT-PEDOT-coated electrodes
Based on the discussion in section 1, CNT-coated austenitic stainless steel electrodes were synthesized using the following steps: the first step involved functionalization of CNTs. 20 mg of CNTs were dispersed and treated with 50 mL of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 concentrated HNO3 and H2SO4 solution for 3 h while sonicating in an ice bath, to functionalize CNTs through the attachment of –COOH groups on the surface of CNTs. The dispersed suspension was kept at 20 °C for ∼12 h. After treatment with acid (i.e. functionalization), the CNTs were washed with water and separated by ultracentrifugation (@15
3 concentrated HNO3 and H2SO4 solution for 3 h while sonicating in an ice bath, to functionalize CNTs through the attachment of –COOH groups on the surface of CNTs. The dispersed suspension was kept at 20 °C for ∼12 h. After treatment with acid (i.e. functionalization), the CNTs were washed with water and separated by ultracentrifugation (@15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for five times), until the pH of the solution was neutral. The functionalized CNTs were dried at 60 °C.
000 rpm for five times), until the pH of the solution was neutral. The functionalized CNTs were dried at 60 °C.
In the second step, functionalized CNTs were immersed in a mixture consisting of 5 mg mL−1 of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) and 5 mg mL−1 of N-hydroxysuccinimide (NHS) for 1 h at room temperature to activate the carboxylic groups. Subsequently, the CNTs were washed with phosphate buffer saline (PBS) and dried at room temperature.
Next, the hybrid CNT-PEDOT nanostructured film was electrodeposited onto austenitic stainless steel electrodes through the electropolymerization process using an aqueous solution comprising of 2 mg mL−1 CNTs and 0.01 mol L−1 ethylenedioxythiophene (EDOT) monomer. The electrochemical set-up for electropolymerization consisted of a three-electrode system (stainless steel working electrode, platinum counter electrode, and an Ag/AgCl reference electrode) coupled to a potentiostat. The optimized electrochemical parameters to obtain a uniform (CNT-PEDOT) film on stainless electrode were current density of 1 mA cm−2 for 5 minutes.
To elucidate the effect of CNT, bare CNTs were deposited onto a stainless steel electrode without PEDOT. The procedure involved the following: a CNT dispersion (0.2 mg mL−1) was made in distilled water by sonicating for 3 h. An identical amount of pyrocatechol violet was added in this dispersion, followed by 1 h stirring at room temperature. This dispersion was used to coat a uniform layer of CNTs onto stainless steel using a potentiostat, as described above in section 2.2 and outlined in Scheme 1.
Considering that the CNT acts as a charge carrier in the electrochemical deposition or electro-polymerization of PEDOT onto stainless steel, bare PEDOT coating on stainless steel electrode was not possible to fabricate. However, bare stainless steel electrode and CNT-coated stainless steel electrodes were used as controls in the determination of impedance, which is an important aspect in the design of the electroactive electrode.
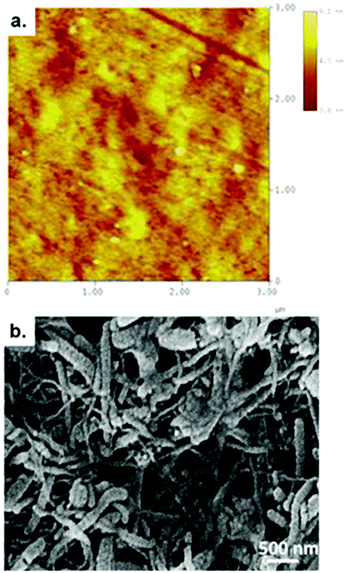
The nanoscale surface morphology and roughness of bare stainless steel was studied using an atomic force microscope (AFM) (Nanoscope TM® IIIa, Digital Instruments, Santa Barbara, CA.). Atomic force microscopy was carried out using tapping mode to prevent possible damage due to continuous contact. All the scans were made in air and the tip used to study the sample was ‘tapping mode etched silicon probe’ (TESM). The number of scan lines for an individual image was 512 with 512 points per line. Five images were taken at a number of locations on the sample to ensure that the presented image was a true representative of the topography of the surface.
2.3 Study of electrochemical properties of the neural electrode
Cyclic voltammetry and electrochemical impedance spectroscopy (EIS) measurements were carried out using a potentiostat (Princeton Applied Research, USA). In brief, cyclic voltammetry was performed in the potential range of −0.6 and 0.7 V at a scan rate of 100 mV s−1 in PBS (pH = 7.3). The area of the current–voltage plot obtained from the time integral of the current in one cycle of the current–voltage was a measure of the charge storage capacity.The electrochemical impedance39–41 was measured in the frequency range of 1–100 kHz using an alternating current sinusoid of 5 mV in amplitude with the direct current potential set at 0 V using a Gamry potentiostat.
The stability of the hybrid CNT-PEDOT nanostructured film on a 316 LN stainless steel electrode was studied by two different methods. The first approach involved cyclic voltammetric stimulation with a potentiostat, using a voltage scan from −0.9 to 0.5 V, at a scan rate of 100 mV s−1 for 3000 cycles.39 The stimulation was conducted using a programmable multichannel stimulator (Multichannel Systems STG2002). An oscilloscope and the WaveStar program measured and recorded the voltage excursion. In the second approach, the CNT-PEDOT coated stainless steel microelectrode was soaked in PBS for ∼14 days, followed by stimulation under a charge balanced, cathodic first, biphasic pulse current at 0.30 mC cm−2 at 50 Hz. The impedance at 1000 Hz and cyclic voltammetry measurements were periodically monitored for change in the impedance and electrochemical behavior with time.
2.4 Structural morphology of the CNT-PEDOT electrode
The microstructure of the CNT-PEDOT film was examined using a scanning electron microscope with a Hitachi S-3000 operated at 15 kV. The CNT-PEDOT coated stainless steel probe was dried in an oven at 80 °C for 1 h, followed by sputter coating with gold of ∼0.8 nm before characterizing the structure via SEM.2.5 Cell culture
NB-39-Nu human neuroblastoma cells (neural cell line: ATCC, Manassas VA, USA) were cultured in Dulbecco's modified Eagle's medium (DMEM) (Gibco BRL, NY, USA) with 10% heat-inactivated fetal bovine serum (FBS; Sigma-Aldrich, St. Louis MO , USA). NB-39-Nu is a human primary neuroblsatoma (NB) cell line. They are transformed, neural crest derived cells, capable of unlimited proliferation and differentiation, in vitro. The cells retain the ability differentiate into neuronal cell types. This ability of NB cells to proliferate and differentiate makes them an excellent cell line for in vitro studies. Next, neural cells at a density of 2 × 105 cells cm−2 were plated onto the CNT-PEDOT coated stainless steel microelectrode in a 30 mm culture dish and placed in a 5% CO2 incubator.To examine the neurite outgrowth and attachment of cells by scanning electron microscopy, CNT-PEDOT coated stainless steel electrodes were first rinsed with PBS to remove the culture medium and non-adherent cells. Next, they were treated with 2.5% glutaraldehyde for 1 h, followed by the dehydration process. The dehydration process involved soaking of the electrodes for 15 minutes in each of the following solutions in the sequence: 30% and 50% ethanol in PBS, 70% and 90% ethanol in water, and 100% ethanol.
The neural cells cultured on the PEDOT-CNT-coated stainless steel electrode were electrically stimulated using a function generator for pulse generation which consists of a switching circuit and variable power supply. This in-house set-up can be used to apply variable voltage with a frequency range of 0.3 Hz–1.0 MHz. Prior to electrical stimulation, the neural electrodes were ultrasonically cleaned and autoclaved. Cultured neural cells on electrodes were stimulated for 5–50 mV mm−1 at a frequency of 100 Hz with a duty cycle of 4%, while keeping the electrodes at least 3 cm apart and keeping the electric field parallel to the cultured cells. The cells were stimulated for 5 min, followed by incubation for 6 h in a humidified CO2 chamber maintained at 37 °C.
2.6 Cell viability
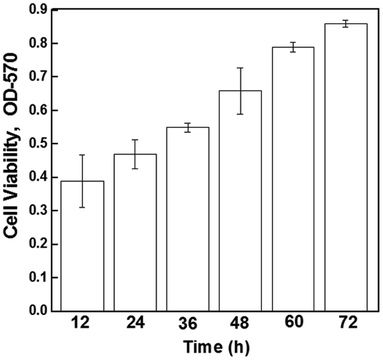
Cell viability of the electrically stimulated cell samples (10 mV mm−1) was determined using the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay (Sigma, St. Louis, USA). The samples were washed with PBS, followed by incubation with fresh culture medium containing MTT (0.5 mg mL−1 medium) at 37 °C for 4 h in darkness. The unreacted dye was removed and DMSO was added to dissolve the intracellular insoluble purple formazan product into a colored solution. The optical density (OD) of the formazan solution was read using a spectrophotometric plate reader (Bio TEK Instrument, EL-307C) at a wavelength of 570 nm, and the results obtained were expressed as OD after subtraction of the blank.2.7 Cell morphology and cell density analysis
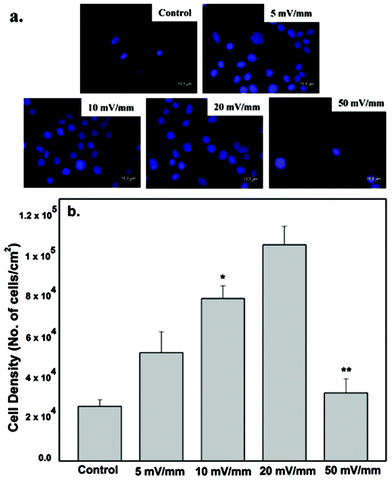
The neural cell-CNT/PEDOT electrode constructs were washed twice with PBS, followed by fixation with 2% v/v glutaraldehyde in cacodylate buffer (pH 7.4) for 30 min and then washed with PBS. After washing the fixatives, the samples were dried with a graded ethanol series, and then sputter coated with gold prior to the study of morphology by SEM.The quantification of cell proliferation was done by determining the cell density using the fluorescence micrographs presented in Fig. 4. At least five non-overlapping locations on each sample were selected to calculate the average cell density. At least 20 micrographs for each experiment were selected for cell density measurements, using the Image J Pro software.
2.8 Effect of electrical field on cytoskeletal proteins: immunocytochemistry and fluorescence microscopy
After electric field stimulation, the neural cells in the culture dish were fixed for 20 min with 4% paraformaldehyde in PBS (pH 7.4) and rinsed with PBS at least three times using the protocol given in ref. 42. Next, the cells were incubated in PBS solution comprising 4% normal goat serum (NGS; Vector Laboratories, CA, USA), 0.2% Triton X-100, 2% bovine serum albumin (BSA; Sigma-Aldrich, St. Louis MO, USA), 2% FBS (Sigma-Aldrich, St. Louis MO , USA) and primary antibodies for 60 min at room temperature. After washing with PBS, secondary Alexa fluor 568 goat anti-mouse IgG (H + L) and Alexa fluor 488 goat anti-rabbit IgG (H + L) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, Molecular Probes, OR) were applied to the cells for 60 min at room temperature in the dark followed by rinsing of cells with PBS. To stain the nucleus, DAPI (1
1000, Molecular Probes, OR) were applied to the cells for 60 min at room temperature in the dark followed by rinsing of cells with PBS. To stain the nucleus, DAPI (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 dilution) was added to the last PBS buffer wash and the cells were incubated for 20 min at 20 °C and washed again prior to mounting. The cells were incubated in a CO2 incubator at 37 °C for 40 min. After final washing with PBS, the cells were mounted with fluorescence mounting medium (DakoCytomation, CA, USA) and covered with a glass cover slip. The primary antibodies used and their dilution are as follows: rabbit anti-fibronectin (1
100 dilution) was added to the last PBS buffer wash and the cells were incubated for 20 min at 20 °C and washed again prior to mounting. The cells were incubated in a CO2 incubator at 37 °C for 40 min. After final washing with PBS, the cells were mounted with fluorescence mounting medium (DakoCytomation, CA, USA) and covered with a glass cover slip. The primary antibodies used and their dilution are as follows: rabbit anti-fibronectin (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500, Millipore, USA); mouse monoclonal anti-F-actin (1
500, Millipore, USA); mouse monoclonal anti-F-actin (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, Millipore, USA); and rabbit anti-vinculin (1
1000, Millipore, USA); and rabbit anti-vinculin (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500, Millipore, USA). After immunostaining, the cells were examined with a fluorescence microscope (DMI6000B, Leica, Germany).
500, Millipore, USA). After immunostaining, the cells were examined with a fluorescence microscope (DMI6000B, Leica, Germany).
Fluorescence micrographs of at least 25 non-overlapping individual regions on each sample were randomly chosen to calculate the fluorescence intensity using the Image J software. The neurite outgrowth was quantified by counting the number of neuroblastoma cells bearing neurites and measuring the neurite length in randomly selected SEM micrographs. A total of 10 micrographs were used to quantify neurite outgrowth using the Image analysis software (Image J; NIH, Bethesda, MD).
2.9 Statistical analysis
All the experiments involved a minimum of five samples and five tests were carried out for the cell culture studies. The obtained values in each experiment were normalized with respect to the control samples. Results are expressed as the mean value of at least five replicates ± SD (standard deviation). Statistical analysis was carried out using the one-way analysis of variance (ANOVA) with 95% confidence interval. The error bars denote ±SD (n ≥ 5).3. Results and discussion
It is important to first describe the characteristics of the CNT-polymer electrode used for stimulation prior to discussing cell-to-cell communication and cellular activity.3.1 Structural and electrochemical characteristics
A high magnification atomic force micrograph of bare stainless steel and a scanning electron micrograph of the CNT-PEDOT-coated stainless steel electrode are presented in Fig. 1a and b, respectively. Metallographically polished mirror surface of the bare stainless steel had atomic scale roughness of 1.45 ± 0.21 nm. The figure shows that CNTs are uniformly dispersed in the PEDOT matrix and are characterized by a network-like structure. This desired structural morphology is attributed to the methodology adopted to fabricate the CNT-PEDOT coated stainless steel electrode. The functionalized CNTs with carboxyl groups were individually dispersed in the aqueous solution. During the electropolymerization of PEDOT in aqueous solution comprising of monomer EDOT and CNTs, the functionalized and negatively charged CNTs with carboxyl groups acted as dopants to neutralize the positive charge present in the backbone of the PEDOT. This facilitates their incorporation into the PEDOT polymer matrix. The combination of highly conducting CNTs with a large surface area (∼1000 m2 g−1 by BET analysis) and a conducting PEDOT polymer renders the microelectrode suitable for neural stimulation and in restoring lost or impaired neurological functions.Furthermore, there was no visible indication of the CNT-PEDOT electrodeposited film experiencing delamination or cracking during neural stimulation at different voltages, and the structural characteristics continued to be similar to the as-synthesized electrode. This behavior is in contrast to PEDOT-PSS coating that experienced delamination during chronic stimulation.43
The charge storage capacity of the CNT-PEDOT coated stainless steel electrode as determined by cyclic voltammetry is presented in Fig. 2. A high charge of ∼70 mC cm−2 was obtained at an electrodeposition charge of ∼70 μC and is preferred for neural stimulation. The underlying reason for high charge storage capacity is CNTs. The CNTs are highly conducting with a large surface area such that the network-like distribution of PEDOT-coated CNTs in the PEDOT matrix effectively increases the electrode/electrolyte interface area and increases the conductivity of the film. This renders the deposited polymer highly conducting for subsequent electrodeposition. Another aspect to be noted from Fig. 2 is that the charge storage capacity of the CNT-PEDOT-coated electrode increases with the electrodeposition charge. This behavior is anticipated because a higher electrodeposition charge results in a thick and rough film with a greater density of electroactive species. Furthermore, the charge capacity increases linearly with electrodeposition charge. This is in contrast to pure PEDOT film, which indicated a linear relationship at low electrodeposition charge (thin film) and a non-linear relationship at high electrodeposition charge (thick film), when the film becomes dense and contains a high density of defects in the lattice.44
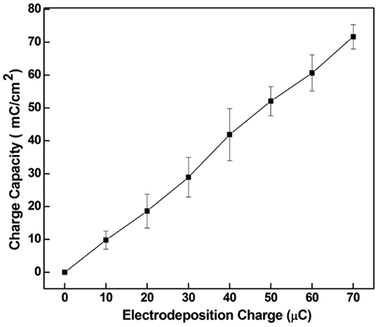 | ||
| Fig. 2 The charge capacity of CNT-PEDOT coated stainless steel electrode as a function of the CNT-PEDOT electrodeposition charge. Bars represented the range over which the values were measured. | ||
The voltage response of an electrode upon current stimulation is a direct indicator of its charge injection capability. An appropriate or desired neural electrode is characterized by high charge injection capability. The underlying reason being that at a given pulse current it will generate lower electrode voltage and is safe for neural stimulation. The CNT-PEDOT-coated electrodes were examined under pulse stimulation current to evaluate their charge injection capacity. The charge injection limit is defined as the maximum cathodic charge that results in cathodic or anodic electrode voltage exceeding 0.6 V versus the Ag/AgCl reference electrode. The average charge injection limit of the CNT-PEDOT electrode was ∼2.3 ± 0.2 mC cm−2 and is comparable to the IrO2 electrode. The results indicate that CNT-PEDOT-coated electrodes can deliver higher charge density without generating high voltage, which is beneficial in preventing detrimental effects on the surrounding tissues.
The impedance of the CNT-PEDOT-coated electrode and the bare stainless steel electrode in the frequency range of 1 and 103 Hz is presented in Fig. 3. In this frequency range, the impedance of the CNT-PEDOT-coated stainless steel electrode was observed to decrease by ∼70% from ∼30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ohm to ∼200 ohm. The significant drop is in contrast to the bare stainless steel electrode and is a consequence of the CNT-PEDOT-coating on stainless steel. The decrease in impedance can be discussed in terms of a circuit model comprising of a solution resistance (SR) in series with a finite-length Warburg diffusion impedance component of a bulk film (DF) and a bulk (electronic) capacitance (EC).45,46 In this model, SR is constant because the solution does not change, EC depends on the electroactive surface area, and in the high frequency region, the impedance due to EC is small, and the impedance is influenced by DF. Based on Fig. 3, there is insignificant change in impedance in the high frequency range, which is DF, implying high conductivity of the CNT-PEDOT electrode material. On the other hand, in the low frequency region, EC contributes to impedance.
000 ohm to ∼200 ohm. The significant drop is in contrast to the bare stainless steel electrode and is a consequence of the CNT-PEDOT-coating on stainless steel. The decrease in impedance can be discussed in terms of a circuit model comprising of a solution resistance (SR) in series with a finite-length Warburg diffusion impedance component of a bulk film (DF) and a bulk (electronic) capacitance (EC).45,46 In this model, SR is constant because the solution does not change, EC depends on the electroactive surface area, and in the high frequency region, the impedance due to EC is small, and the impedance is influenced by DF. Based on Fig. 3, there is insignificant change in impedance in the high frequency range, which is DF, implying high conductivity of the CNT-PEDOT electrode material. On the other hand, in the low frequency region, EC contributes to impedance.
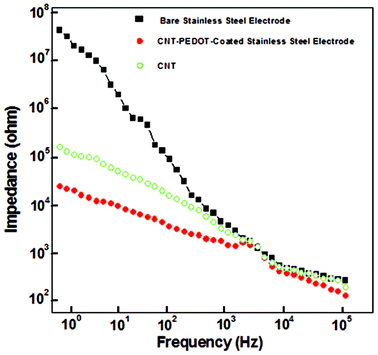 | ||
| Fig. 3 Electrochemical impedance of bare stainless steel, CNT-coated stainless steel, and CNT-PEDOT coated-stainless steel electrodes. | ||
3.2 Cell viability, proliferation and morphological changes in relation to electrical field strength
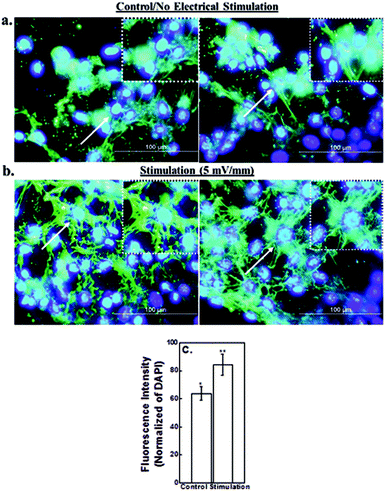
The nucleus area of neural cells increased after electrical stimulation and is illustrated in Fig. 4. It is clear from Fig. 4 that the area of the nucleus and the number of cells increased with increase in applied electrical field strength, with a maximum increase at 20 mV mm−1 stimulation, as compared with the control cells (no stimulation). However, a marked decrease was observed when the cells were stimulated at an applied electrical field strength of 50 mV mm−1. There was a significant decrease in the number of proliferating cells cultured on the CNT-PEDOT coated stainless steel electrode. The effect of the applied electrical field certainly affects the bio-physicochemical functions of the stimulated cells such that the cellular proliferation and nuclear area is increased. Neuronal cells and excitable cells respond to changes in external field through voltage gated-ion channels. Their opening modifies the permeability of the neuronal cells’ membrane and potentially leads to action potential. In contrast, electrical stimulation of the non-excitable cells promote alteration in extracellular ion concentration (including H+ and hence pH). This ultimately leads to alteration in the electrochemical gradient across the plasma-membrane and promotes modification of membrane permeability to ions. Calcium signaling across the cells via connexins (other ion channels) is another possibility.23 This affects the cell proliferation and metabolic activities, with a higher rate of protein and DNA synthesis under the influence of the applied electrical stimulation. Similarly, the applied electrical field had significant influence on the synthesis of actin, implying greater cytoplasmic extension and reorganization (see also section 4.3). The decrease in the number of cells at high electrical field strength is envisaged to be related to high current flow through the cells, which presumably alters the pH of the culture medium.As shown in Fig. 4b, the cell proliferation was significantly influenced by electrical stimulation. The cell proliferation increased with increase in the externally applied voltage. The highest neural cell proliferation was observed at 20 mV mm−1. At electrical field greater than 20 mV mm−1 the cell death was observed.
The higher proliferation of neural cells in the presence of an externally applied electrical field suggest the non-toxic nature of the electrode material and a positive effect of stimulation and the cells did not experience any observable cell death/apoptosis, as confirmed by the MTT assay (Fig. 5). It may be seen that after electrical stimulation, the cells remained viable and proliferated up to 72 hours (experimental study time).
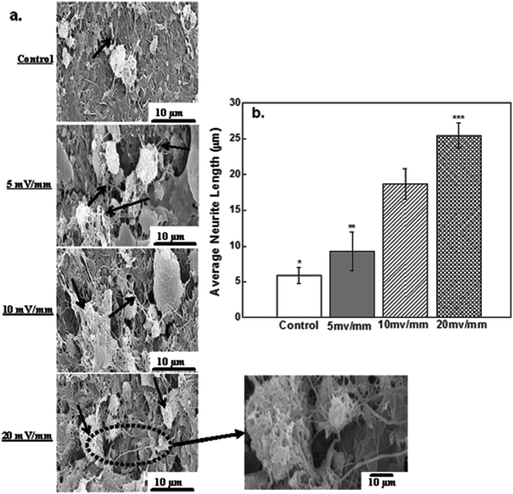
The success of a neural prosthetic device is governed by the successful electrical stimulation of neural cells and the ability of the cells to attach to the surface of the CNT-PEDOT-coated stainless steel electrode. Fig. 6 shows scanning electron microscopy micrographs of attached neural cells on the surface of the CNT-PEDOT-coated electrode after electrical stimulation. The attachment of neural cells (control) can be seen in Fig. 6a. In contrast, a number of cells were observed on electrical stimulation such that long neuritis extended and developed into extensive secondary processes. The extensive proliferation and branching in different directions and a strong attachment to the surface with long neurite extension is indicative of an interface that is favorable for electrical stimulation. It also confirms the biocompatible nature of the surface that is favorable for the growth of neural cells.
The average neurite length for neural cells cultured on the PEDOT-CNT-coated electrode and subjected to electrical stimulation for 20 mV mm−1 was nearly quadrupled with control cells (no stimulation). This represents a 4 times increase in the neurite length, which is significantly greater than the 2–3 times increase in the neurite length observed for neural cells after stimulation at 5 mV mm−1 and 10 mV mm−1. Electrophoretic redistribution of cytoskeletal proteins or conformational changes in extracellular matrix proteins are responsible for the changes in the neurite length. In the present work, it is also shown that the electrical stimulation increases the expression level of proteins, actin and fibronectin. Among these, fibronectin is an extracellular matrix (ECM) adhesive glycoprotein and is crucial for the cellular attachment to the substrate surface. Hence, an enhanced ECM protein expression on electrical stimulation increases neuronal attachment and neurite outgrowth, as shown in Fig. 6b.
In the SEM micrographs (Fig. 6), the neurites were identified as elongated projections from the main cell body of neural cells. These elongated long fibers were in the range of 10–25 μm, where the neurite length is governed by the electric field strength. At low field (5 mV mm−1), the average neurite length was ∼10 μm, while at highest electrical field (20 mV mm−1), the neurite length was increased from ∼10 μm to 20 μm, as shown in Fig. 6b. Furthermore, the presence of neurites, which are indicators of viability and adhesion capability of neural cells, confirmed that PEDOT-CNT is an excellent material for neural cells and can be used in neural tissue regeneration. The fiber-like thin projections are characteristic of neurites and neuronal outgrowth.
Neurite outgrowth is known to be induced by depolarization and a potential difference exists between the intracellular fluids of nerve cells.47 When neural cells are inactive, the potential difference is nearly constant (steady), but on electrical stimulation, the cell membrane experiences an intensity-dependent depolarization. Once a threshold is attained, an action potential is generated that propagates across the membrane. Our results at 20 mV mm−1 indicate significant impact on neurite extension.
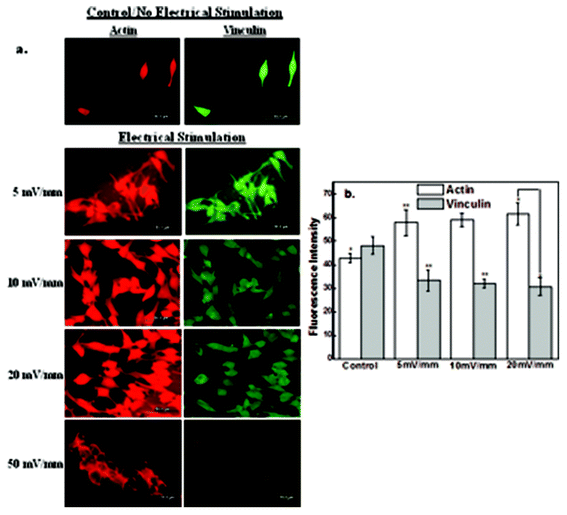
3.3 The impact of electric field stimulation on cytoskeleton proteins
In this study, we have investigated the effects of the electrical field-induced cell-to-cell coupling behavior on protein synthesis and mechanisms by immunocytochemistry. Neuroblastoma cell line (NB-39-Nu) has abundant adhesion proteins distributed throughout the cytoplasm and different proteins play a significant role in cell motility and cell-to-cell contact44,48,49. Actin is a primary cytoskeleton protein related to cellular mobility. Vinculin focal adhesion contact and actin contribute to morphological adhesive changes in the cell.44,48Immunocytochemical analysis of actin and vinculin is presented in Fig. 7a. It may be noted from Fig. 7a that the number of cells increased with electrical field strength from 5 mV mm−1 to 20 mV mm−1, but at high electrical field strength of 50 mV mm−1 majority of the cells died, even though their nuclear area was greater than the control experiments (no stimulation). The expression level of primary cytoskeletal protein, actin, was increased with stimulation up to the electrical field strength of 20 mV mm−1 (Fig. 7b), while that of vinculin was slightly decreased. The increase in actin expression suggests that the cell mobility was promoted or enhanced by the applied electrical field. On the other hand, the small decrease in vinculin expression implies that the focal adhesions formed at the interface between the cells and the CNT-PEDOT film were weakened and undermined because of the protrusion edge of the contacting cell with the adjacent neighboring cells.
It is clear that the electric field influences the expression level of intracellular proteins, actin and vinculin, which contribute to cell mobility and cell adhesion, respectively and thereby enhances cell-to-cell contact beyond normal cell membrane protrusion. The aforementioned observations are of particular significance to neural cell transplantation therapy, given that cell therapy is being utilized to cure neuronal cell loss or neuronal functional loss.42
Immunocytochemical analysis of extracellular matrix protein, fibronectin, in control neural cells (no simulation) and under electric field strength of 5 mV mm−1 is presented in Fig. 8. An increase in the expression level of fibronectin suggests upregulation of fibrillar adhesions in the presence of electric field. The dissemination or distribution of fibronectin protein was changed from the wider sites in the cytoplasm to perinuclear location. This led to high concentration of fibronectin around the perinucleus (Fig. 8). The localization of fibronectin at the center indicates that the cellular edge was actively modified to a greater degree in the presence of electric field in contrast to the central cellular part. Cell-to-cell coupling is relevant in functional neuronal circuits and therefore, contributes to the success of the transplantation.42
The study described here provides a strong evidence and potential for the consideration of CNT PEDOT-coated metallic microelectrodes (stainless or platinum) for neural stimulation. Besides the evidence of the effect of electrical field on cytoskeleton proteins and cell-to-cell contact using CNT PEDOT-coated metallic steel electrodes, the microelectrode exhibited the desired attributes of stability, high charge injection limit, and low impedance at high frequency. The chemical and structural design of the microelectrode can be potentially extended to other microfabrication processes related to neural electrodes such as microelectrochemical systems.
3.4 Implications of CNTs in neural tissue engineering
Carbon nanotubes (CNTs) are being increasingly researched for neural tissue engineering applications and can be used in the design of neuronal interfaces or for synthesis of scaffolds to promote neuronal growth, as illustrated in our study. Given that the architecture of the scaffold structure is important in the success of implant, the introduction of longitudinal tubular structure such as that of CNTs provides physical guidance for axonal regrowth and cell migration and enhances nerve regeneration.50 Based on the crucial role of process entanglements in neuronal anchoring, the appearance of a rough surface and large surface area of CNTs is expected to influence the contact between neuronal membrane and CNTs, and hence favorably impact direct electrical coupling.51The nanoscale roughness and its intrinsic conductivity make CNTs excellent candidates for neural tissue regeneration, because the nanotopography and physical and chemical properties of CNTs promote neural contact to CNTs, activating multiple and complex adhesion-mediated, intracellular signaling cascades.52 In general, neural cells respond favorably to a surface which consists of extracellular matrix components such as fibronectin, collagen, and laminin. In such a case, the neural cells first attach to the surface and with the help of biochemical cues, the dendrites start to elongate, while the extracellular signals stimulate the process of neurite outgrowth followed by the axonal growth. This means that the cell attachment and spreading is highly crucial for cell differentiation. In our case, the expression of ECM protein, fibronectin, provided favorable biochemical cues to stimulate neurite outgrowth.
Previous studies examined the relationship between CNTs and neuronal growth, and proposed that CNTs may affect neurite outgrowth.53 However, we envisage that PEDOT may also induce signal transduction for neurite outgrowth. Thus, a detailed mechanism pertaining to the role of CNTs and PEDOT in facilitating neural cell attachment and neurite outgrowth still needs to be determined. Similarly, the interaction of these components with intra- and extracellular proteins requires further examination. These aspects are being currently studied and will be reported in future.
4. Conclusions
The study illustrates the potential and desired attributes of the CNT-PEDOT-coated stainless steel electrodes for electrical stimulation of neural cells. The CNT-PEDOT-stainless steel electrodes were characterized by significantly low impedance (∼200 ohm, at a frequency of 105 Hz compared with the bare electrode), high charge storage capacity, and a high charge injection limit of ∼70 mC cm−2. The CNT-PEDOT coated microelectrodes indicated no signs of delamination or cracking indicative of superior mechanical stability. The study of the effects of electrical field strength-induced stimulation of neural cells on protein synthesis and mechanism indicated cell-to-cell interaction. This implied change in the regulation of endogenous cytoskeletal proteins, actin, vinculin, and fibronection involving cell mobility and cell adhesion. The observed magnificent characteristics are promising for clinical trials.References
- B. S. Wilson, D. T. Lawson, J. M. Muller, R. S. Tyler and J. Kiefer, Cochlear implants: some likely next steps, Annu. Rev Biomed. Eng., 2003, 5, 207–249 CrossRef CAS PubMed.
- J. D. Weiland, W. Liu and M. S. Humayun, Retinal prosthesis, Annu. Rev. Biomed. Eng., 2005, 7, 361–401 CrossRef CAS PubMed.
- X. Navarro, T. B. Krueger, N. Lago, S. Micera, T. Stieglitz and P. Dario, A critical review of interfaces with the peripheral nervous system for the control of neuroprostheses and hybrid bionic systems, J. Peripher. Nerv. Sys., 2007, 10, 229–258 CrossRef PubMed.
- A. L. Benabid, Deep brain stimulation for Parkinson's disease, Curr. Opin. Neurobiol., 2003, 13, 696–706 CrossRef CAS PubMed.
- R. A. Green, G. J. Suaning, L. A. Poole-Warren and N. L. Lovell, Bioactive conducting polymers for neural interfaces: application to vision prosthetics, International IEEE/EMBS Conference Neural Engineering, 2009, 60–63.
- S. B. Brummer and M. J. Turner, Electrochemical considerations for safe electrical stimulation of the nervous system with Platinum electrodes, IEEE Trans. Biomed. Eng., 1997, 24, 59–63 Search PubMed.
- J. O. Winter, S. F. Cogan and J. F. Rizzo 3rd, Retinal prostheses: current challenges and future outlook, J. Biomater. Sci., Polym. Ed., 2007, 18, 1031–1055 CrossRef CAS PubMed.
- Y. Nam, Materials consideration for in vitro interface technology, Mater. Res. Bull., 2012, 37, 566–572 CrossRef CAS.
- S. Chen and M. G. Allen, Extracellular matrix-based materials for neural interfacing, Mater. Res. Bull., 2012, 37, 606–619 CrossRef CAS.
- L. Di, L. P. Wang, Y. N. Lu, L. He, Z. X. Lin and K. J. Wu, Protein adsorption and peroxidation of rat retinas under stimulation of a neural probe coated with polyaniline, Acta Biomater., 2011, 7, 3738–3745 CrossRef CAS PubMed.
- Y. Lu, Z. X. Cai, Y. L. Cao, H. X. Yang and Y. Y. Duan, Activated iridium oxide films fabricated by asymmetric pulses for electrical neural micro stimulation and recording, Electrochem. Commun., 2008, 10, 778–782 CrossRef CAS PubMed.
- S. F. Cogan, A. A. Guzelian, W. F. Agnew, T. G. H. Yuen and D. B. McCreery, Over-pulsing degrades activated iridium oxide films used for intracortical neural stimulation, J. Neurosci. Methods, 2004, 137, 141–150 CrossRef CAS PubMed.
- V. S. Polikov, P. A. Tresco and W. M. Reichert, Response of brain tissue to chronically implanted neural electrodes, J. Neurosci Methods, 2005, 148, 1–18 CrossRef PubMed.
- Y. Zhong and R. V. Bellamkonda, Controlled release of anti-inflammatory agent α-MSH from neural implants, J. Controlled Release, 2006, 106, 309–318 CrossRef PubMed.
- J. Ordonez, M. Schuetter, C. Boehler, T. Boretium and T. Stieglitz, Thin films as microelectrode array for neuroprosthetics, Mater. Res. Bull., 2012, 37, 590–598 CrossRef CAS.
- D. R. Merrill, M. Bikson and J. G. R. Jefferys, Electrical stimulation of excitable tissue: design of efficacious and safe protocols, J. Neurosci. Methods, 2005, 141, 171–198 CrossRef PubMed.
- T. L. Rose and L. S. Robblee, Electrical-stimulation with Pt electrodes. VIII. Electrochemically safe charge injection limits with 0.2 ms pulses, IEEE Trans, Biomed. Eng., 1990, 37, 1118–1120 CrossRef CAS PubMed.
- E. W. Keefer, B. R. Botterman, M. I. Romero, A. F. Rossi and G. W. Gross, Carbon nanotube coatings improves neuronal recordings, Nat. Nanotechnol., 2008, 3, 434–439 CrossRef CAS PubMed.
- J. D. Weiland and D. J. Anderson, Chronic neural stimulation with thin-film, iridium oxide electrodes, IEEE Trans. Biomed. Eng., 2000, 47, 911–918 CrossRef CAS PubMed.
- K. Gobbels, T. Kuenzel, A. V. Ooyen, W. Baumgartner, U. Schnakenberg and P. Braunig, Neuronal cell growth on Iridium Oxide, Biomaterials, 2010, 31, 1055–1067 CrossRef PubMed.
- I. Lee, C. N. Whang, K. Choi, M. S. Choo and Y. H. Lee, Characterization of iridium film as a stimulating neural electrode, Biomaterials, 2002, 23, 2375–2380 CrossRef CAS.
- S. C. Mailley, M. Hyland, P. Mailley, J. M. McLaughlin and E. T. McAdams, Electrochemical and structural characterizations of electrodeposited iridium oxide thin-film electrodes applied to neuro stimulating electrical signal, Mater. Sci. Eng., C, 2002, 21, 167–175 CrossRef.
- Y. Zeng, X. H. Lu, S. Q. Zeng, S. L. Tian, M. Li and J. Shi, Sustained depolarization-induced propagation of [Ca2+] oscillations in cultured DRG neurons: The involvement of extracellular ATP and P2Y receptor activation, Brain Res., 2008, 1239, 12–23 CrossRef CAS PubMed.
- L. Y. Hung and E. Neher, Ca2+ dependent exocytosis in the somata of dorsal root ganglion neurons, Neuron, 1996, 17, 135–145 CrossRef.
- C. Zhang, W. Xiong and H. Zheng, Calcium- and dynamin-independent endocytosis in dorsal root ganglion neurons, Neuron, 2004, 42, 225–236 CrossRef CAS.
- K. Ouyang, H. Zheng, X. Qin, C. Zhang, D. Yang, X. Wang, C. Wu, Z. Zhou and H. Cheng, Ca2+ sparks and secretion in dorsal root ganglion neurons, Proc. Natl. Acad. Sci. U. S. A., 2005, 23, 12259–12264 CrossRef PubMed.
- S. Baek, R. Green, A. Granville, P. Martens and L. Poole-Warren, Thin film hydrophilic electroactive polymer coatings for bioelectrodes, J. Mater. Chem. B, 2013, 1, 3803–3810 RSC.
- E. Jan, J. L. Hendricks, V. Husaini, S. M. Richardson-Burns, A. Sereno, D. C. Martin and N. A. Kotov, Layered Carbon Nanotube-Polyelectrolyte Electrodes Outperform Traditional Neural Interface Materials, Nano Lett., 2009, 9, 4012–4018 CrossRef CAS PubMed.
- V. Benfenati, S. Toffanin, S. Bonetti, G. Turatti, A. Pistone, M. Chiappalone, A. Sagnella, A. Stefani, G. Generali, G. Ruani, D. Saguatti, R. Zamboni and M. Muccini, A transparent organic transistor structure for bidirectional stimulation and recording of primary neurons, Nat. Mater., 2013, 12, 672–680 CrossRef CAS PubMed.
- T. Cramer, B. Chelli, M. Murgia, M. Barbalinardo, E. Bystrenova, D. M. de Leeuw and F. Biscarini, Organic ultra-thin film transistors with a liquid gate for extracellular stimulation and recording of electric activity of stem cell-derived neuronal networks, Phys. Chem. Chem. Phys., 2013, 21, 3897–3905 RSC.
- G. Seifert, K. Schilling and C. Steinhauser, Astrocyte dysfunction in neurological disorders: a molecular perspective, Mat. Rev. Neurosci., 2006, 7, 194–206 CrossRef CAS PubMed.
- E. A. Nagelhus, M. Amiry-Moghaddam, L. H. Bergersen, J. G. Bjaalie, J. Eriksson, V. Gundersen, T. B. Leergaard, J. P. Morth, J. Storm-Mathisen, R. Torp, K. B. Walhovd and T. Tonjum, The glia doctrine: addressing the role of glial cells in healthy brain ageing, Mach Ageing Dev., 2013, 134, 449–599 CrossRef CAS PubMed.
- V. Benfenati, N. Martino, M. R. Antognazza, A. Pistone, S. Toffanin, S. Ferroni, G. Lanzani and M. Muccini, Photostimulation of whole-cell conductance in primary rat neocortical astrocytes mediated by organic semiconducting thin films, Adv. Healthcare Mater., 2014, 3, 392–399 CrossRef CAS PubMed.
- B. L. Groenendaal, F. Jonas, D. Freitag, H. Pielartzik and J. R. Reynolds, Poly(3,4-hylenedioxythiophene) and its derivatives: past, present, and future, Adv. Mater., 2000, 12, 481–494 CrossRef.
- T. I. Chao, S. Xiang, C. S. Chen, W. C. Chin, A. J. Nelson and C. Wang, Carbon nanotubes promote neuron differentiation from human embryonic stem cells, Biochem. Biophys. Res. Commun., 2009, 384, 426–430 CrossRef CAS PubMed.
- K. Matsumoto, C. Sato, Y. Naka, R. Whitby and N. Shimizu, Stimulation of neuronal neurite outgrowth using functionalized carbon nanotubes, Nanotechnology, 2010, 21, 115101 CrossRef CAS PubMed.
- G. Cellot, E. Cilia, S. Cipollone, V. Rancic, A. Sucapane and S. Giordani, Carbon nanotubes might improve neuronal performance by favoring electrical shortcuts, Nat. Nanotechnol., 2009, 4, 126–133 CrossRef CAS PubMed.
- H. Hu, Y. Ni, S. K. Mandal, V. Montana, B. Zhao and R. C. Haddon, Polyethyleneimine functionalized single-walled carbon nanotubes as a substrate for neuronal growth, J. Phys. Chem. B, 2005, 109, 4285–4289 CrossRef CAS PubMed.
- X. Luo, C. L. Weaver, D. D. Zhou, R. Greenberg and X. T. Cui, Highly stable carbon nanotube doped Poly(3,4-ethylenedioxythiophene) for chronic neural stimulation, Biomaterials, 2011, 32, 5551–5557 CrossRef CAS PubMed.
- K. Wang, H. A. Fishman, H. Dai and J. S. Harris, Neural stimulation with a carbon nanotube microelectrode assay, Nano Lett., 2006, 6, 2043–2048 CrossRef CAS PubMed.
- Y. Lu, T. Li, X. Zhao, M. Li, Y. Cao and H. Yang, Electrodeposited electrodes for neural interfaces, Biomaterials, 2010, 31, 5169–5181 CrossRef CAS PubMed.
- C. Heo, J. Yoo, S. Lee, A. Jo, S. Jung, H. Yao, Y. H. Lee and M. Suh, The control of neural cell-to-cell interactions through non-contact electrical field stimulation using graphene electrodes, Biomaterials, 2011, 32, 19–27 CrossRef CAS PubMed.
- E. Jan, J. L. Hendricks, V. Husaini, S. M. Richardson-Burns, A. Serene and D. C. Marten, Layered carbon nanotubes polyelectrolyte electrodes outperform traditional neural interface materials, Nano Lett., 2009, 9, 4012–4018 CrossRef CAS PubMed.
- X. T. Cui and D. D. Zhou, Poly (3,4-ethylenedioxythiophene) for chronic neural stimulation, IEEE Trans. Neural Rehabil. Eng., 2007, 15, 502–508 CrossRef PubMed.
- J. Bobacka, A. Lewenstam and A. Ivaska, Electrochemical impedance spectroscopy of oxidized poly (3,4-ethylenedioxythiophene) film electrodes in aqueous solution, J. Electroanal. Chem., 2000, 489, 17–27 CrossRef CAS.
- J. R. Macdonald, Impedance spectroscopy: emphasizing solid materials and systems, Wiley, New York, 1987 Search PubMed.
- G. Matthews, Cellular Physiology of nerve and muscle, Blackwell Science Ltd., Malden, MA, 4th edn, 2003 Search PubMed.
- J. V. Small, B. Geiger, I. Kaverina and A. Bershadsky, How do microtubules guide migrating cells?, Nat. Rev. Mol. Cell Biol., 2002, 3, 957–964 CrossRef CAS PubMed.
- M. R. Morgan, M. J. Humphries and M. D. Bass, Synergistic control of cell adhesion by integrins and syndecan, Nat. Rev. Mol. Cell Biol., 2007, 8, 957–969 CrossRef CAS PubMed.
- F. C. Spivey, Z. Z. Khaniz, J. B. Shear and C. E. Schmidt, The fundamental role of subcellular topography in peripheral nerve repair therapies, Biomaterials, 2012, 33, 4264–4276 CrossRef PubMed.
- G. Cellot, E. Cilia, S. Cipollone, V. Rancic and A. Sucapane, et al., Carbon nanotubes might improve neuronal performance by favoring electrical shortcuts, Nat. Nanotechnol., 2009, 4, 126–133 CrossRef CAS PubMed.
- T. Dvir, B. P. Timko, D. S. Kohane and R. Langer, Nanotechnological strategies for engineering complex tissues, Nat. Nanotechnol., 2011, 6, 13–22 CrossRef CAS PubMed.
- H. Hu, Y. Ni, V. Montana, R. C. Haddon and V. Parpura, Chemically functionalized carbon nanotubes as substrates for neuronal growth, Nano Lett., 2004, 4(3), 507–511 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |