Examination of native chemical ligation using peptidyl prolyl thioesters†‡
Takahiro
Nakamura
,
Akira
Shigenaga
,
Kohei
Sato
,
Yusuke
Tsuda
,
Ken
Sakamoto
and
Akira
Otaka
*
Institute of Health Biosciences and Graduate School of Pharmaceutical Sciences, The University of Tokushima, 1-78-1 Shomachi, Tokushima 770-8505, Japan. E-mail: aotaka@tokushima-u.ac.jp; Fax: +81-88-633-9505; Tel: +81-88-633-7283
First published on 22nd October 2013
Abstract
Peptidyl prolyl thioesters, which have been regarded as too unreactive for practical use in native chemical ligations (NCLs), were proven to be versatile reagents in peptide coupling. Tuning the reactivity of prolyl thioesters enabled a one-pot/sequential NCL reaction based on kinetically controlled ligation.
The development of native chemical ligation (NCL) by Kent and co-workers has placed the chemical synthesis of proteins with up to about 100 amino acid residues within our reach.1 NCL involves the use of peptide thioesters in chemoselective reactions with N-terminal cysteinyl residues. Through the examination of reactivity of 20 proteinogenic amino acyl thioesters, peptidyl prolyl thioesters have been reported to hardly participate in NCL at practical reaction rates (Fig. 1).2,3
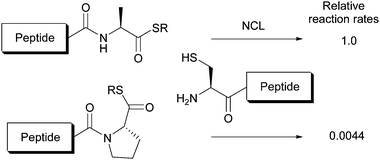 | ||
| Fig. 1 Comparison of reactivity of alanyl and prolyl thioesters in NCL reaction.3a NCL conditions: 6 M Gn·HCl–0.2 M phosphate–20 mM TCEP–30 mM MPAA, pH 7.0, room temperature. | ||
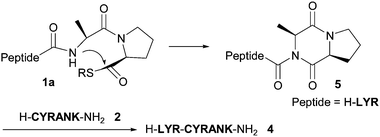
Therefore, an innovative method using prolyl selenoesters as thioester equivalents has been investigated. Although the use of the selenoester enables rapid NCL at the proline site, the NCL requires an excess amount of N-terminal cysteinyl peptides for quantitative NCL conversion and is incompatible with nonligation site cysteines.4 As an alternative, we re-examined the potential use of peptidyl prolyl thioesters for exploring suitable conditions for NCL at the proline site. Initially, a model peptidyl prolyl thioester, H-LYRAP-SCH2CH2CO-L-NH2 (1a), was subjected to NCL with H-CYRANK-NH2 (2) in 3 M guanidine (Gn)·HCl–0.5 M sodium phosphate in the presence of 40 mM tris(2-carboxyethyl)phosphine hydrochloride (TCEP)5 and 30 mM (4-carboxymethyl)thiophenol (MPAA),6 pH 7.3, at 37 °C. Contrary to our expectations, a significant amount of an NCL product, H-LYRAPCYRANK-NH2 (3a), was observed after 24 h, with concomitant formation of the alanyl proline-deleted peptide, H-LYRCYRANK-NH2 (4), even though the attempted reaction did not go to completion. The deletion peptide probably has its origin in the formation of N-arginyl diketopiperazine 5, followed by nucleophilic attack of the N-terminal cysteinyl residue of 2 (Fig. 2). Although the reaction conditions that afforded the ligated products 3a and 4 were far from being satisfactory for practical use in NCL, this unexpected result encouraged us to continue our study of the use of peptidyl prolyl thioesters in NCL.
To evaluate the NCL conditions for prolyl thioesters, model peptides without affording deletion peptides were needed. Since kinetic analysis of diketopiperazine formation from Xaa-Pro-p-nitroanilide (Xaa = Ala, Val, Gly, Phe, or Arg) indicated that the Val–Pro peptide was insensitive to diketopiperazine formation,7 Val was substituted for Ala in model peptide 1a. This replacement suppressed the side reaction. Further evaluations were carried out using this model prolyl thioester, H-LYRVP-SCH2CH2CO-L-NH2 (1b); the results are given in Table 1.
| Entry | Conditionsa | Fraction ligatedb | Rate constants kc (M−1 s−1) |
|---|---|---|---|
| a All reactions were performed in the presence of 6 M Gn·HCl at pH 7.0 using equimolar amounts of peptides. b The fraction ligated was determined by integration of 3b (integ. 3b) as a fraction of the sum of the unreacted 2 (integ. 2) + integ. 3b. c Second order rate constants were derived from the integrated rate equation 1/[2] = kt + 1/[2]0. d 6 h reaction. e 24 h reaction. f Ala-substituted peptide (H-LYRVA-SR) was subjected to the model ligation reaction. g See ref. 8. | |||
| 1 | 0.1 M phosphate, 4% benzylmercaptan, 4% thiophenol, 37 °C | 0.07d | 4.70 × 10−3 |
| 0.22e | |||
| 2 | 0.1 M phosphate, 4% thiophenol, 37 °C | 0.06d | 4.30 × 10−3 |
| 0.39e | |||
| 3 | 0.1 M phosphate, 4% thiophenol, 20 mM TCEP, 37 °C | 0.08d | 7.10 × 10−3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) g g |
| 0.33e | |||
| 4 | 0.1 M phosphate, 20 mM TCEP, 30 mM MPAA, 37 °C | 0.20d | 7.30 × 10−3 |
| 0.63e | |||
| 5 | 0.4 M phosphate, 20 mM TCEP, 30 mM MPAA, 37 °C | 0.32d | 12.1 × 10−3 |
| 0.77e | |||
| 6 | 0.1 M phosphate, 20 mM TCEP, 30 mM MPAA, 25 °C | 0.06d | 3.10 × 10−3 |
| 0.36e | |||
| 7 | 0.4 M phosphate, 20 mM TCEP, 250 mM MPAA, 37 °C | 0.67d | 55.5 × 10−3 |
| 8 | 0.4 M phosphate, 20 mM TCEP, 250 mM MPAA, 50 °C | 0.84d | 143 × 10−3 |
| 9 | 0.4 M phosphate, 20 mM TCEP, 30 mM MPAA, 50 °C | 0.58d | 39.4 × 10−3 |
| 0.93e | |||
| 10 | 0.4 M phosphate, 167 mM TCEP, 250 mM MPAA, 50 °C | 0.85d | 158 × 10−3 |
| 0.99e | |||
| 11 | 0.1 M phosphate, 167 mM TCEP, 250 mM MPAA, 50 °C | 0.83d | 129 × 10−3 |
| 0.98e | |||
| 12f | 0.1 M phosphate, 20 mM TCEP, 30 mM MPAA, 37 °C | 0.97d | 800 × 10−3 |
| 0.98e | |||
| 13f | 0.4 M phosphate, 167 mM TCEP, 250 mM MPAA, 37 °C | 1.0d | 2650 × 10−3 |
NCLs between equimolar amounts of 1b and 2 were conducted. A comparison of entries 3 and 4 indicates that MPAA is a more efficient catalyst for NCL involving prolyl thioesters than thiophenol.8 Furthermore, increasing the concentration of MPAA enhances the progress of the NCL (Table 1, entry 5 vs. 7). The reaction temperature is also a vital factor for the NCL (e.g., Table 1, entry 4 vs. 6; 5 vs. 9). Although the enhancement of reaction by increasing the concentration of phosphate salts is not precluded at this stage, enough experimental data are yet to be accumulated. Based on data in Table 1, we speculated that NCL at the proline site, which originally proceeded two orders of magnitude more slowly than NCL of the corresponding alanyl thioester (Table 1, entry 4 vs. 12), could be put to practical use by using a high concentration of MPAA (250 mM) and a relatively high reaction temperature (50 °C) (Table 1, entry 10 vs. 12). NCL of 1b with 2 in 6 M Gn·HCl–0.4 M phosphate–167 mM TCEP–250 mM MPAA at 50 °C for 24 h yielded the desired ligated peptide 3b in 74% isolated yield, without the accompanying epimerized product (see ESI‡).
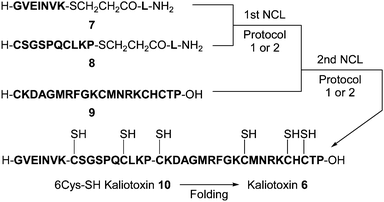
We next investigated one-pot/sequential NCLs operating under kinetically controlled NCL (KCL) conditions9,10 using prolyl thioesters. For the desired KCL, enhancement of the reactivity of the prolyl thioesters in the second NCL step is necessary, and this could be achieved by increasing the MPAA concentration and/or raising the reaction temperature. To verify the feasibility of KCL using prolyl thioesters, one-pot syntheses of the 37-residue peptide kaliotoxin 6 (H-GVEINVKCSGSPQCLKP-CKDAGMRFGKCMNRKCHCTP-OH) were attempted by applying two protocols (Scheme 1).
Protocol 1 used only temperature adjustment to enhance the reactivity of the prolyl thioester in the second NCL step, and the progress of the reactions is shown in Fig. 3. The first NCL, in protocol 1 of 7 (1.5 μmol) with 8 (1.5 μmol) proceeded in 6 M Gn·HCl–0.4 M phosphate in the presence of 167 mM TCEP and 250 mM MPAA, at 25 °C, pH 7.0, to yield the desired ligated peptidyl prolyl thioester (7 + 8), along with a small amount of a cyclic peptide derived from intramolecular NCL of 8 (Fig. 3A and B). Peptide 9 (1.5 μmol), in the same buffer as that used for the first NCL, was then added, and the reaction temperature was increased (50 °C) to initiate the second NCL. After 24 h, the reaction went almost to completion to give the ligated peptide (10) in a one-pot manner in 30% isolated yield (Fig. 3C and D).
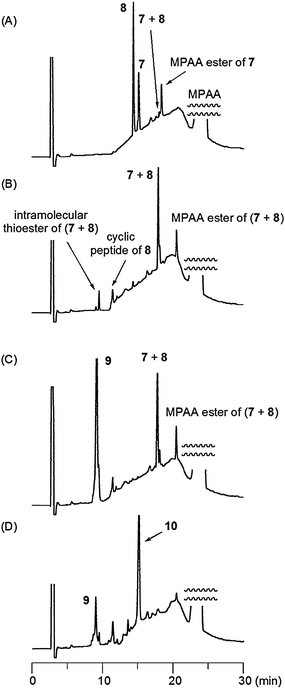 | ||
| Fig. 3 HPLC analysis of progress of reaction for 10 using protocol 1: (A) first NCL (t < 3 min); (B) first NCL (t = 3 h); (C) second NCL (t < 3 min); (D) second NCL (t = 24 h). HPLC conditions: see ESI.‡ | ||
Protocol 2 used modulation of both the MPAA concentration and the reaction temperature. The first NCL of 7 with 8 in 6 M Gn·HCl–0.4 M phosphate in the presence of 20 mM TCEP and 30 mM MPAA at 37 °C, followed by addition of a solution of 9 in 6 M Gn·HCl–0.4 M phosphate–246 mM TCEP–370 mM MPAA (final MPAA 200 mM),11 pH 7.0, and subsequent raise in the reaction temperature (50 °C), gave the ligated peptide 10 in a one-pot manner in 29% isolated yield. Although protocols 1 and 2 proceed with comparable efficiency, protocol 1 is recommended because of the ease of operation.11
Purified 10 (0.32 mg peptide per mL) was then subjected to a folding process in 1 M Gn·HCl–16 mM phosphate–42 mM Tris·HCl–0.0025% (v/v) Tween 20 in the presence of 1.7 mM reduced glutathione and 0.17 mM oxidized glutathione at 25 °C, pH 8.0, to give kaliotoxin 6 in 48% isolated yield (calculated on the basis of purified 10).
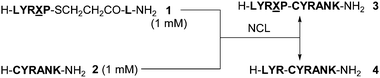
We then further examined the limitations of prolyl thioesters in terms of the formation of deletion peptides. Model prolyl thioesters (H-LYR![[X with combining low line]](https://www.rsc.org/images/entities/char_0058_0332.gif) P-SCH2CH2CO-L-NH2 (1), where X is any of the 20 naturally occurring amino acids) were subjected to NCLs with peptide 2 in 6 M Gn·HCl–0.4 M phosphate–167 mM TCEP–250 mM MPAA at 50 °C, pH 7.0, to examine the ratio of deleted peptide 4 to the desired peptides 3 (Table 2). As the data in Table 2 show, the thioesters 1, except those with X = Gly, Asn, Ser, Thr or Cys, can participate in NCL with 2 to afford the desired ligated peptides 3 with a fraction of over 0.73 ligated. Ligation of Ser/Thr-containing peptides gave considerable amounts of the hydrolyzed peptide (H-LYR-OH) instead of 4. Nucleophilic attack of side-chain hydroxyl groups on the activated carbonyl of peptidyl diketopiperazines led to the formation of the hydrolyzed peptide. Acidic deprotection of the Cys-peptide resin afforded H-LYR-SCH2CH2CO-L-NH2 as a major product via a mechanism similar to that involved in Aimoto's cysteinyl prolyl ester unit.12
P-SCH2CH2CO-L-NH2 (1), where X is any of the 20 naturally occurring amino acids) were subjected to NCLs with peptide 2 in 6 M Gn·HCl–0.4 M phosphate–167 mM TCEP–250 mM MPAA at 50 °C, pH 7.0, to examine the ratio of deleted peptide 4 to the desired peptides 3 (Table 2). As the data in Table 2 show, the thioesters 1, except those with X = Gly, Asn, Ser, Thr or Cys, can participate in NCL with 2 to afford the desired ligated peptides 3 with a fraction of over 0.73 ligated. Ligation of Ser/Thr-containing peptides gave considerable amounts of the hydrolyzed peptide (H-LYR-OH) instead of 4. Nucleophilic attack of side-chain hydroxyl groups on the activated carbonyl of peptidyl diketopiperazines led to the formation of the hydrolyzed peptide. Acidic deprotection of the Cys-peptide resin afforded H-LYR-SCH2CH2CO-L-NH2 as a major product via a mechanism similar to that involved in Aimoto's cysteinyl prolyl ester unit.12
| Entry | X | Ratiob | Fraction ligatedc |
|---|---|---|---|
| a All NCLs of an equimolar mixture of thioesters 1 and 2 were carried out in 6 M Gn·HCl–0.4 M phosphate–167 mM TCEP–250 mM MPAA at 50 °C, pH 7.0, for 24 h. b Ratio was determined by HPLC separation and integration of the deletion peptide 4 (integ. 4) detected at 220 nm as a ratio to the desired ligated peptides 3 (integ. 3). c The fraction ligated was calculated from the equation (integ. 3/(integ. 2 (unreacted) + integ. 3 + integ. 4)). d Five-residue-extended peptide (H-LRANK-LYRXP-SR) was used for satisfactory resolutions in the HPLC analyses. e The deletion peptide 4 was not observed, but a hydrolyzed peptide (H-LYR-OH) was detected. f Requisite peptide (H-LYRCP-SR) was not obtained. | |||
| 1 | Ala (1a) | 0.24 | 0.75 |
| 2 | Val (1b) | 0 | 0.97 |
| 3 | Gly (1c) | 2.3 | 0.29 |
| 4 | Asp (1d) | 0.33 | 0.73 |
| 5 | Asnd (1e) | 0.97 | 0.51 |
| 6 | Glu (1f) | 0.07 | 0.92 |
| 7 | Glnd (1g) | 0.02 | 0.93 |
| 8 | Sere (1h) | 0 | 0.29 |
| 9 | Thre (1i) | 0 | 0.51 |
| 10 | Leu (1j) | 0 | 0.84 |
| 11 | Ile (1k) | 0 | 0.92 |
| 12 | Met (1l) | 0.05 | 0.84 |
| 13 | Pro (1m) | 0 | 0.96 |
| 14 | Phe (1n) | 0.04 | 0.94 |
| 15 | Tyr (1o) | 0 | 0.82 |
| 16 | Trp (1p) | 0 | 0.84 |
| 17 | His (1q) | 0.28 | 0.73 |
| 18 | Lys (1r) | 0.03 | 0.81 |
| 19 | Arg (1s) | 0 | 0.80 |
| 20 | Cysf | — | — |
In conclusion, peptidyl prolyl thioesters with an appropriate amino acid at the position adjacent to proline can be used in NCL by tuning the reaction conditions, i.e., the catalyst concentration and the reaction temperature. Our protocol for NCL at the proline site enabled the ligation between equimolar amounts of coupling partners, and was extended to one-pot/sequential ligation due to its controllable reactivity. It is of note that prolyl thioesters, which have been thought to be of no use in NCLs, are shown to be versatile reagents in ligation chemistry.
This research was supported in part by a Grant-in-Aid for Scientific Research (KAKENHI) and research grants from the Takeda Science and the Uehara Memorial Foundations. K.S. is grateful for a scholarship from the Yoshida Scholarship Foundation.
Notes and references
- S. B. H. Kent, Chem. Soc. Rev., 2009, 38, 338 RSC.
- (a) T. M. Hackeng, J. H. Griffin and P. E. Dawson, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 10068 CrossRef CAS; (b) S. D. Townsend, Z. Tan, S. Dong, S. Shang, J. A. Brailsford and S. J. Danishefsky, J. Am. Chem. Soc., 2012, 134, 3912 CrossRef CAS PubMed.
- (a) S. B. Pollock and S. B. H. Kent, Chem. Commun., 2011, 47, 2342 RSC; (b) A. Choudhary, C. G. Fry, K. J. Kamer and R. T. Raines, Chem. Commun., 2013, 49, 8166 RSC.
- T. Durek and P. F. Alewood, Angew. Chem., Int. Ed., 2011, 50, 12042 CrossRef CAS PubMed.
- H. Rohde, J. Schmalisch, Z. Harpaz, F. Diezmann and O. Seitz, ChemBioChem, 2011, 12, 1396 CrossRef CAS PubMed.
- E. C. B. Johnson and S. B. H. Kent, J. Am. Chem. Soc., 2006, 128, 6640 CrossRef CAS PubMed.
- C. Goolcharran and R. T. Borchardt, J. Pharm. Sci., 1998, 87, 283 CrossRef CAS PubMed.
- Rate constants were calculated on the basis of disappearance of 2. Presence of thiophenol resulted in unproductive consumption of peptide 2 although its reason is unclear. Therefore, the use of fraction ligated is adequate for the comparison of entries 3 and 4.
- (a) D. Bang, B. L. Pentelute and S. B. H. Kent, Angew. Chem., Int. Ed., 2006, 45, 3985 CrossRef CAS PubMed; (b) T. Durek, V. Y. Torbeev and S. B. H. Kent, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 4846 CrossRef CAS PubMed; (c) K. Sato, A. Shigenaga, K. Kitakaze, K. Sakamoto, D. Tsuji, K. Itoh and A. Otaka, Angew. Chem., Int. Ed., 2013, 52, 7855 CrossRef CAS PubMed.
- (a) A. Otaka, K. Sato, H. Ding and A. Shigenaga, Chem. Rec., 2012, 12, 479 CrossRef CAS PubMed; (b) L. Raibaut, N. Ollivier and O. Melnyk, Chem. Soc. Rev., 2012, 41, 7001 RSC.
- Although MPAA solution (470 mM) is needed to increase the concentration of MPAA (up to 250 mM), high concentration of MPAA (470 mM) is difficult to prepare due to supersaturation of MPAA.
- T. Kawakami and S. Aimoto, Tetrahedron, 2009, 65, 3871 CrossRef CAS PubMed.
Footnotes |
| † During the reviewing process, related literature on prolyl thioester appeared. See L. Raibaut, P. Seeberger and O. Melnyk, Org. Lett., 2013, 15, 5516, DOI: 10.1021/ol402678a |
| ‡ Electronic supplementary information (ESI) available: Experimental details of peptide syntheses including HPLC charts and rate measurements. See DOI: 10.1039/c3cc47228k |
| This journal is © The Royal Society of Chemistry 2014 |