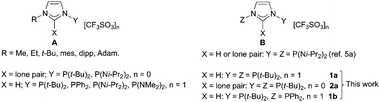A novel, rigid diphosphine with an active NHC spacer; di- and trinuclear complexes of d10 coinage metals†‡
Pengfei
Ai
a,
Andreas A.
Danopoulos
*ab,
Pierre
Braunstein
*a and
Kirill Yu.
Monakhov
c
aLaboratoire de Chimie de Coordination, Institut de Chimie (UMR 7177 CNRS), Université de Strasbourg, 4 rue Blaise Pascal, 67081 Strasbourg Cedex, France. E-mail: danopoulos@unistra.fr; braunstein@unistra.fr
bInstitute for Advanced Study, USIAS, Université de Strasbourg, France
cInstitut für Anorganische Chemie, RWTH Aachen University, Landoltweg 1, 52074 Aachen, Germany
First published on 24th October 2013
Abstract
A novel N,N′-diphosphanyl-imidazol-2-ylidene acts as a stable, hybrid PCNHCP ligand for M2 or linear M3 (M = Cu, Ag, Au) arrays with metallophilic interactions.
The coordination of both NHC and P donors to the same metal has led to complexes with superior catalytic properties to their all-NHC or all-P analogues.1 Although the origins of this synergism are mechanistically diverse and not yet fully understood,2 advancements can be anticipated by precisely tuning the metal coordination sphere through the use of multidentate P-functionalized NHC ligands, featuring donor atoms in fixed positions relative to each other. P-functionalized NHC pro-ligands and ligands3 are thus of high current interest.4 Very recently, a few N-phosphanyl imidazoliums and their corresponding NHCs have been described (Scheme 1).5 Distinctive characteristics of these ligands include: (i) a narrow bite angle and high rigidity; (ii) covalent P–N(heterocycle) bonds facilitating direct electronic communication between the donors; (iii) aptitude of the phosphanyl fragment for 1,2-migration from N(heterocycle) to C2(heterocycle) which, in order to be suppressed, places constraints on the nature of the P-substituents. Recent focus5 has mainly been on bidentate (pro)-ligands of type A and their complexes with group 10 and 11 metals. In contrast, molecules of type B are only known for Y = Z = P(Ni-Pr2)2,5a which limits their scope in coordination chemistry; although combining P- and NHC-strong σ-donors should have obvious potential in multimetallic chemistry and relevant applications, especially with coinage metals.6
Here we describe novel rigid diphosphines of type B (Scheme 1) with an imidazolium or an NHC spacer, investigate their stability by experimental and DFT methods with respect to phosphanyl migration aptitude from the N to the NCNHCN carbon atom, and characterise rare NHC, phosphine complexes,3c,5c,7 with either κ2-PCNHCP or unprecedented, rigid κ3-PCNHCP bonding to two or three coinage metals, respectively.
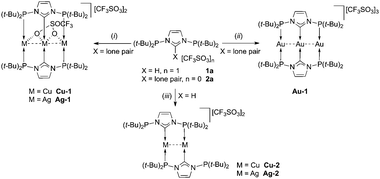
Critical for the successful isolation of 1a,b is the use of the non-nucleophilic triflate anion (Scheme 2)‡.5a,b
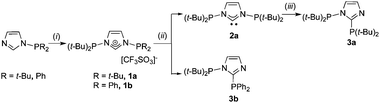 | ||
| Scheme 2 Reagents and conditions: (i) NaO3SCF3, PBr(t-Bu)2, THF, 0 °C to RT; (ii) NaN(SiMe3)2, ether, 0 °C to RT; (iii) heating to 120 °C, overnight. | ||
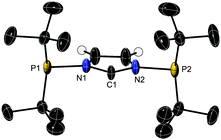
Deprotonation of 1a with NaN(SiMe3)2 in ether gave 2a while similar attempts with 1b under various conditions led to the non-symmetric diphosphine 3b, after PPh2 migration (see the DFT study below). The air sensitive 2a is thermally stable at room temperature (t1/2 ≃ 6 h at 120 °C) and has been fully characterised, including by X-ray diffraction (Fig. 1) (for comparison the structure of 1a was also determined; Fig. S1 and Table S1, ESI‡).
In the highly symmetrical 1a and 2a,‡ the orientation of the lone pairs at P differs: in 1a it is syn to the proton at (crystallographic)C1 but in 2aanti to the lone pair at the CNHC. These syn, anti-orientations were also found by DFT calculations at the BP86/TZ2P level to be energetically favoured (Tables S2 and S3, ESI‡). Similar conformations have been observed in other ‘pincer’ type NHCs8 and mono-N-phosphanyl-substituted NHC ligands.5e
The stability of 2a in conjunction with unsuccessful efforts to isolate or detect spectroscopically analogous NHCs with PR2 groups where R ≠ t-Bu (Scheme 2 for R = Ph) prompted us to attempt to rationalize our observations by DFT methods.‡ Molecule 3a§ with the N(C–PR2) bond is thermodynamically more stable than 2a with the Nimid–PR2 bonds by ca. 90 kJ mol−1 (R = t-Bu); the same applies to R = Ph, the energy difference being 100 kJ mol−1; Scheme S1, ESI.‡ In contrast, the HOMO–LUMO gaps (ΔEH–L) of the N(C–PR2) and Nimid–PR2 isomers (R = t-Bu, Ph) are very different and follow the order for R = t-Bu: ΔEH–L[Nimid–PR2] = 4.05 eV > ΔEH–L[N(C–PR2)] = 3.75 eV and for R = Ph: ΔEH–L[Nimid–PR2] = 2.87 eV < ΔEH–L[N(C–PR2)] = 3.53 eV (Scheme S1, ESI‡). These results may suggest that the formation of 2a is kinetically controlled. This is also supported by a Hirshfeld charge analysis for the R2P+ and (t-Bu)2P-NHC− fragments. The charge on P in Ph2P+ (+0.32) is less positive than that in t-Bu2P+ (+0.36).9 In turn, the charges on the Nimid and CNHC sites in the anionic fragment are −0.30 and −0.19, respectively. Combining these fragments leads preferentially to 2a and 3b. The rearrangement from the Nimid–PR2 to N(C–PR2) bond isomer requires a higher energy barrier for R = t-Bu than for R = Ph. Further theoretical analyses of these and related molecules are in progress.
Complexation studies of 2a (Scheme 3) showed that the nature of the reaction product dramatically depends on the combination of a metal precursor and the nature of the ligand source (i.e. imidazolium vs. free NHC).
All complexes were air stable as solids for short periods of time (ca. 1–2 h). Particularly diagnostic are: (i) the 31P NMR singlet at δ 102.1 for Cu-1, two doublets centered at δ 119.4 for Ag-1 (JP–109Ag = 541.0 Hz, JP–107Ag = 467.3 Hz) and one singlet at δ 141.0 for Au-1; (ii) the shift of the 13C NMR signals due to CNHC from δ 232.7 in 2a to δ 194.2 (t, 2JP–C = 22.4 Hz) in Cu-1, to δ 200.3 (overlapping m, 1JC–109Ag = 213.1 Hz, 1JC–107Ag = 184.2 Hz) for Ag-1, and to δ 194.3 (t, 2JP–C = 14.6 Hz) for Au-1.
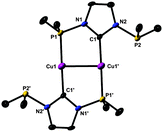
The structures of Cu-1 and Ag-1 (Fig. 2 left and Fig. S3, ESI‡) are very similar: the complex cation features an almost linear (171.81° and 171.41°, respectively) trinuclear metal arrangement with two bridging ligands 2a coordinated in such a way that the internal metal is bound to two NHC donors, and the two terminal metals to two P donors from different ligands.
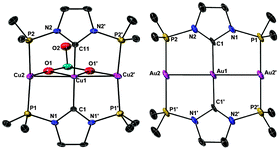 | ||
| Fig. 2 Structures of the cation in Cu-1·2CH2Cl2 (left) and one, centrosymmetric cation in Au-1·2MeCN (right). H atoms, CF3 group of coordinated triflate, uncoordinated triflate anions, the t-Bu Me groups and solvent molecules are omitted.‡ Selected bond lengths (Å) and angles [°] for Cu-1·2CH2Cl2: Cu1–C1 1.947(8), Cu1–C11 1.943(8), Cu2–P1 2.222(2), Cu2–P2 2.226(2), Cu1–Cu2 2.5761(9), Cu2–O1 2.611, Cu1–O1 2.700, C1–Cu1–C11 177.1(3), Cu2–Cu1–Cu2′ 171.81(7), P1–Cu2–P2 161.86(9). For Au-1·2MeCN: Au1–C1 2.068(5), Au2–P2 2.3168(14), Au2′–P1 2.3141(15), Au1–Au2 2.7584(2), P1–Au2′–P2′ 178.69(5). | ||
The terminal and internal metal centres are μ2-bridged pairwise by two different oxygen atoms of a triply-bridging triflate. We are not aware of any other example of triflate bridging three different metals in a linear arrangement via two oxygen atoms. The geometry around the internal metal (not considering the M–M interactions) can be described as ‘sawhorse’ and that of the terminal metals as distorted ‘T-shaped’. The short metal–metal distances (2.5761(9) Å for Cu-1 and 2.7599(3) Å for Ag-1) imply fully-supported metallophilic d10–d10 interactions.6d Discrete molecular, open (i.e. non-triangular), nearly linear M3 species (M = CuI, AgI) are rare.10Cu-1 and Ag-1 constitute unique examples of linear M3 species with NHC donors.
The structure of Au-1 contains two different trinuclear Au3 cations in the unit cell (Fig. 2 right),‡ one featuring strictly linear (symmetry imposed) metal arrangement and the other slightly deviating from it. There are also two bridging 2a ligands coordinated as in the Cu and Ag analogues; however, no triflate coordination is observed. Here too, metallophilic d10–d10 interactions are present (2.7584(2) and 2.7628(2) Å). Discrete molecular, linear Au3 species are uncommon, fully-supported by linear triphosphines with ethylene or methylene linkers, semi-supported or unsupported.10d,11 Amongst these examples, Au-1 shows the shortest Au–Au distances.
Further insight into the coordination chemistry of 2a was obtained by attempts to form dinuclear complexes (Scheme 3). Particularly revealing is the reaction of 1a with CuN(SiMe3)2 (in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio in THF), which led cleanly to the new dinuclear complex Cu-2. This molecule (Fig. 3) features a dinuclear complex with the [Cu2(2a)2] core,‡ with each metal exhibiting an identical coordination sphere comprising one NHC and one P donor in an almost linear trans-arrangement; the second P donor remains dangling. This behaviour is analogous to that of bidentate ligands recently reported,5c,g where NHC coordination and dangling P donors have also been observed. Interestingly, the orientation of the lone pairs of the dangling P donors in Cu-2, as judged from the observed conformation in the crystal, is towards the metal. The NHC rings and the coordinated Cu centres are coplanar. The internuclear distance [2.6827(12) Å] is longer than that in the trinuclear Cu-1. The Cu–CNHC or Cu–P bond lengths in Cu-2 are shorter compared to those in Cu-1. The solution structure of Cu-2 at room temperature cannot be deduced with certainty due to the broad NMR resonances. Thus, the 31P-NMR spectrum shows one broad peak at δ 94.5; broad features are also observed for the t-Bu groups in 1H NMR and 13C NMR spectra implying nonrigidity in solution which could originate from intermolecular ligand exchange or partial dissociation of the P donors followed by rotation around the Cu–CNHC bond; this will be addressed in future studies.
1 ratio in THF), which led cleanly to the new dinuclear complex Cu-2. This molecule (Fig. 3) features a dinuclear complex with the [Cu2(2a)2] core,‡ with each metal exhibiting an identical coordination sphere comprising one NHC and one P donor in an almost linear trans-arrangement; the second P donor remains dangling. This behaviour is analogous to that of bidentate ligands recently reported,5c,g where NHC coordination and dangling P donors have also been observed. Interestingly, the orientation of the lone pairs of the dangling P donors in Cu-2, as judged from the observed conformation in the crystal, is towards the metal. The NHC rings and the coordinated Cu centres are coplanar. The internuclear distance [2.6827(12) Å] is longer than that in the trinuclear Cu-1. The Cu–CNHC or Cu–P bond lengths in Cu-2 are shorter compared to those in Cu-1. The solution structure of Cu-2 at room temperature cannot be deduced with certainty due to the broad NMR resonances. Thus, the 31P-NMR spectrum shows one broad peak at δ 94.5; broad features are also observed for the t-Bu groups in 1H NMR and 13C NMR spectra implying nonrigidity in solution which could originate from intermolecular ligand exchange or partial dissociation of the P donors followed by rotation around the Cu–CNHC bond; this will be addressed in future studies.
Similarly, Ag-2, tentatively formulated as a related dinuclear species, was obtained from 1a and a solution of AgN(SiMe3)2 generated in situ. The 31P-NMR spectrum showed two broad multiplets, also observed in the reaction of 1a with Ag2O in CH2Cl2. However, after attempting crystallisation in CH2Cl2 the product was converted to Ag-1. Understanding the details of this process is under study.
In conclusion, we have prepared a novel stable and rigid tridentate diphosphanylated-imidazol-2-ylidene and gained experimental and computational information on its stability. It serves as a unique platform for the synthesis of novel complexes with the coinage metals, exhibiting rare structural features. Dinuclear complexes with this tridentate ligand have also been isolated and exhibit one dangling P-donor. We are currently investigating their potential as precursors towards trinuclear species. Preliminary data show that Au-1 is luminescent under sunlight or UV-light in acetonitrile solution. The study of further aspects of the donor properties, stability and the versatile coordination chemistry of phosphorylated-imidazol-2-ylidenes is in progress in our laboratory.
A.A.D. thanks the CNRS, the Région Alsace, the Département du Bas-Rhin and the Communauté Urbaine de Strasbourg for a Gutenberg Excellence Chair (2010–2011). K.Y.M. is grateful to the DFG for a postdoctoral reintegration fellowship. We thank the CNRS, the MESR (Paris), the UdS, China Scholarship Council, and the ucFRC (www.icfrc.fr) for financial support and the Service de Radiocristallographie (Institut de Chimie, Strasbourg) for the determination of the crystal structures.
Notes and references
- (a) M. Scholl, S. Ding, C. W. Lee and R. H. Grubbs, Org. Lett., 1999, 1, 953 CrossRef CAS; (b) G. D. Frey, J. Schütz, E. Herdtweck and W. A. Herrmann, Organometallics, 2005, 24, 4416 CrossRef CAS; (c) T. E. Schmid, D. C. Jones, O. Songis, O. Diebolt, M. R. L. Furst, A. M. Z. Slawin and C. S. J. Cazin, Dalton Trans., 2013, 42, 7345 RSC.
- K. Albert, P. Gisdakis and N. Rösch, Organometallics, 1998, 17, 1608 CrossRef CAS.
- (a) W. A. Herrmann, C. Köcher, L. J. Gooßen and G. R. J. Artus, Chem.–Eur. J., 1996, 2, 1627 CrossRef CAS; (b) A. A. Danopoulos, S. Winston, T. Gelbrich, M. B. Hursthouse and R. P. Tooze, Chem. Commun., 2002, 482 RSC; (c) X. Liu and P. Braunstein, Inorg. Chem., 2013, 52, 7367 CrossRef CAS PubMed and references therein.
- (a) O. Kühl, Functionalised N-Heterocyclic Carbene Complexes, Wiley, Weinheim, 2010 Search PubMed; (b) P. G. Edwards and F. E. Hahn, Dalton Trans., 2011, 40, 10278 RSC; (c) S. Gaillard and J.-L. Renaud, Dalton Trans., 2013, 42, 7255 RSC and references therein.
- (a) A. P. Marchenko, H. N. Koidan, A. N. Huryeva, E. V. Zarudnitskii, A. A. Yurchenko and A. N. Kostyuk, J. Org. Chem., 2010, 75, 7141 CrossRef CAS PubMed; (b) A. P. Marchenko, H. N. Koidan, I. I. Pervak, A. N. Huryeva, E. V. Zarudnitskii, A. A. Tolmachev and A. N. Kostyuk, Tetrahedron Lett., 2012, 53, 494 CrossRef CAS PubMed; (c) E. Kühnel, I. V. Shishkov, F. Rominger, T. Oeser and P. Hofmann, Organometallics, 2012, 31, 8000 CrossRef; (d) A. P. Marchenko, H. N. Koidan, E. V. Zarudnitskii, A. N. Hurieva, A. A. Kirilchuk, A. A. Yurchenko, A. Biffis and A. N. Kostyuk, Organometallics, 2012, 31, 8257 CrossRef CAS; (e) P. Nägele, U. Herrlich, F. Rominger and P. Hofmann, Organometallics, 2012, 32, 181 CrossRef; (f) H. Salem, M. Schmitt, U. Herrlich, E. Kühnel, M. Brill, P. Nägele, A. L. Bogado, F. Rominger and P. Hofmann, Organometallics, 2013, 32, 29 CrossRef CAS; (g) A. P. Marchenko, H. N. Koidan, A. N. Hurieva, O. V. Gutov, A. N. Kostyuk, C. Tubaro, S. Lollo, A. Lanza, F. Nestola and A. Biffis, Organometallics, 2013, 32, 718 CrossRef CAS.
- (a) J. C. Garrison and W. J. Youngs, Chem. Rev., 2005, 105, 3978 CrossRef CAS PubMed; (b) J. C. Y. Lin, R. T. W. Huang, C. S. Lee, A. Bhattacharyya, W. S. Hwang and I. J. B. Lin, Chem. Rev., 2009, 109, 3561 CrossRef CAS PubMed; (c) S. Sculfort and P. Braunstein, Chem. Soc. Rev., 2011, 40, 2741 RSC; (d) H. Schmidbaur and A. Schier, Chem. Soc. Rev., 2012, 41, 370 RSC; (e) R. Visbal, I. Ospino, J. M. López-de-Luzuriaga, A. Laguna and M. C. Gimeno, J. Am. Chem. Soc., 2013, 135, 4712 CrossRef CAS PubMed and references therein.
- P. L. Chiu and H. M. Lee, Organometallics, 2005, 24, 1692 CrossRef CAS.
- A. A. Danopoulos, S. Winston and W. B. Motherwell, Chem. Commun., 2002, 1376 RSC.
- J. M. Slattery and S. Hussein, Dalton Trans., 2012, 41, 1808 RSC.
- (a) J. Beck and J. Strähle, Angew. Chem., Int. Ed. Engl., 1985, 24, 409 CrossRef; (b) X. He, K. Ruhlandt-Senge, P. P. Power and S. H. Bertz, J. Am. Chem. Soc., 1994, 116, 6963 CrossRef CAS; (c) Y. Takemura, T. Nakajima, T. Tanase, M. Usuki, H. Takenaka, E. Goto and M. Mikuriya, Chem. Commun., 2009, 1664 RSC; (d) G. S. M. Tong, S. C. F. Kui, H.-Y. Chao, N. Zhu and C.-M. Che, Chem.–Eur. J., 2009, 15, 10777 CrossRef CAS PubMed; (e) V. T. Yilmaz, E. Soyer and O. Büyükgüngör, Polyhedron, 2010, 29, 920 CrossRef CAS PubMed.
- (a) W. Schuh, H. Kopacka, K. Wurst and P. Peringer, Chem. Commun., 2001, 2186 RSC; (b) S. D. Hanna, S. I. Khan and J. I. Zink, Inorg. Chem., 1996, 35, 5813 CrossRef CAS; (c) S. J. Hsu, K. M. Hsu, M. K. Leong and I. J. B. Lin, Dalton Trans., 2008, 1924 RSC.
Footnotes |
| † Dedicated to Michael I. Bruce on this occasion of his 75th birthday, with our warmest congratulations. |
| ‡ Electronic supplementary information (ESI) available: Experimental details and full characterisation of all compounds; crystal structure data for 1a, 2a, Cu-1·2CH2Cl2, Ag-1·2CH2Cl2, Au-1·2MeCN and Cu-2 (CCDC 963084–963089); and computational details. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3cc47370h |
| § This molecule has been obtained in a direct way: see M. Brill, L. Weigel, K. Rübenacker, F. Rominger and P. Hofmann, Heidelberg Forum of Molecular Catalysis, 28 June 2013, poster P60. |
| This journal is © The Royal Society of Chemistry 2014 |