Robust production of purified H2 in a stable, self-regulating, and continuously operating solar fuel generator†
Miguel A.
Modestino
abc,
Karl A.
Walczak
ab,
Alan
Berger
abc,
Christopher M.
Evans
ab,
Sophia
Haussener
ad,
Carl
Koval
a,
John S.
Newman
ac,
Joel W.
Ager
ab and
Rachel A.
Segalman
*abc
aJoint Center for Artificial Photosynthesis, Lawrence Berkeley National Laboratory, 1 Cyclotron Road, Mail Stop 976, Berkeley, CA 94720, USA. E-mail: segalman@berkeley.edu; Tel: +1-510-642-7998
bMaterials Sciences Division, Lawrence Berkeley National Laboratory, Berkeley, CA 94720, USA
cDepartment of Chemical and Biomolecular Engineering, University of California, Berkeley, CA 94720, USA
dInstitute of Mechanical Engineering, Ecole Polytechnique Fédérale de Lausanne, 1015 Lausanne, Switzerland
First published on 28th November 2013
Abstract
The development of practical solar-driven electrochemical fuel generators requires the integration of light absorbing and electrochemical components into an architecture that must also provide easy separation of the product fuels. Unfortunately, many of these components are not stable under the extreme pH conditions necessary to facilitate ionic transport between redox reaction sites. By using a controlled recirculating stream across reaction sites, this work demonstrates a stable, self-regulating and continuous purified solar-hydrogen generation from near neutral pH electrolytes that yield continuous nearly pure H2 streams with solar-fuel efficiencies above 6.2%.
Broader contextPractical solar fuel generators will likely require the use of economically viable components, the operation under environmentally benign conditions and the robust, stable and continuous production of purified fuels over long periods of time. To date, there have been no reports on solar fuel generators that satisfy all these constraints. One of the main challenges is the development of material systems that are stable under strong acid or basic electrolytes, or the development of alternate ion-transport pathways that can lead to continuous water splitting at moderate pH while evolving pure hydrogen streams. This manuscript presents a robust solution to this fundamental challenge, and demonstrates for the first time a continuous and self-regulating solar-hydrogen generator operated under near-neutral buffered electrolytes. |
Converting solar energy directly into chemical fuel (i.e. artificial photosynthesis) could potentially provide a carbon-neutral alternative to fossil fuels.1–5 Since the discovery of light driven electrochemical water splitting in the early 1970s by Fujishima and Honda,6 there has been continuing research into the development of photoelectrochemical (PEC) cells which has resulted in systems with solar to hydrogen conversion efficiencies as high as 18%.7–9 These laboratory-scale demonstrations have tended to rely on multi-junction photovoltaic (PV) components, water splitting catalysts, and other complex components that have limited stability under electrolytes necessary for efficient operation (i.e. strong acids or bases).10,11 Recently, systems composed of multi-junction amorphous silicon PV cells and cobalt-based catalysts have been developed that operate at high efficiencies and under near-neutral buffered electrolytes.12,13 Importantly, with few exceptions,9 previous device demonstrations co-evolve hydrogen and oxygen in a common electrolyte solution. For practical implementation of solar-fuel generators, the reduction (i.e. H2 for water splitting) and oxidation (O2) products need to be produced at physically separated sites. This is of vital importance for several reasons. (1) The gaseous fuel can be collected in its pure form avoiding the need for expensive gas separation steps. (2) Potential losses due to crossover of the reduction product to the oxidation site can be minimized and (3) the accumulation of explosive mixtures of fuels and oxidants can be avoided. To achieve the required product separation, integrated devices incorporate ion-conducting and product impermeable membranes to physically separate the electrodes and allow for the electrochemical production of fuels,14,15 an approach analogous to that used in commercial electrolysis systems.16–19
Operation under moderate pH conditions relaxes the stability constraints of practical light absorbing and catalyst components (e.g. silicon based PV components and earth-abundant catalysts)12,13 and mitigates the risks associated with managing large volumes of highly corrosive solutions. However, operation of water splitting systems under near neutral conditions poses significant challenges for ion-transport. In PEC devices, the ionic current between the two electrodes needs to match the photocurrent generated in the device. Under strong basic or acidic conditions, this ionic current is carried by either hydroxide ions or protons, respectively, at steady state. Under moderate pH conditions, the concentration of protons and hydroxide ions is low, requiring a supporting electrolyte that can carry the ionic current, lower the solution resistance, and allow the device to operate at reasonable overpotentials and, consequently, efficiencies. The consequence of having supporting electrolytes carrying the current in the system is the formation of undesirable concentration gradients between the oxidation and reduction sites as the migrating ions are not consumed or regenerated by either reaction. These concentration gradients lead to large overpotentials and can prevent the device from operating efficiently and continuously. Furthermore, the ionic depletion from the oxidation side can result in a significant increase in solution resistance further affecting the device operation. Approaches to mitigate this issue include the use of large volumes of electrolytes per device area to avoid depletion of buffer capacity, but those quantities of electrolytes make the system impractical for large scale implementation.15 This report presents a practical, i.e. membrane-separated, stable and robust solar hydrogen generator that mitigates the formation of large concentration gradients by the introduction of controlled convection streams between oxidation and reduction sites. This allows for continuous, robust and stable system operation and enables the use of a large number of PEC components that, similar to the natural photosynthesis system,20 are only stable under near-neutral pH conditions.12,21 The approach proposed here has the potential to propel the large scale deployment of cost-effective integrated PEC fuel generators.
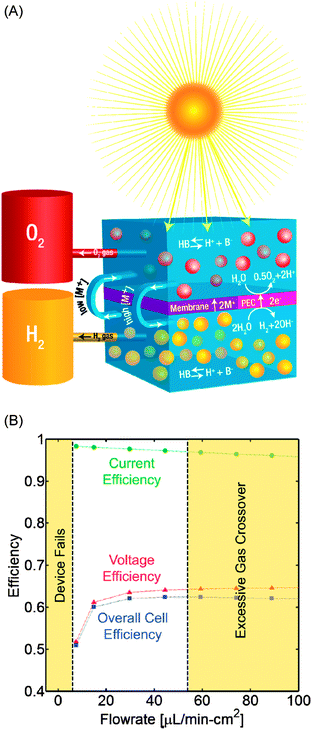
The method proposed to mitigate the formation of large concentration gradients involves the use of small and controlled recirculation streams to balance the electrolyte concentration between the two sides of the membrane separated system while maintaining a relatively low crossover of the product gases (shown in Fig. 1(A)). Since the solubility of gases in solution is small compared to the electrolyte concentration, the convective transport of significant quantities of ions across reaction chambers brings with it only a small fraction of the product gases. It is important to point out that the membrane used in the system is a critical component that allows the system to operate with low ion transport resistance (less than a 30 mV ohmic drop at current densities lower than 10 mA cm−2), while preventing large amounts of gas crossover. Fig. S5 of the ESI† demonstrates the critical role of the membrane at providing low resistance paths for ion transport. Numerical modeling work, detailed in the ESI,† indicates that without the recirculating stream, the device will not be able to function continuously at steady state. However, if the recirculating stream is implemented with flow rates as low as 10 μL min−1 for each cm2 of device the limiting current can be increased above 10 mA cm−2 (i.e. 1 mL min−1 for a 100 cm2 PEC device operating above 10% solar-fuel efficiency). Under these operating conditions, more than 97% of the fuel generated can be collected, with a Faradaic efficiency also above 97%. The output hydrogen stream is predicted to be nearly pure, with an O2 concentration below the flammability limit. The electrolyte concentration difference between the two sides of the device is lower than 0.2 M, corresponding to less than 50 mV concentration overpotential loss in the PEC device.
Fig. 1(B) shows the predicted device operation efficiency associated with the introduction of recirculating streams across the membrane. By increasing the recirculation rate it is possible to reduce the concentration differences between the anode and cathode sides, and by doing so the voltage efficiency of the device increases due to the reduction of the concentration overpotential and solution resistance in the anodic side. On the other hand, an additional gas crossover is introduced by the convective pathways, leading to decreased current efficiencies. The maximum overall cell efficiency at 25 °C is expected to be achieved with a recirculation rate of 44 μL min−1 cm−2, and is fairly insensitive to changes in the recirculation rate for values above 20 μL min−1 cm−2. Furthermore, in the presence of the recirculation stream the solar-fuel generator has the ability to operate at steady state and the concentration profiles would adapt to changes in the current density due to natural fluctuations in the solar adsorption of the device. Importantly, if the recirculation rate is too low (<6 μL min−1 cm−2) the potential drop in the device reaches values that are too high to sustain device operation. In other words, for a given set of conditions, it is impossible to run the device at steady state at a current above the limiting current associated with those conditions. On the other hand, if the recirculation rate is too high, the concentration of O2 in the H2 stream reaches values above the flammability limit (4% v/v) making the device operation impractical and unsafe.22,23 This problem can be alleviated if the device operates at temperatures higher than ambient conditions (likely to happen for systems operated under solar illumination), as the concentration of dissolved gases will decrease and so will the product crossover.24
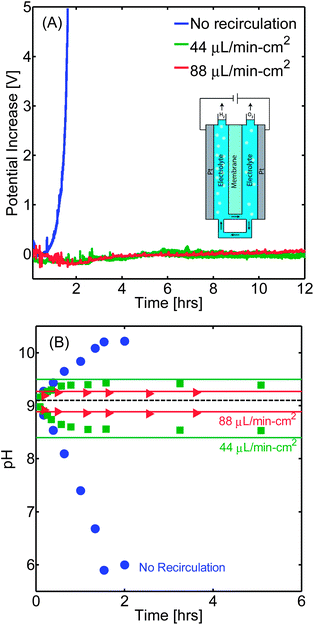
Device operation was demonstrated experimentally in a membrane-separated (ion-conducting 22.5 cm2 potassium-exchanged Nafion® 117) two-compartment electrolysis cell with recirculation channels that was custom-built using a 3D printer. Platinum electrodes were used for both the reduction and oxidation reactions. As an electrolyte, a 1 M potassium borate buffer solution (pH 9.3) was used at a volume corresponding to 2 mL per cm2 of membrane at each compartment. The current through the membrane was set at a value of 10 mA cm−2, which is in the relevant range for integrated solar-driven PEC hydrogen generation. Stable operations for over 12 h were achieved for flow rates above 22 μL min−1 cm−2 without significant concentration differences between compartments or noticeable overpotential build-up (Fig. 2). Furthermore, a continuous stream of nearly pure hydrogen (<3.0% v/v of O2) and oxygen (<2.8% v/v of H2) was collected at the cathode and anode sides, respectively, for 22 μL min−1 cm−2, demonstrating the practical generation of hydrogen fuel from near-neutral pH electrolytes. This is in rough agreement with model predictions of 2.3% v/v and 3.7% v/v, respectively.
In contrast, if the system is operated without recirculation, the buffer capacity is exhausted and, due to the increase in solution resistance and concentration overpotential, the potential to maintain a 10 mA cm−2 current density increases by more than 5 V in less than 2 h of operation. We also showed that the use of recirculating streams could enable the electrolysis of salt water by moderating the pH differences between the oxidation and reduction sides. Systems operated under NaCl solutions with concentrations equivalent to that of natural seawater (3.5% w/v) reached pH values of 1.8 and 13.0 at the anode and cathode sides respectively when no recirculation was used, while upon introducing a recirculating stream of 44 μL min−1 cm−2 the pHs stabilized at 7.3 and 11.0 enabling the operation of the device under milder conditions and with a lower concentration overpotential.
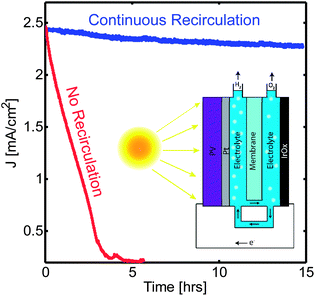
The concept demonstrated for an electrolyzer can be directly applied to a solar-fuel generator where the electrochemical splitting of water is driven by sunlight. The cell described above was modified to accommodate the components of a solar-fuel generator. A commercial triple junction amorphous silicon (tj-a-Si) solar cell with a planar platinum electrode contacted to the cathode was used in the hydrogen-evolution side of the cell, and the front of the PV component was connected to an iridium oxide electrode in the oxygen-evolution side of the cell (Fig. 3(A)). We used here a parallel-plate design to minimize the solution resistance in the system25 but our recirculation approach can also be applied to a wireless design.12 The cell was irradiated with a solar simulator (AM 1.5) for periods of up to 15 h. Under these conditions we demonstrated stable and continuous operation of a cell with a solar-to-fuel efficiency over 2.8% for a complete solar cycle with the use of recirculating streams (Fig. 3). Without the use of recirculating streams the current density in the device decreases monotonically, as a result of the continuous depletion of the buffer ions in the anodic side. Moreover, if a high-efficiency triple-junction solar cell (GaInP2/GaAs/Ge) is used instead of the tj-a-Si cell, the current density through the catalyst and membrane components can reach values up to 9.7 mA cm−2 (corresponding to a 6.2% solar to hydrogen efficiency when normalized by the irradiation area) while still operating stably and continuously with the implementation of recirculating streams (details are presented in the ESI†).
Conclusions
The development of practical PEC devices at moderate pH regimes has been limited because of their inability to operate continuously without significant decrease in their performance. The alternate ion transport pathways proposed in this report enables the incorporation of a wide range of catalytic and light adsorbing components that otherwise would degrade under strong basic or acidic environments. Common challenges arising when operating devices under near-neutral electrolytes include reduced catalyst activity at lower proton or hydroxide concentration. Additionally, when approaching pH 7, gradients created between the electrode surface and the bulk solution start to become important and can be detrimental to the operation of the device unless local mixing elements are introduced.26 The majority of previous reports on direct solar-fuel generators have lacked the robust production of separated product streams, i.e. pure hydrogen and oxygen, via ion-transport membrane components.6,7,9–12,21,27–29 The addition of a recirculation scheme to a membrane-separated PEC system to balance the concentration across the membrane allows for practical and robust solar hydrogen generation. Also wireless PEC systems12 can benefit from this approach, since the use of membranes and convection could allow them to continuously produce pure streams of fuel in the presence of sunlight and under mild electrolyte conditions. The implementable solution presented in this report will allow solar-fuels generators to function continuously and robustly with the ability to self-regulate and operate under safe electrolytes even from abundant natural water sources. Although noble metal catalysts were selected for this demonstration, other efficient earth-abundant catalysts can be incorporated and could operate continuously by using small recirculation flow rates. Furthermore, the system can be easily designed so that the catalyst surface area and operating pH are optimized for maximum solar-to-hydrogen efficiency. The results presented here are universal and the methodology described above provides a practical and easy-to-implement path for the fabrication of solar-fuel generators. This will ease the scale-up process of artificial-photosynthesis systems and open avenues for their incorporation in tomorrow's world energy landscape.Acknowledgements
This material is based on the work performed by the Joint Center for Artificial Photosynthesis, a DOE Energy Innovation Hub, supported through the Office of Science of the U.S. Department of Energy under Award number DE-SC0004993. The authors would like to thank Kostas Goulas and Peter Soler for experimental assistance in measuring gas concentrations and developing the recirculating cell.Notes and references
- S. Chu and A. Majumdar, Nature, 2012, 488, 294–303 CrossRef CAS PubMed.
- N. S. Lewis and D. G. Nocera, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 15729–15735 CrossRef CAS PubMed.
- J. Barber and P. D. Tran, J. R. Soc. Interface, 2013, 10, 20120984 CrossRef PubMed.
- T. A. Faunce, W. Lubitz, A. W. Rutherford, D. MacFarlane, G. F. Moore, P. Yang, D. G. Nocera, T. A. Moore, D. H. Gregory, S. Fukuzumi, K. B. Yoon, F. A. Armstrong, M. R. Wasielewski and S. Styring, Energy Environ. Sci., 2013, 6, 695–698 Search PubMed.
- Y. Tachibana, L. Vayssieres and J. R. Durrant, Nat. Photonics, 2012, 6, 511–518 CrossRef CAS.
- A. Fujishima and K. Honda, Nature, 1972, 238, 37–38 CrossRef CAS.
- O. Khaselev and J. A. Turner, Science, 1998, 280, 425–427 CrossRef CAS.
- S. Licht, B. Wang, S. Mukerji, T. Soga, M. Umeno and H. Tributsch, J. Phys. Chem. B, 2000, 104, 8920–8924 CrossRef CAS.
- G. Peharz, F. Dimroth and U. Wittstadt, Int. J. Hydrogen Energy, 2007, 32, 3248–3252 CrossRef CAS PubMed.
- Y. Yamada, N. Matsuki, T. Ohmori, H. Mametsuka, M. Kondo, A. Matsuda and E. Suzuki, Int. J. Hydrogen Energy, 2003, 28, 1167–1169 CrossRef CAS.
- R. E. Rocheleau, E. L. Miller and A. Misra, Energy Fuels, 1998, 12, 3–10 CrossRef CAS.
- S. Y. Reece, J. A. Hamel, K. Sung, T. D. Jarvi, A. J. Esswein, J. J. H. Pijpers and D. G. Nocera, Science, 2011, 334, 645–648 CrossRef CAS PubMed.
- F. F. Abdi, L. Han, A. H. M. Smets, M. Zeman, B. Dam and R. van de Krol, Nat. Commun., 2013, 4 DOI:10.1038/ncomms3195 , Article number: 2195.
- S. Haussener, C. Xiang, J. M. Spurgeon, S. Ardo, N. S. Lewis and A. Z. Weber, Energy Environ. Sci., 2012, 5, 9922–9935 CAS.
- E. A. Hernandez-Pagan, N. M. Vargas-Barbosa, T. Wang, Y. Zhao, E. S. Smotkin and T. E. Mallouk, Energy Environ. Sci., 2012, 5, 7582–7589 CAS.
- K. A. Mauritz and R. B. Moore, Chem. Rev., 2004, 104, 4535–4585 CrossRef CAS.
- A. S. Aricò, S. Siracusano, N. Briguglio, V. Baglio, A. Blasi and V. Antonucci, J. Appl. Electrochem., 2013, 43, 107–118 CrossRef PubMed.
- J. G. Garche, C. K. Dyer, P. T. Moseley, Z. Ogumi, D. A. Rand and B. Scrosati, Encyclopedia of electrochemical power sources, Elsevier, 2009 Search PubMed.
- T. Smolinka, Encyclopedia of Electrochemical Power Sources, Fraunhofer Institute for Solar Energy Systems ISE, Freiburg, Germany, 2009, pp. 394–413 Search PubMed.
- J. Barber, Philos. Trans. R. Soc., A, 2007, 365, 1007–1023 CrossRef CAS PubMed.
- N. A. Kelly and T. L. Gibson, Int. J. Hydrogen Energy, 2006, 31, 1658–1673 CrossRef CAS PubMed.
- H. F. Coward and G. W. Jones, Limits of flammability of gases and vapors, Bureau of Mines, Washington, DC, 1952 Search PubMed.
- H. Le, S. Nayak and M. S. Mannan, Ind. Eng. Chem. Res., 2012, 51, 9396–9402 CrossRef CAS.
- S. Haussener, S. Hu, C. Xiang, A. Z. Weber and N. S. Lewis, Energy Environ. Sci., 2013, 6, 3605–3618 CAS.
- J. Newman, J. Electrochem. Soc., 2013, 160, F309–F311 CrossRef CAS PubMed.
- C. Delacourt, P. L. Ridgway, J. B. Kerr and J. Newman, J. Electrochem. Soc., 2008, 155, B42–B49 CrossRef CAS PubMed.
- A. J. Bard and M. A. Fox, Acc. Chem. Res., 1995, 28, 141–145 CrossRef CAS.
- O. Khaselev, A. Bansal and J. A. Turner, Int. J. Hydrogen Energy, 2001, 26, 127–132 CrossRef CAS.
- K. Ohashi, J. McCann and J. O. M. Bockris, Nature, 1977, 266, 610–611 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details on the electrolyte preparation, electrochemical cell fabrication and characterization, gas composition measurements and device efficiency calculations. See DOI: 10.1039/c3ee43214a |
| This journal is © The Royal Society of Chemistry 2014 |