Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
An energy-efficient route to the rapid synthesis of organically-modified SBA-15 via ultrasonic template removal†
Cyril
Pirez
*ab,
Karen
Wilson
c and
Adam F.
Lee
*bd
aCardiff Catalysis Institute, School of Chemistry, Cardiff University, Cardiff, UK
bDepartment of Chemistry, University of Warwick, Coventry CV4 7AL, UK. E-mail: A.F.Lee@warwick.ac.uk
cEuropean Bioenergy Research Institute, Aston University, Aston Triangle, Birmingham B4 7ET, UK
dSchool of Chemistry, Monash University, Victoria 3800, Australia
First published on 17th September 2013
Abstract
A low energy route for the removal of Pluronic P123 surfactant template during the synthesis of SBA-15 mesoporous silicas is explored. The conventional reflux of the hybrid inorganic–organic intermediate formed during co-condensation routes to Pr-SO3H-SBA-15 is slow, utilises large solvent volumes, and requires 24 h to remove ∼90% of the organic template. In contrast, room temperature ultrasonication in a small methanol volume achieves the same degree of template extraction in only 5 min, with a 99.9% energy saving and 90% solvent reduction, without compromising the textural, acidic or catalytic properties of the resultant Pr-SO3H-SBA-15.
Introduction
The past two decades have seen a host of new applications for silica-derived materials following the discovery of the MCM family of ordered mesoporous silicas1 and subsequent HMS,2 SBA3 and KIT4 families. Among the most widely exploited of all mesoporous silicas is the hexagonal close-packed SBA-15, first reported by Zhao et al. in 1998,5 which possesses large pore diameters spanning 5–30 nm, coupled with excellent thermal, mechanical and chemical resistance properties which underpin its application in catalysis,6–11 enzyme immobilisation12 and separation science.13 Since its discovery, SBA-15 has received over 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 citations in the scientific literature, and now features in approximately 800 new publications every year (Fig. 1).
000 citations in the scientific literature, and now features in approximately 800 new publications every year (Fig. 1).
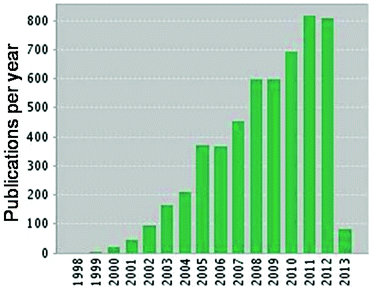 | ||
| Fig. 1 Annual new publications relating to SBA-15 materials. Citation data from Web of Knowledge (02/03/13). | ||
Conventional SBA-15 syntheses employ a nonionic, triblock co-polymer (ethylene oxide–propylene oxide–ethylene oxide) template in acidic media to direct the hydrolysis and condensation of silica precursors and thereby form an intermediate, ordered inorganic–organic mesostructure. Researchers have also reported one pot, co-condensation routes to sulfonic acid functionalised SBA-15, wherein e.g. propyl sulfonic acid (Pr-SO3H) moieties can be inserted directly into the silica walls14 while preserving the parent SBA-15 texture and structure. Such approaches thus offer higher acid site densities than achievable via post-modification of SBA-15 by grafting protocols.10
A key step in the synthesis of mesoporous SBA-15 materials is the removal of the organic surfactant template. This has been explored via several approaches, including photocalcination using vacuum UV,15 ozone treatment16 and oxidation by acidified KMnO4;17 however, by far the most common routes are high temperature calcination (typically around 550 °C) and solvent extraction. Calcination and extraction by reflux under acidic solvents (e.g. 1 M HCl–EtOH) are effective means of template removal,18 but are inapplicable for organically-functionalised silicas such as Pr-SO3H-SBA-15 due to respective combustion or hydrolysis of the desired surface modifier. Solvent extraction under reflux also incurs significant volumes of contaminated waste, and long processing times (usually 24 h) via energy-intensive reflux to achieve a template-free SBA-15 product. Lai et al. recently reported a microwave-assisted, solvent extraction route to unfunctionalised SBA-15, which afforded fast and complete template removal in 2 min, while delivering a material with similar structural properties to calcined SBA-15.19 Ultrasonication has also been shown to be an effective means to remove the organic micelles during MCM-41 synthesis.20 Here, we demonstrate that ultrasonication is also an efficient method to remove Pluronic triblock co-polymer templates from organically-functionalised PrSO3H-SBA-15, thereby facilitating the rapid synthesis of functionalised, mesoporous silicas, without any loss of structural or chemical properties, for separation and catalytic applications.
Experimental
PrSO3H-SBA-15 was synthesized by co-condensation (one-pot synthesis) according to the protocol of Margolese et al.14 4 g of Pluronic 123 was first dissolved with 125 cm3 of 2 M HCl at 40 °C. Subsequently, 8.2 cm3 of tetraethylorthosilicate (TEOS) was added, and after 45 min reaction, 0.76 mL of (3-mercaptopropyl)trimethoxysilane (MPTS) and 3.8 cm3 of H2O2 simultaneously added and the solution stirred for 24 h. The mixture was then aged at 100 °C for a further 24 h, and the resulting solid filtered and washed three times with water and finally dried overnight at 80 °C. This silica material contains the P123 template both on the external surface and entrapped in the pore channels. Reflux is commonly employed to remove Pluronic templates from mesoporous frameworks, however, the success of extraction depends heavily on the solvent (cm3)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) material (g) ratio. Our finished PrSO3H-SBA-15 materials were thus obtained from the preceding dried (organic–inorganic) solid following either (i) literature reflux methods using 50 (or 100) cm3 of solvent per 100 mg of solid material at 60 °C for 24 (or 48) h, or (ii) ultrasonic extraction employing either 10 cm3 or 50 cm3 methanol (ethanol) per 100 mg of solid, whereby the crushed slurry was placed in a polypropylene bottle immersed in an Elma Ultrasound bath (50 Hz) at room temperature. The water temperature was constant during the 5 min treatments. For both processes, post-extraction, materials were recovered by filtration with methanol washing and drying in oven at 80 °C.
material (g) ratio. Our finished PrSO3H-SBA-15 materials were thus obtained from the preceding dried (organic–inorganic) solid following either (i) literature reflux methods using 50 (or 100) cm3 of solvent per 100 mg of solid material at 60 °C for 24 (or 48) h, or (ii) ultrasonic extraction employing either 10 cm3 or 50 cm3 methanol (ethanol) per 100 mg of solid, whereby the crushed slurry was placed in a polypropylene bottle immersed in an Elma Ultrasound bath (50 Hz) at room temperature. The water temperature was constant during the 5 min treatments. For both processes, post-extraction, materials were recovered by filtration with methanol washing and drying in oven at 80 °C.
The resulting dried powders were characterised by nitrogen physisorption using a Quantachrome Nova 2000e porosimeter using NOVAWin software. Samples were degassed at 120 °C for 2 h prior to analysis by N2 adsorption at −196 °C. BET surface areas were calculated over the relative pressure range 0.01–0.2. Pore diameters and volumes were calculated applying the BJH method to the desorption isotherm for relative pressures >0.35. Low angle powder XRD patterns were recorded on a PANalytical X'pertPro diffractometer fitted with an X'celerator detector and Cu Kα (1.54 Å) source calibrated against a Si standard (PANalytical). Low angle patterns were recorded for 2θ = 0.3–8° with a step size of 0.01°. TEM micrographs were obtained with a JEOL 2100 transmission electron microscope operated at 200 kV, with images recorded by a Gatan Ultrascan 1000XP digital camera. Image analysis was undertaken using ImageJ software. XPS was performed using a Kratos Axis HSi X-ray photoelectron spectrometer fitted with a charge neutraliser and magnetic focusing lens employing Al Kα monochromated radiation (1486.6 eV). Surface elemental analysis was undertaken on Shirley background-subtracted spectra applying the appropriate instrument and element-specific response factors. Spectral fitting was conducted using CasaXPS version 2.3.14, with binding energies corrected to the C 1s peak at 284.5 eV and high-resolution C 1s, O 1s, S 2p and Si 2p XP spectra fitted using a common Gaussian/Lorentzian peak shape. Errors were estimated by varying the Shirley background subtraction procedure across reasonable limits and re-calculating fits. Thermogravimetric analysis (TGA) was performed using a Stanton Redcroft STA780 thermal analyser on ∼10–20 mg samples under helium (20 cm3 min−1 total flow) during heating at 20 °C min−1 between 20 °C and 1000 °C. Acid site densities were measured via NH3 pulse chemisorption using a Quantachrome ChemBET 3000 instrument at 100 °C on samples degassed at 150 °C. DRIFT spectra were obtained using a Nicolet Avatar 370 MCT with Smart Collector accessory, mid/near infrared source and mercury cadmium telluride (MCT-A) photon detector at −196 °C (liquid N2). Samples were diluted with KBr powder (10 wt% in KBr) for analysis and then loaded into an environmental cell and subjected to additional drying under vacuum at 200 °C for 2 h prior to measurements to remove moisture physisorbed during air exposure.
Hexanoic acid esterification was performed under stirred batch conditions at atmospheric pressure in a Radley's carousel reaction station using a 25 mm diameter glass reactor vessel. Reactions were conducted using 10 mmol hexanoic acid at 60 °C in 12.5 mL of methanol (molar ratio nMeOH/nacid = 30 under which conditions the organic acid and methanol were completely miscible) with 50 mg of the catalyst and 0.6 mL of dihexylether as an internal standard. Reaction profiles were obtained via periodic sampling and off-line GC analysis, with product calibration curves used to verify mass balances (>98%). Esterification was monitored using a Varian 450-GC equipped with a CP-Sil 5 CB 15 m × 0.25 mm × 0.25 μm capillary column. Dichloromethane was used to dilute samples for GC analysis. Catalytic profiles are an average of two separate runs with 3 injections per sample. Methyl hexanoate was the sole product observed. Turnover frequencies (TOF) were determined from the linear portion of the initial reaction rate profile for conversions below 25%, normalized to the surface acid site concentration determined by NH3 titration.
Results and discussion
Table 1 summarises the results of PrSO3H-SBA-15 extraction by either reflux or ultrasonication as quantified by thermal analysis. TGA profiles for propylsulfonic acid-functionalised SBA-15 (Fig. S1†) show three weight loss regimes: (i) water desorption from the hydrophilic environment of the sulfonic acid sites between room temperature and 150 °C; (ii) residual surfactant decomposition from 150 °C to 350 °C, as evidenced by comparative analyses on unfunctionalised SBA-15 (Fig. S2 and Table S1†); and (iii) thermal decomposition of grafted propyl sulfonic acid groups >350 °C.21 Few literature reports quantify surfactant template extraction from SBA-15 and its derivatives, or detail the reflux conditions, with such limited observations suggesting that ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) silica ratios of 300–500 cm3 g−1 can remove 84–94% of P123 in 12–24 h. For pure SBA-15 materials obtained via calcination, the volume of solvent and associated moisture content employed in a pre-wash step can influence the resultant surface area and pore-size distribution.22 We therefore first examined the impact of different solvents upon template extraction under conventional (i.e. non-Soxhlet) reflux. TGA revealed that only 74% of the template could be extracted from our PrSO3H-SBA-15 material under reflux with 50 cm3 methanol over 24 h. This was slightly more than achievable using ethanol, and significantly greater than possible with aprotic, polar solvents such as acetone or THF, or non-polar toluene, as expected in view of the polar, hydrophilic nature of P123. Hence a second 50 cm3 reflux was required to achieve 89% template removal, with each reflux consuming 3600 W. In contrast, only 5 min ultrasonication with MeOH
silica ratios of 300–500 cm3 g−1 can remove 84–94% of P123 in 12–24 h. For pure SBA-15 materials obtained via calcination, the volume of solvent and associated moisture content employed in a pre-wash step can influence the resultant surface area and pore-size distribution.22 We therefore first examined the impact of different solvents upon template extraction under conventional (i.e. non-Soxhlet) reflux. TGA revealed that only 74% of the template could be extracted from our PrSO3H-SBA-15 material under reflux with 50 cm3 methanol over 24 h. This was slightly more than achievable using ethanol, and significantly greater than possible with aprotic, polar solvents such as acetone or THF, or non-polar toluene, as expected in view of the polar, hydrophilic nature of P123. Hence a second 50 cm3 reflux was required to achieve 89% template removal, with each reflux consuming 3600 W. In contrast, only 5 min ultrasonication with MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PrSO3H-SBA-15 ratios of 500 cm3 g−1 at room temperature were required to remove 90% of the P123 template, consuming a mere 3 W. Template extraction was equally efficient even when the MeOH
PrSO3H-SBA-15 ratios of 500 cm3 g−1 at room temperature were required to remove 90% of the P123 template, consuming a mere 3 W. Template extraction was equally efficient even when the MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PrSO3H-SBA-15 ratio was lowered to 100, while extending the sonication treatment to 60 min conferred no additional benefit.
PrSO3H-SBA-15 ratio was lowered to 100, while extending the sonication treatment to 60 min conferred no additional benefit.
| Protocol | Solvent | Time | Solvent![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sample/cm3 g−1 sample/cm3 g−1 |
%P123 removed/%a |
|---|---|---|---|---|
| a Proportion of template removed relative to as-synthesised PrSO3H-SBA-15 from TGA between 150 and 350 °C. b 3-(Propylsulfonyl)propane-1-SO3H-SBA-15. c Two consecutive 50 cm3 reflux cycles. | ||||
| Reflux | EtOH | 24 h | 267 | 9414 |
| Reflux | EtOH | 24 h | 267 | 9123 |
| Reflux | EtOH | 12 h | 500 | 84b,24 |
| Reflux | Toluene | 24 h | 500 | 62 |
| Reflux | THF | 24 h | 500 | 69 |
| Reflux | Acetone | 24 h | 500 | 68 |
| Reflux | EtOH | 24 h | 500 | 72 |
| Reflux | MeOH | 24 h | 500 | 74 |
| Refluxc | MeOH | 24 h | 1000 | 89 |
| Ultrasonication | MeOH | 5 min | 500 | 89 |
| Ultrasonication | MeOH | 5 min | 100 | 90 |
| Ultrasonication | MeOH | 1 h | 100 | 90 |
Fig. 2 shows the resulting adsorption–desorption isotherms for PrSO3H-SBA-15 extracted via reflux or ultrasonication, which reveal essentially identical type IV behaviour characteristic of PrSO3H-SBA-15. The corresponding XRD patterns in Fig. 3 both exhibit low-angle (100), (110) and (200) reflections indicative of PrSO3H-SBA-15, evidencing preservation of the expected 2D hexagonal structure, with their similar peakwidths and intensities showing comparable mesopore ordering.
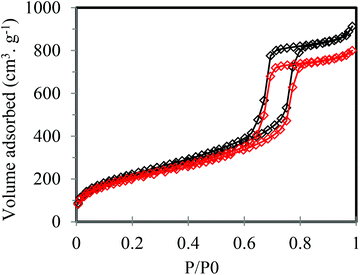 | ||
| Fig. 2 N2 isotherms of PrSO3H-SBA-15 extracted by MeOH reflux – 2 × 50 cm3 (black curve) and 5 min MeOH ultrasonication (red curve). | ||
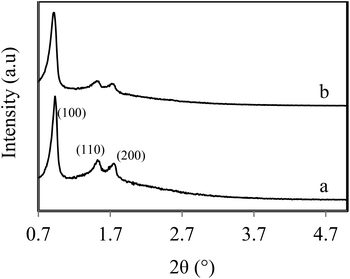 | ||
| Fig. 3 Low angle XRD patterns of PrSO3H-SBA-15 extracted by: (a) MeOH reflux (2 × 50 cm3); and (b) 5 min MeOH ultrasonication (offset to aid comparison). | ||
Table 2 compares the textural and structural properties of PrSO3H-SBA-15 obtained via 100 cm3 methanol reflux, versus 5 min ultrasonication in 50 cm3 methanol. It is clear that the two extracted mesoporous silicas are essentially indistinguishable, exhibiting common surface areas, lattice parameters, pore diameters and surface sulphur loadings, with sulfonic acid groups equally accessible to ammonia titration. We note the excellent quantitative agreement between the XPS-derived RSO3H content of 3 wt% (which equates to 0.9 mmolH+ g−1) and the corresponding acid site loading determined by NH3 chemisorption, in line with our previous observations.25 Since bulk elemental analysis measures the total sulfur content, irrespective of whether present as fully oxidised sulphonic acid groups or SH/disulphide residues, it often over-estimates acid site loadings in sulphonic acid silicas, whereas XPS provides a more accurate estimate due to the ability to discriminate and quantify SO3H moieties specifically. Surface elemental analysis (Table S2 and Fig. S4†) confirms the presence of propylsulfonic acid moieties, with a single, high binding energy (BE) sulfur species at 168.6 eV characteristic of –SO3H8,10,26 observed for as-synthesised and extracted PrSO3H-SBA-15. Two carbon species are observed for as-synthesised PrSO3H-SBA-15, with C 1s BE of 284.5 and 286.1 eV associated with adventitious carbon from sample handling and the P123 template respectively. Three carbon components are observed for both reflux and ultrasonic extracted PrSO3H-SBA-15 at 284.5 eV (adventitious carbon), 285.6 eV and 286.6 eV. The latter two components are in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 intensity ratio, which, together with their binding energies, confirms assignment to the respective –O3Si–CH2–CH2– and R–CH2–SO3H carbons of the propyl backbone.
1 intensity ratio, which, together with their binding energies, confirms assignment to the respective –O3Si–CH2–CH2– and R–CH2–SO3H carbons of the propyl backbone.
| Extraction protocol | BET/m2 g−1 | Vpa/cm3 g−1 |
W
BJH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b/nm b/nm |
Plane spacingc/nm | Unit cell parameterd/nm | Wall thicknesse/nm | Surface S contentf/wt% | Acid site loadingg/mmolH+ g−1 |
|---|---|---|---|---|---|---|---|---|
a Mean pore volume.
b Mean mesopore diameter calculated via BJH analysis of desorption isotherm.
c Calculated from Bragg's Law, d = nλ/(2sinθ).
d Calculated from a = 2d(100)/√3 for hexagonal close-packed mesopores.
e Unit cell parameter-WBJH.
f Calculated from XPS.
g Calculated from NH3 pulse titration.
h MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PrSO3H-SBA-15 = 1000 for 24 h at 60 °C.
i MeOH PrSO3H-SBA-15 = 1000 for 24 h at 60 °C.
i MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PrSO3H-SBA-15 = 500 for 5 min at RT. PrSO3H-SBA-15 = 500 for 5 min at RT.
|
||||||||
| Refluxh | 797 | 1.55 | 6.7 | 9.5 | 11 | 4.9 | 3 | 0.97 |
| Ultrasonicationi | 740 | 1.36 | 6.7 | 9.7 | 11.2 | 5.1 | 3.1 | 0.99 |
TEM (Fig. 4 and Fig. S5†) also shows that the desired 2D hexagonal ordered array of mesopores extends throughout both PrSO3H-SBA-15 materials. These observations confirm that ultrasonication yields a final PrSO3H-SBA-15 of identical quality to that obtained by the more lengthy and energy-intensive conventional solvent reflux. Microporosity in SBA-15 type materials is believed to result from silica templating polyethyleneoxide (PEO) fingers,27,28 in which part of the PEO template inserts into the condensing silica wall, rendering it more difficult to remove by mild/moderate treatments such as reflux or sonication.
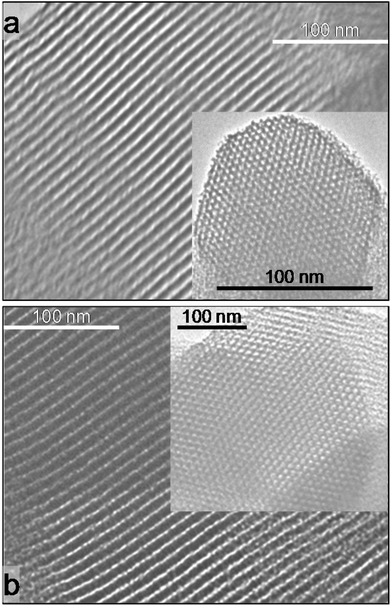 | ||
| Fig. 4 TEM images of PrSO3H-SBA-15 extracted by: (a) MeOH reflux (2 × 50 cm3); and (b) 5 min MeOH ultrasonication. | ||
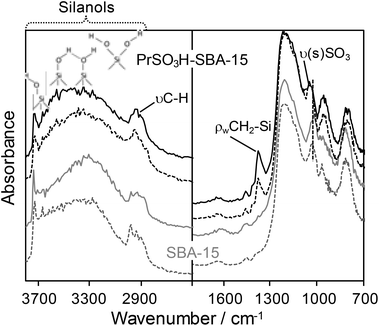
In situ DRIFTS provides some insight into the relative ease with which the surfactant template is removed from PrSO3H-SBA-15 by ultrasonication. Fig. 5 compares DRIFT spectra of PrSO3H-SBA-15 obtained by ultrasonication versus conventional reflux, and analogous spectra for pure SBA-15 obtained by both extraction methods. In all cases, materials were outgassed at 200 °C for 2 h to remove physisorbed water. The pure SBA-15 mesoporous silicas exhibit essentially identical spectra, possessing the characteristic bands between 700–1300 cm−1 and 3000–3800 cm−1 associated with the silica framework and surface silanols respectively. A weak feature around 2900 cm−1 reflects the presence of alkoxy residues arising from either the TEOS precursor or the P123 template. Spectra for both the propylsulfonic acid functionalised silicas are likewise almost indistinguishable from each other, but exhibit subtle but important differences from the pure silicas. Extracted PrSO3H-SBA-15 possess lower concentrations of isolated (3740 cm−1) versus vicinal and geminal silanols, which would reduce the strength of hydrogen bonding with ethylene oxide monomers within the P123 template, and thus facilitate the latter's extraction during sonication which is known to readily break hydrogen bonds. This may explain the significantly higher degree of P123 extraction from ultrasonicated PrSO3H-SBA-15 relative to ultrasonicated SBA-15 seen in Table S1.† The extracted PrSO3H-SBA-15s also exhibit new features at 1370 cm−1 (due to the propylsilane backbone) and 1035 cm−1 (due to the asymmetric vibrational mode of SO3−),29 in accordance with their functionalisation.
The nature of the organic residue extracted from PrSO3H-SBA-15s by reflux or ultrasonication, whether intact P123 or decomposition fragments thereof, was also qualitatively probed by 1H and 13C NMR. Fig. S6† shows that the ultrasonic extract is an almost perfect match for the parent P123 surfactant, evidencing a negligible decomposition of the pluronic template during either the hydrolysis step generating the silica framework, or the subsequent ultrasonication process.
As a final verification that the material obtained by rapid ultrasonic extraction was not only structurally, but chemically, indistinguishable from that obtained under energy intensive reflux, the catalytic performance of both PrSO3H-SBA-15 solid acids was compared towards hexanoic acid esterification with methanol. The resulting reaction profiles for methyl hexanoate production shown in Fig. 6 are virtually superimposable, following the expected pseudo first-order kinetics with respect to acid concentration in the presence of excess methanol, confirming that ultrasonication impairs neither the accessibility of acid sites, nor their strength. The corresponding TOFs are also essentially indistinguishable, being 30.3 versus 31.3 h−1 for reflux and ultrasonic extracted PrSO3H-SBA-15 respectively after a two-hour reaction. It is interesting to consider whether the frequency of ultrasonication influences the structural and reactive properties of our extracted PrSO3H-SBA-15; however, there are no commercially available, variable frequency ultrasonic baths or probes able to deliver a constant power, amplitude and contact area with which to investigate this.
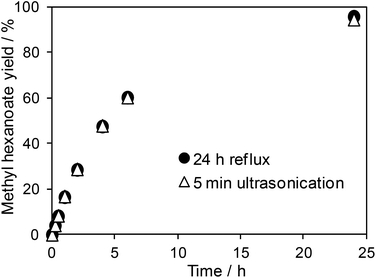 | ||
| Fig. 6 Hexanoic acid esterification over PrSO3H-SBA-15 extracted by MeOH reflux (2 × 50 cm3) versus 5 min MeOH ultrasonication. | ||
Conclusions
Ultrasonication offers an extremely rapid and energy-efficient route to the extraction of the P123 surfactant template employed in the widespread synthesis of SBA-15 materials, and specifically the preparation of sulfonic acid-functionalised SBA-15 via a co-condensation route for heterogeneous catalysis applications. Around 90% of P123 can be removed from SBA-15 via 5 min ultrasonication in 10 mL methanol at room temperature, comparable to that achievable after a conventional 48 h reflux using ten times the methanol volume. This represents a 99% reduction in energy consumption, and a 90% reduction in the volume of solvent required. The new protocol is easily implemented on the >10 g scale. Eco-friendly, ultrasonic template removal yields a propylsulfonic acid functionalised SBA-15 with an identical catalytic performance to that derived by more laborious reflux in the esterification of hexanoic acid with methanol. Our new synthetic protocol offers a greener route to the accelerated design of mesoporous silicas.We thank the EPSRC (EP/G007594/2 and EP/F063423/2) for financial support and a Leadership Fellowship (AFL), and the Royal Society for the award of an Industry Fellowship (KW).
Notes and references
- C. T. Kresge, M. E. Leonowicz, W. J. Roth, J. C. Vartuli and J. S. Beck, Nature, 1992, 359, 710–712 CrossRef CAS.
- S. A. Bagshaw, E. Prouzet and T. J. Pinnavaia, Science, 1995, 269, 1242–1244 CrossRef PubMed.
- D. Zhao, Q. Huo, J. Feng, B. F. Chmelka and G. D. Stucky, J. Am. Chem. Soc., 1998, 120, 6024–6036 CrossRef CAS.
- T.-W. Kim, F. Kleitz, B. Paul and R. Ryoo, J. Am. Chem. Soc., 2005, 127, 7601–7610 CrossRef CAS PubMed.
- D. Zhao, J. Feng, Q. Huo, N. Melosh, G. H. Fredrickson, B. F. Chmelka and G. D. Stucky, Science, 1998, 279, 548–552 CrossRef CAS.
- N. Rahmat, A. Z. Abdullah and A. R. Mohamed, Am. J. Appl. Sci., 2010, 7, 1579–1586 CrossRef CAS.
- C. M. A. Parlett, K. Wilson and A. F. Lee, Chem. Soc. Rev., 2013, 42, 3876–3893 RSC.
- J. Dhainaut, J.-P. Dacquin, A. F. Lee and K. Wilson, Green Chem., 2010, 12, 296–303 RSC.
- A. F. Lee, C. V. Ellis, J. N. Naughton, M. A. Newton, C. M. A. Parlett and K. Wilson, J. Am. Chem. Soc., 2011, 133, 5724–5727 CrossRef CAS PubMed.
- J. P. Dacquin, A. F. Lee, C. Pirez and K. Wilson, Chem. Commun., 2012, 48, 212–214 RSC.
- C. M. A. Parlett, D. W. Bruce, N. S. Hondow, M. A. Newton, A. F. Lee and K. Wilson, ChemCatChem, 2013, 5, 939–950 CrossRef CAS.
- Y. Hu, S. Tang, L. Jiang, B. Zou, J. Yang and H. Huang, Proc. Biochem., 2012, 47, 2291–2299 CrossRef CAS PubMed.
- T. Yasmin and K. Müller, J. Chromatogr., A, 2011, 1218, 6464–6475 CrossRef CAS PubMed.
- D. Margolese, J. A. Melero, S. C. Christiansen, B. F. Chmelka and G. D. Stucky, Chem. Mater., 2000, 12, 2448–2459 CrossRef CAS.
- A. Hozumi, H. Sugimura, K. Hiraku, T. Kameyama and O. Takai, Chem. Mater., 2000, 12, 3842–3847 CrossRef CAS.
- M. T. J. Keene, R. Denoyel and P. L. Llewellyn, Chem. Commun., 1998, 0, 2203–2204 RSC.
- A.-H. Lu, W.-C. Li, W. Schmidt and F. Schuth, J. Mater. Chem., 2006, 16, 3396–3401 RSC.
- C.-Y. Chen, H.-X. Li and M. E. Davis, Microporous Mater., 1993, 2, 17–26 CrossRef CAS.
- T.-L. Lai, Y.-Y. Shu, Y.-C. Lin, W.-N. Chen and C.-B. Wang, Mater. Lett., 2009, 63, 1693–1695 CrossRef CAS PubMed.
- S. Jabariyan and M. A. Zanjanchi, Ultrason. Sonochemistry, 2012, 19, 1087–1093 CrossRef CAS PubMed.
- G. Morales, G. Athens, B. F. Chmelka, R. van Grieken and J. A. Melero, J. Catal., 2008, 254, 205–217 CrossRef CAS PubMed.
- J. P. Thielemann, F. Girgsdies, R. Schlögl and C. Hess, Beilstein J. Nanotechnol., 2011, 2, 110–118 CAS.
- J. A. Melero, L. F. Bautista, G. Morales, J. Iglesias and R. Sánchez-Vázquez, Chem. Eng. J., 2010, 161, 323–331 CrossRef CAS PubMed.
- A. J. Crisci, M. H. Tucker, M.-Y. Lee, S. G. Jang, J. A. Dumesic and S. L. Scott, ACS Catal., 2011, 1, 719–728 CrossRef CAS.
- J.-P. Dacquin, H. E. Cross, D. R. Brown, T. Duren, J. J. Williams, A. F. Lee and K. Wilson, Green Chem., 2010, 12, 1383–1391 RSC.
- C. Pirez, J.-M. Caderon, J.-P. Dacquin, A. F. Lee and K. Wilson, ACS Catal., 2012, 2, 1607–1614 CrossRef CAS.
- Y. Bennadja, P. Beaunier, D. Margolese and A. Davidson, Microporous Mesoporous Mater., 2001, 44–45, 147–152 CrossRef CAS.
- A. Galarneau, H. Cambon, F. Di Renzo, R. Ryoo, M. Choi and F. Fajula, New J. Chem., 2003, 27, 73–79 RSC.
- K. Wilson, A. F. Lee, D. J. Macquarrie and J. H. Clark, Appl. Catal., A, 2002, 228, 127–133 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Additional bulk and surface materials characterisation. See DOI: 10.1039/c3gc40474a |
| This journal is © The Royal Society of Chemistry 2014 |