Microwave-assisted flash conversion of non-edible polysaccharides and post-harvest tomato plant waste to levulinic acid
Silvia
Tabasso
a,
Enzo
Montoneri
a,
Diego
Carnaroglio
b,
Marina
Caporaso
b and
Giancarlo
Cravotto
*b
aDipartimento di Chimica, University of Turin, Via P. Giuria 7, I-10125 Turin, Italy
bDipartimento di Scienza e Tecnologia del Farmaco, University of Turin, Via P. Giuria 9, I-10125 Turin, Italy. E-mail: giancarlo.cravotto@unito.it; Fax: +39 011 6707687
First published on 18th September 2013
Abstract
A microwave-assisted protocol for the conversion of non-edible polysaccharides and tomato plant waste to levulinic acid has been developed. Full conversion has been achieved in all cases at 2 min irradiation and clean levulinic acid was obtained without any purification in high yields (63–95%).
Although fossil fuels and their derivatives are still the main feedstock for the chemical industry,1 the demand for renewable sources has steadily increased over the last two decades. Biomass has been recognized as a major worldwide renewable source of fixed carbon and one which can be used for the production of biofuels and biochemicals.2
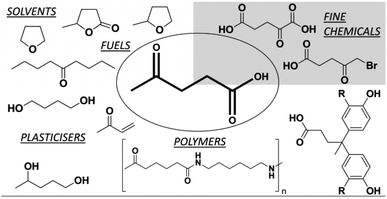
We have recently investigated the catalytic conversion of starch-based industrial waste into reducing sugars under microwave and ultrasound irradiation used alone and in a joint fashion.3 This efficient non-conventional depolymerization process4 has paved the way for further studies in the search for more selective end-product isolation. Levulinic acid (LA), a sugar derived keto-acid (4-oxopentanoic acid), is a key intermediate for the production of fine chemicals and biofuels. LA may be converted into numerous value-added chemicals, such as fuels, solvents, anti-freeze agents, herbicides, polymers, resins, pharmaceutical agents and food fragrances (Fig. 1).5
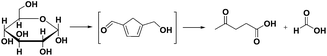
Cellulose, starch or C6 sugars can be converted into LA and formic acid via degradation of monosaccharides (MSC) formed by chemical or enzymatic hydrolysis. Sugar dehydration first generates 5-(hydroxymethyl)furfural (HMF) and finally LA (Scheme 1).6 LA can also be produced from hemicellulose in a 3 step process in which furfural and furfuryl alcohol are intermediates.7 Cellulose, hemicellulose and lignin are the main components of several types of lignocellulose waste and non-food feedstock.
Numerous catalysts, acids and solvents have been described in the LA forming process: trifluoroacetic acid in fluorinated solvents,8 solid catalysts including amberlites9 and zeolites,10,11 acetone, water, supercritical carbon dioxide fluids,12 and ionic liquids.13,14
In general, the most common procedure for the hydrolysis of cellulose and biomass entails the use of mineral acids. Cha and Hanna showed that the main mineral acids’ order of reactivity was HBr > HCl > H2SO4 corresponding to the strength of their primary dissociation constants.15 Volatile acids (e.g. HCl) are preferred because they make the recovery of LA simpler,16 although they usually give around 7–10% yield (wt%) in long reaction times. The treatment of typical biomasses like rice hulls and straw, corn stalks and saw dust with HCl at 160–190 °C generates numerous by-products including small molecules, heterogeneous sugar and lignin-like polymeric products.5,17 Despite the high cost of fractionation and purification,18 in southern USA the industrial production of LA has already a capacity of 3000 tons of feedstock per year (Biofine Technology LCC),19 mainly from local tobacco bagasse and paper mill sludge.
Non-conventional energy sources such as microwaves (MW) can dramatically enhance reaction rates in organic synthesis.20,21 Dielectric heating is a valid response to stubborn, time consuming reactions,22 and can be applied on a range of scales, from millilitres to kilograms.23
In this work, we address all the requirements for the potential industrial production of LA using cost-effective feedstock17 and a very fast MW process. A suitable lignocellulosic feedstock was found in the form of tomato plant waste at the end of the crop harvest season (Ragusa, Sicily).24 The inherent chemical complexity of food waste makes it a very attractive source to convert crop residues and agro-industrial byproducts into value-added chemicals. This goal can be achieved by means of sustainable and integrated processes using low environmental impact technologies and efficient energy sources such as microwaves.25 Although several chemicals are derived from agricultural residues,26 so far tomato plants as a source of LA have not been investigated.
We propose a flash procedure that maximised conversion and product selectivity. This was carried out using a latest generation MW reactor (SynthWave by Milestone) designed to rapidly reach high temperatures and pressures (up to 300 °C and 200 bar) and then quickly cool down to room temperature. This LA production technology was first applied to various pure polysaccharides such as cellulose, chitin and chitosan. Szabolcs et al.16 have recently reported significantly improved LA yields (wt%) under MW irradiation: 31% from cellulose (with 2 M HCl at 190 °C for 50 min) and 22.7% from chitosan (190 °C, 20 min). On the basis of these promising results, it was decided that optimisation of the reaction conditions for the production of LA from post-harvest tomato plants (PHTP) should be carried out. The chemical composition of the starting biomass was investigated by solid-state 13C-NMR spectroscopy. To determine the cellulose content, the dry matter was fractionated into its constituents using various solvents, as reported in a previous work.27 This procedure is expected to separate the major biomass proximate on the basis of the components’ solubility in benzene and in HCl at different temperatures, i.e. lipids and apolar compounds, hemicellulose and proteins, cellulose and lignin fractions. The 13C-NMR data of the starting biomass and the separated fractions (Table 1) demonstrate, however, that the supposed cellulose fractions recovered after solvent evaporation are not pure cellulose. It contained not only the expected anomeric C and OX (X = H, R) functional groups, but also other C types and functional groups which were similar to those found in the lignin fraction, although present at different relative ratios. As the isolation of a pure cellulose fraction was not demonstrated, a reliable value for the concentration of this proximate could not be obtained.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28
28
| Fraction | Aliphatic | NR + OMe | OR | Anomeric | Ph | PhOH | COX | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
|---|---|---|---|---|---|---|---|---|
| a F2: hemicellulose; F3: cellulose; F4: lignin. | ||||||||
| PHTP | 14.34 | 7.22 | 49.60 | 11.62 | 6.82 | 3.44 | 6.28 | 0.61 |
| F2 | 25.61 | 9.25 | 47.43 | 3.09 | 0.00 | 0.00 | 14.61 | 0.00 |
| F3 | 13.98 | 8.23 | 47.29 | 10.80 | 9.25 | 5.36 | 4.11 | 0.98 |
| F4 | 20.69 | 8.20 | 45.68 | 9.44 | 4.75 | 2.79 | 7.21 | 1.25 |
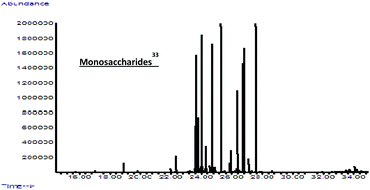
In this work, biomass conversion and product yields were calculated on the basis of the dry weight of the starting biomass and of its organic content. Firstly, we evaluated the effect of HCl concentration on MW-assisted degradation of biomass to LA. Although it has recently been reported that low acid concentrations favour the formation of 5-hydroxymethyl-2-furaldehyde (5-HMF) rather than LA,29 no 5-HMF was detected under our conditions when the concentration was changed from 12 M to 0.1 M. Neither biomass conversion nor LA yield was affected by acid concentrations ranging from 12 M to 0.5 M, while no LA was produced and biomass conversion was lower at 0.1 M HCl concentration. As shown in Table 2,30 lower temperatures (≤150 °C) favour the selective hydrolysis of carbohydrates to simple MSC, as demonstrated by GC-MS analysis after sample derivatisation (Fig. 2),31 with no LA. PHTP conversions and LA yields were higher at temperatures above 150 °C and at HCl concentration >0.1 M. Under these conditions, LA was the only product detected by 1H-NMR, 13C-NMR and GC-MS, as confirmed by the spectral patterns in Fig. 3–5.
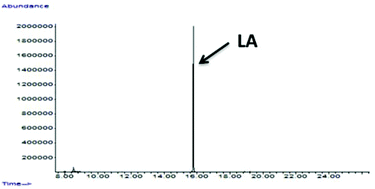 | ||
| Fig. 3 GC of PHTP (conditions as in Table 3). | ||
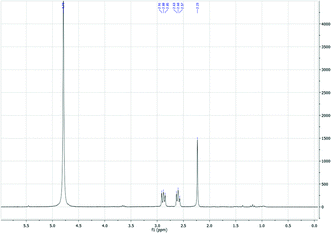 | ||
| Fig. 4 1H-NMR (D2O) of PHTP (conditions as in Table 3). | ||
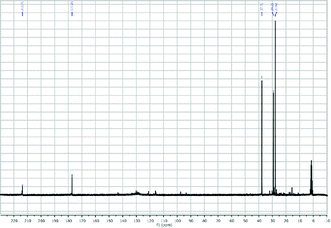 | ||
| Fig. 5 13C-NMR (D2O) of PHTP (conditions as in Table 3). | ||
| Entry | HCl (mol L−1) | T (°C) | Conversion (%) | Yield (%)32 | |
|---|---|---|---|---|---|
| LA | MSC | ||||
| a Reaction conditions: PHTP/HCl solution = 1/10 (w/v), 2 min MW, N2 pressure (40 bar). b PHTP/HCl solution = 1/5 (w/v). c Conventional heating: PHTP/HCl solution = 1/10 (w/v), 2 h. | |||||
| 1 | 12 | 225 | 80 | 58 | 0 |
| 2 | 5 | 225 | 79 | 60 | 0 |
| 3 | 1 | 225 | 78 | 63 | 0 |
| 4 | 0.5 | 225 | 74 | 51 | 0 |
| 5 | 0.1 | 225 | 67 | 0 | 49 |
| 6 | 1 | 60 | 30 | 0 | 11 |
| 7 | 1 | 100 | 30 | 0 | 30 |
| 8 | 1 | 150 | 62 | 0 | 52 |
| 9 | 1 | 190 | 81 | 61 | 0 |
| 10 | 1 | 250 | 80 | 56 | 0 |
| 11b | 1 | 225 | 73 | 59 | 0 |
| 12c | 12 | Reflux | 50 | 5 | 20 |
After an easy product recovery via solvent evaporation, entries 3 and 11 showed the best results in both PHTP/HCl ratios 1/10 and 1/5 (w/v). For the sake of comparison, experiments under conventional heating have been performed. As shown in Table 2 (entry 12), both conversion and selectivity were very low even with 12 mol L−1 HCl. We extended our investigation to non-edible polysaccharides such as cellulose and chitosan (Table 3), revealing that full conversion and high LA yields (90 and 95%, respectively) were achieved under the same conditions reported in Table 2.
Conclusions
A highly selective protocol for the direct conversion of post-harvest tomato plants into LA via a MW-assisted flash reaction under mild acidic conditions at high temperature has been developed for the first time. This versatile procedure is applicable to numerous cellulosic biomasses and non-edible polysaccharides and can selectively give either simple sugars or pure LA by varying the reaction temperature. These impressive results come from the outstanding performance of the MW reactor that enables fast heating/cooling and high gas pressure. The use of dielectric heating technology for waste feedstock valorisation offers intriguing future perspectives that go over and beyond the development of a sustainable process for LA production. High-throughput applications of MW-assisted flash hydrolysis would entail the use of flow MW reactors.Notes and references
- Y. Yu, X. Lou and H. Wu, Energy Fuels, 2008, 22, 46 CrossRef CAS.
- G. W. Huber, S. Iborra and A. Corma, Chem. Rev., 2006, 106, 4044 CrossRef CAS PubMed; D. M. Alonso, S. G. Wettsteinb and J. A. Dumesic, Green Chem., 2013, 15, 584 RSC.
- A. Hernoux-Villière, U. Lassi, T. Hu, A. Paquet, L. Rinaldi, G. Cravotto, S. Molina-Boisseau, M.-F. Marais and J.-M. Lévêque, ACS Sustainable Chem. Eng., 2013, 1, 995 CrossRef.
- G. Cravotto and P. Cintas, Chem.–Eur. J., 2007, 13, 1902 CrossRef CAS PubMed.
- D. W. Rackemann and W. O. S. Dohertyand, Biofuels, Bioprod. Bioref., 2011, 5, 198 CrossRef CAS.
- B. Girisuta, L. P. B. M. Janssen and H. J. Heeres, Green Chem., 2006, 8, 701 RSC; J.-P. Lange, E. van der Heide, J. van Buijtenen and R. Price, ChemSusChem, 2012, 5, 150 CrossRef CAS PubMed.
- G. M. Gonzalez Maldonado, R. S. Assary, J. Dumesic and L. A. Curtiss, Energy Environ. Sci., 2012, 5, 6981 Search PubMed; R. Weingarten, J. Cho, R. Xing, W. C. Conner Jr. and G. W. Huber, ChemSusChem, 2012, 5, 1280 CrossRef CAS PubMed.
- H. Heeres, R. Handana, D. Chunai, C. B. Rasrendra, B. Girisuta and H. J. Heeres, Green Chem., 2009, 11, 1247 RSC; C. Chang, X. Ma and P. Cen, Chin. J. Chem. Eng., 2006, 14, 708 CrossRef CAS.
- B. C. Redmon, US patent2738367, 1956 Search PubMed.
- J. Jow, G. L. Rorrer, M. C. Hawley and D. T. A. Lamport, Biomass, 1987, 14, 185 CrossRef CAS.
- A. Corma, S. Iborra and A. Velty, Chem. Rev., 2007, 107, 2411 CrossRef CAS PubMed.
- A. Boisen, T. B. Christensen, W. Fu, Y. Y. Gorbanev, T. S. Hansen, J. S. Jensen, S. K. Klitgaard, S. Pedersena, A. Riisager, T. Stahlberg and J. M. Woodley, Chem. Eng. Res. Des., 2009, 87, 1318 CrossRef CAS PubMed; M. Bicker, D. Kaiser and H. Vogel, Green Chem., 2003, 5, 280 RSC; W. Sangarunlert, P. Piumsomboon and S. Ngamprasertsith, Korean J. Chem. Eng., 2007, 24, 936 CrossRef.
- S. Hu, Z. Zhang, Y. Zhou, B. Han, H. Fan, W. Li, S. Jinliang and X. Ye, Green Chem., 2008, 10, 1280 RSC; C. Moreau, A. Finiels and L. Vanoye, J. Mol. Catal. A: Chem., 2006, 253, 165 CrossRef CAS PubMed.
- H. Ren, Y. Zhou and L. Liu, Bioresour. Technol., 2013, 129, 616 CrossRef CAS PubMed.
- J. Y. Cha and M. A. Hanna, Ind. Crop. Prod., 2002, 16, 109 CrossRef CAS.
- A. Szabolcs, M. Molnár, G. Dibó and L. Mika, Green Chem., 2013, 15, 439 RSC.
- A. M. R. Galletti, C. Antonetti, E. Ribechini, M. P. Colombini, N. N. Nassi and E. Bonari, Appl. Energy, 2013, 102, 157 CrossRef CAS PubMed; G. A. Sheldon Coulson, Production of levulinic acid in urban refineries. Available from Massachusetts Institute of Technology Libraries, Cambridge, MA 02139, USA, 2011, pp. 1–89 Search PubMed.
- L. Yan, N. Yang, H. Pang and B. Liao, Clean, 2008, 36, 158 CAS.
- http://www.biofinetechnology.com/index.php?p=1_7_Biofine-Technology-LLC .
- Microwaves in Organic Synthesis, ed. A. De La Hoz and A. Loupy, 3rd edn, Springer, U.S.A., 2012 Search PubMed.
- C. O. Kappe, Angew. Chem., Int. Ed., 2004, 43, 6250 CrossRef CAS PubMed.
- G. Cravotto, D. Garella, L. Beltramo, D. Carnaroglio, S. Mantegna and C. M. Roggero, Chemosphere, 2013, 92, 299 CrossRef CAS PubMed; P. Cintas, D. Carnaroglio, L. Rinaldi and G. Cravotto, Chem. Today, 2012, 30, 58 Search PubMed; G. Palmisano, W. Bonrath, L. Boffa, D. Garella, A. Barge and G. Cravotto, Adv. Synth. Catal., 2007, 349, 2338 CrossRef.
- R. Morschhäuser, M. Krull, C. Kayser, C. Boberski, R. Bierbaum, P. A. Püschner, T. N. Glasnov and C. O. Kappe, Green Process Synth., 2012, 1, 281 Search PubMed.
- http://www.unctad.info/en/Infocomm/AACP-Products/COMMODITY-PROFILE-Tomato .
- C. S. Ki Lin, L. A. Pfaltzgraff, L. Herrero-Davila, E. B. Mubofu, S. Abderrahim, J. H. Clark, A. A. Koutinas, N. Kopsahelis, K. Stamatelatou, F. Dickson, S. Thankappan, Z. Mohamed, R. Brocklesby and R. Luque, Energy Environ. Sci., 2013, 6, 426 Search PubMed.
- R. Luque and J. H. Clark, Sustainable Chem. Process., 2013, 1, 10 CrossRef.
- P. L. Genevini, F. Adani and C. Villa, Soil Sci. Plant Nutr., 1997, 43, 135 CrossRef.
- E. Montoneri, V. Boffa, P. Savarino, D. G. Perrone, M. Ghezzo, C. Montoneri and R. Mendichi, Waste Manage., 2011, 31, 10 CrossRef CAS PubMed.
- G. R. Akien, L. Qi and I. T. Horváth, Chem. Commun., 2012, 48, 5850 RSC.
- PHTP was ground in a blade mill; in a typical experiment 2 g of the sample were suspended in HCl (20 or 10 mL). The mixture was irradiated in a MW reactor for 2 min at 225 °C (average power 1000 W) under N2 (40 bar) under magnetic stirring. After 2 min, the reactor was cooled down. The reaction mixture was passed through laboratory filter paper to remove the insoluble residue. This solid was washed three times with distilled water (3 × 5 mL) and all filtrates were combined. After evaporating the solvent, a brownish viscous solid remained. It was analyzed by 1H-NMR (300 MHz, D2O), 13C-NMR (75 MHz, D2O) and GC-MS. NMR spectra were recorded using a Bruker Avance spectrometer.
- GC-MS analyses were carried out after prior derivatisation. In a typical derivatisation, 10 mg of the sample was solubilized in 1 mL of pyridine. Then, 300 μL of BSTFA (N,O-bis(trimethylsilyl) trifluoroacetamide) was added to a solution. The mixture was heated for 45 min at 60 °C under magnetic stirring. GC-MS analyses were performed using a GC Agilent 6890 (Agilent Technologies – USA) fitted with a mass detector Agilent Network 5973, using a 30 m long capillary column, i.d. 0.25 mm and film thickness 0.25 μm. Temperature program: from 80 °C (3 min) to 300 °C at 5 °C min−1.
- Theoretical yield was calculated as soluble organic yield % relative to organic feed recovered after reaction, i.e. 100 × (organic content in soluble fraction (g)/organic content in PHTP (g)). The organic content was calculated as the weight loss after calcination of the sample at 650 °C for 5 hours.
- Monosaccharides identified as ribitol, D-ribose, mannose, α-galactofuranoside, arabinose, β-D-galactofuranose, D-altrose, xylitol, α-D-galactopyranose, glucopyranose, D-glucose, and maltose (library: NIST05).
| This journal is © The Royal Society of Chemistry 2014 |