Rapid high-performance sample digestion for ICP determination by ColdBlock™ digestion: part 1 environmental samples
Yong
Wang
a,
Ravi
Kanipayor
b and
Ian D.
Brindle
*a
aDepartment of Chemistry, Brock University, St. Catharines, Ontario, Canada L2S 3A1. E-mail: ibrindle@brocku.ca; Fax: +1 905 688-0748; Tel: +1 905 688 5550 ext. 4559
bChemquant Laboratories, 1210 Larny Court, Mississauga, Ontario, Canada L4W 3N4. E-mail: ravi_kanipayor@yahoo.ca; Tel: +1 705 942 8646
First published on 29th October 2013
Abstract
Validation, using a prototype of a novel sample digestion device (ColdBlock™ (patent pending, WO 2011/054086 A1)), for elemental determination, has been conducted. This device utilizes focused infra-red radiation to induce speedy sample breakdown and offers an alternative technique of wet sample dissolution that combines reduced digestion time with excellent accuracy and precision. The current study explores the digestion capacity of ColdBlock™ for a variety of solid environmental samples that include difficult matrices. Two certified materials (contaminated soil SS-1, bio-solid CRM030-040) were analyzed. The technology was also used to compare results from a recent inter-laboratory evaluation of an uncertified commercial sludge sample (LPTP12-S2) using inductively coupled plasma optical emission spectroscopy (ICP-OES). Quantification of eleven major metals (Al, Ca, Cr, Cu, Fe, Mg, Mn, Ni, Pb, V, Zn) yielded recoveries from 89–108% and relative standard deviations from 0.7–3.6%, employing a common aqua regia leach that required reduced volumes of acid. Digestion times were satisfactory, since the samples required only 15–20 minutes to deliver the results described above. This technique shows great potential to improve sample throughput while maintaining the quality of analytical results for both research and commercial applications.
1. Introduction
Elemental analysis is essential in many real-world applications, e.g., environmental monitoring, mining, food, and forensics, where concentrations of elements are required for a variety of reasons, both regulatory, in the case of human and animal health, but also for economic reasons, where geological samples are analyzed for their concentrations of valuable elements. While modern state-of-the-art analytical instruments, such as ICP-OES and ICP-MS, provide high sample-throughput capability and superb detection limits, the overall analytical efficiency, in terms of the total time period for the analysis, has changed little since the development of microwave digestion technology in 1975.1 Contemporary analytical laboratories generally rely heavily on the conventional hot-block and microwave equipment to accomplish wet digestions of a broad range of sample types.Ideally, sample dissolution should be achieved in an accurate, fast, uncomplicated, cost-effective, risk-free, environmentally friendly, and highly reproducible manner. The intrinsic shortcomings for open-vessel hot-block digestions are well-known, particularly for their lengthy digestion time,2,3 relatively large consumption of both sample and mineral acids,4,5 together with concomitant increased potential for contamination. Unlike hot-block methods, that employ conductive heat transfer and often takes hours of heating, microwave assisted digestion (MAD) dramatically expedites the digestion process by virtue of its ability to promote complex redox reactions by raising the temperature through the transfer of energy directly to the solvent and increasing it further by sealing the digestion vessel, thereby increasing the pressure. The overall performance of MAD has been evaluated thoroughly in a number of previous publications.6–8 Excessive build-up of pressure in closed digestion system can, however, result in the rupture of sealed vessels. Moreover, the safe working temperature of Teflon™ (PTFE) vessels, frequently used in MAD applications, is generally limited to temperatures below 260 °C. Above this temperature the vessel is subject to deformation and consequently can compromise the analysis.9 Consequently, some decomposition reactions that require high activation energy may not be achieved, due to inadequate energy input. Aside from those limitations, sample pre-digestion for organic-rich or gas-releasing samples and extended cooling stages may be required.10,11 Ybáñez et al.12 reported rather uneven distribution of microwave radiation within the oven cavity, resulting in compromised method reproducibility. Taylor et al.13 proposed an open-vessel digestion method with focused microwave radiation to resolve some of the limitations of conventional close-vessel microwave operations. However, despite the facilitation of using hydrofluoric acid for silicate-rich samples, multiple acid-addition steps and consumption of relatively large amounts of acids was still required and the reproducibility and digestion time were improved only fractionally. In addition, since many Teflon reaction tubes are made by sintering powdered PTFE, cleanup of the tubes prior to use, or between uses, can be difficult and time-consuming, due to the retention of contaminants by the reticulated surface of the tubes. Those additional handling steps, together with the need to clean complex parts, such as the lid of the digestion vessels, inevitably compromise the overall digestion efficiency and can lead to increased variation in analytical results. From the perspective of many production labs, the slowness of current digestion methods represents the rate-determining step in the analytical process. This slowness presents a significant barrier to automation of the digestion process and, ultimately, automation of the entire analytical process. Therefore, the need for new dissolution methods that address the above-mentioned weaknesses in current digestion technologies has been growing in the analytical community.
While microwave technology (MW) has successfully demonstrated speedy digestion using the microwaves to boost kinetic energy, the potential of accelerating wet digestion using other kinetic sources, such as infrared (IR) lamps, has been sought by several device manufacturers and research groups with limited success. Infrared (IR) heating sources are commercially available and can provide more intense irradiation than microwave, due to its higher energy (usually by 2–4 orders of magnitude).†14 Lopes et al.15 developed an IR-MW procedure using a domestic IR lamp to facilitate sample pre-digestion, and achieved significantly improved reproducibility. Two European manufacturers, Büchi and Gerhardt, market IR-based digesters for elemental determination. These products utilize dispersive, unfocused IR radiation to promote convective–conductive heating. The digestion time is still relatively long (typically around two hours).
In the current investigation, the IR digester employs focused short-wavelength IR irradiation which transmits infrared energy directly to the surface of sample particles and is the first IR-based digester of its kind. Its digestion performance was systematically evaluated with a variety of environmental matrices, as the first step in validating the device and exploring its potential for extension to other matrices. The device's uniquely small, focused heating zone, in contrast to its relatively cool operating environment, led to its being called the ‘ColdBlock’.
2. Experimental
2.1. Design of digestion apparatus
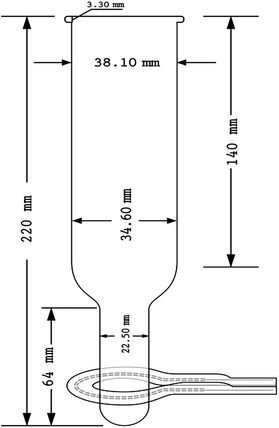
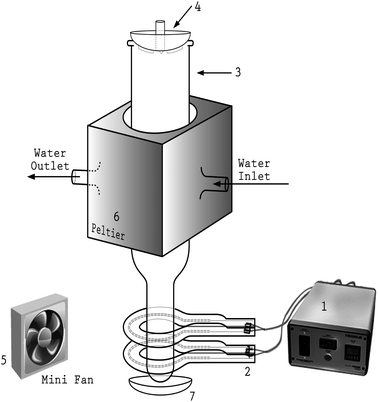
The prototype of the ‘ColdBlock™’ used in the current study comprises seven functional units (see Fig. 2): (1) adjustable AC power supply (Heraeus AccuPower 120, maximum power output 1800 W, with timer); (2) up to two infra-red emitter (Anderson Omega IR rings with ceramic coating, tungsten filament, maximum power: 250 W); (3) high purity quartz digestion vessel (125 mL); (4) ribbed watch glass; (5) mini-fans; (6) Peltier cooling block; and (7) concave reflective disk. A customized quartz digestion vessel, made of high purity quartz to maximize the transmission of IR and to minimize the background contamination, is vertically positioned in a metal chamber and the lower, smaller diameter section of the tube is encircled by the IR ring(s) such that loaded sample–acid mixture can be perfectly irradiated with IR radiation. The quartz IR ring(s), powered by the AccuPower 120 unit, guide maximum IR energy to the digestion mixture, focused by the ceramic coating on the outside of IR rings. In order to improve the efficiency of IR irradiation, a concave reflective disk, made of polished aluminum metal, is placed about 1 cm below the bottom of quartz tube to ensure that unfocused and deflected IR beams are directed back to the target zone. The quartz tube transmits much of the IR radiation so that maximum energy absorption by the sample occurs. The effective IR heating zone covers as much as 25 mm in length from the bottom of the quartz tube, depending on the number of IR rings installed. The structure and orientation of quartz tube and IR rings are illustrated in Fig. 1.A semi-closed cooling system, incorporating a pair of mini-fans, a Peltier cooling block, and a ribbed watch glass, was devised to handle the excess heat generated during the digestion (Fig. 2). Rapid removal of the heat generated by the IR rings and by the exothermic reaction of the sample provides an advantage in that the upper part of the tube remains cool, which facilitates handling. Mini-fans are positioned at the bottom of heating chamber that blow cool air directly onto the lower part of quartz tube, enabling rapid cooling of the digestion vessel at the end of the run. The water-cooled Peltier cooling block (typically operating at 4 °C), fitted tightly into the upper-body of the thimble, thermally moderates the release of acid fume evaporated in the wake of extreme heat from IR irradiation. The selection of this powerful cooling accessory, in contrast to other ordinary cooling methods, is necessitated by its application for tougher geological matrices, that must be more completely dissolved, and where the promotion of reflux of high boiling point acids is critical. The ribbed watch glass is intended to prevent airborne contaminants from entering the reaction vessel. In addition, once a digestion starts, the gap between the ribbed watch glass and the top of quartz tube allows gaseous product, such as CO2 and NO2, to escape. Meanwhile, the chilled upper part of the quartz tube recovers much of the acid fume and thus reduces the use of acid and minimizes sample loss.
2.2. Analytical instrumentation
A PerkinElmer (Sheldon, CT, USA) OPTIMA® DV3300 ICP-OES was used for analyzing all samples and standards and was operated in the axial viewing mode. The sample introduction system consists of a Teflon™ Scott double-pass spray chamber and a cross-flow nebulizer. Plasma stability tests were conducted daily, after the plasma had been running for two hours, using a multi-element calibration standard (SCP28AES). Important operating parameters of the instrument are listed in Table 1.| Plasma RF power (W) | 1300 |
| Plasma gas flow (L min−1) | 15.0 |
| Auxiliary gas flow (L min−1) | 0.5 |
| Nebulizer gas flow (L min−1) | 0.8 |
| Sample uptake (mL min−1) | 1.5 |
| Detector | UV |
| Delay time (s) | 30 |
| Integration period (s) | Automatic (3–4 s) |
| Sweeps | 3 |
| Number of replicates | 3 |
2.3. Reagents
Reagent-grade (single distilled) nitric acid (68–70% m/v) and hydrochloric acid (36.5–38% m/v) were purchased from Caledon (Georgetown, ON, Canada). Elemental stock solutions were purchased from High-Purity Standards (Charleston, SC, USA). All sample and working solutions were prepared with ultra-pure de-ionized water (18.2 MΩ resistivity) from an Elgastat-Maxima purification system (High Wycombe, UK). Certified reference materials (contaminated soil SS-1, biosolid CRM031-040) were acquired from SCP Science (Baie d'Urfé, QC, Canada) and RTC (Laramie, WY, USA), respectively. Uncertified sewage sludge material for inter-laboratory comparison, LPTP12-S2, was obtained from ACLASS (Milwaukee, WI, USA). Certified QC standard (QC.STD4) and calibration standard (SCP28AES) were obtained from SCP Science (Baie d'Urfé QC, Canada). ICP grade liquid nitrogen (99.999%) and argon (99.998%) were supplied by Praxair (Hamilton, ON, Canada).2.4. Detection limits and calibration
The instrument was calibrated frequently to eliminate the effect of detector signal drift. Four calibration standards prepared in 0.5% (v/v) HNO3, wavelengths for determination, and the detection limits of the elements, based on 8 continuous measurements of the blank and using the equation: (SDL = Sblank + 3σblank),16 are listed in Table 2. All calibration curves were linear through zero (blank subtracted) and delivered correlation coefficients of at least 0.9999.| Elements | Low (μg mL−1) | High (μg mL−1) | Wavelength (nm) | LOD (ng mL−1) |
|---|---|---|---|---|
| Al | 1.0 | 20 | 394.401 | 5.4 |
| Ca | 1.0 | 20 | 317.933 | 2.1 |
| Cr | 1.0 | 20 | 267.716 | 2.6 |
| Cu | 1.0 | 20 | 327.393 | 1.4 |
| Fe | 1.0 | 20 | 238.204 | 1.9 |
| Mg | 1.0 | 20 | 285.213 | 0.6 |
| Mn | 1.0 | 20 | 257.610 | 0.1 |
| Ni | 1.0 | 20 | 231.604 | 3.2 |
| Pb | 1.0 | 20 | 220.353 | 9.3 |
| Ti | 1.0 | 20 | 334.940 | 0.3 |
| V | 1.0 | 20 | 290.880 | 0.5 |
| Zn | 1.0 | 20 | 206.200 | 1.8 |
2.5. Digestion method and sample preparation procedure
The appropriate dissolution treatment depends primarily on the nature of the sample under investigation, elements of interest, and the analytical purpose. In the current study, difficult environmental matrices, including soil and sludge materials, were selected for examining the performance of the ColdBlock™ Employing customized digestion profiles and modified recipes for the aqua regia leach, the accuracy and precision of the concentrations of eleven major elements (Al, Ca, Cr, Cu, Fe, Mg, Mn, Ni, Pb, V, Zn) present in samples were evaluated.All digestions were performed in a well-vented fume hood to address safety concerns. Approximately 0.2–0.25 grams of dried samples were accurately weighed and carefully loaded into the bottom of the quartz digestion tube. Compared with conventional EPA hot-block methods (3050, 3050B) that adopt a repeated acidifying-drying approach, sample digestion is straightforward with the ColdBlock™ digester – the reaction is completed without the sample being taken to dryness. Due to the high-organic nature of the environmental materials being tested, the first stage in the current method was to add 2 mL of concentrated nitric acid to the sample. This step aimed to remove as much organic carbon content as possible and helped to prevent excessive foaming in the following aqua regia leaching stage. Oxidation of organics was immediately observed upon starting IR heating. After approximately two minutes, the evolution of reddish NO2 fumes, a by-product of the oxidation reaction, subsided. The quartz tube was then left to cool by the mini-fans for one minute before 3 mL of concentrated HCl was added to form a modified aqua regia leaching mixture. IR heating for 10–15 minutes at higher power was allowed for the aqua regia leaching. Finally, digestates were treated with 1 mL of H2O2 to further oxidize carbon residues as well as to convert remaining NOx to more stable NO3−. The detailed digestion profiles for each environmental sample are listed in Table 3. The resultant digestates were diluted to 25 mL with ultra-pure water and filtered with a syringe-filter of 0.22 μm porosity. Further dilutions were applied to the filtrate, with appropriate dilution factors to ensure that elemental concentrations are comfortably bracketed within the calibration range.
| Sample ID | SS-1 (min) | CRM031-040 (min) | LPTP12-S2 (min) |
|---|---|---|---|
| Step 1: add 2 mL of HNO 3 | |||
| Power used: | |||
| 55% | 1 | ||
| 60% | 1 | 3 | 1 |
| 70% | 1 | ||
| Step 2: add 3 mL of HCl | |||
| 60% | 1 | 1 | |
| 70% | 1 | 1 | 1 |
| 80% | 10 | 15 | 15 |
| Step 3: add 2 mL of H 2 O 2 | |||
| 80% | 1 | 1 | 1 |
| Total | 15 | 20 | 20 |
3. Results and discussion
3.1. Temperature profile of the ColdBlock™
Both the temperature that digestion mixture can reach and the rate at which exhaust heat is removed, are critical to the efficiency of wet digestion. The ring-shaped IR lamp minimizes uneven heating of the sample–acid mixture and ultimately improves the reproducibility of the digestion methods. Maximum emission from the IR rings occurs around 1 micron, at which wavelength the radiation is absorbed primarily by the sample particles rather than by liquid acids and the quartz tube.17 As the sample absorbs the infrared radiation, it is very likely that local heating of the sample particles takes place, leading to more effective reaction with the acid mixture that surrounds them.3.2. Effect of in situ cooling
The energy dynamics in a digestion system can be very complicated. In the current design of the ColdBlock™ prototype, we considered that in situ cooling is necessary. In situ cooling of the quartz tube can help reduce the violent spattering of the digestion mixture and largely confine the movement of liquid to a lower level. Such control of reaction violence is desirable, as the digestion can be performed in a more reproducible manner, with little loss of sample and acid. Such a ‘greener’ performance characteristic presents a net benefit to many analytical labs as it enables lower consumption of acid and drastically reduces emission of acid fumes to the environment and the concomitant cost for control of those emissions.3.3. Validation of analytical performance
In Tables 4 and 5, analytical results for eleven major elements (Al, Ca, Cr, Cu, Fe, Mg, Mn, Ni, Pb, V, Zn) in the two CRMs are presented. Compared with the certified values, which were generated with EPA method 3050B, a hot-plate method, recoveries of analytes using the ColdBlock™ digestions were satisfactory, except for the slightly lower Cr values (89%) in CRM031-040. This low recovery may be ascribed to the relatively high inertness of chromium in more refractory phases.18,19 Precision at the 95% confidence level‡ was calculated from 4 between-run analyses for each CRM and yielded a smaller spread than certified values. The RSDs range from 0.7–3.6% for the major elements. This markedly improved between-run reproducibility at varying elemental concentration levels, compared with reported microwave data on similar soil types,20–22 may be a function of the efficiency of the focused IR heating during digestion or could, as a referee has noted, be due to other variables that might include sample homogeneity and sample size.
| SS-1 (n = 4) | ||||
|---|---|---|---|---|
| Element | Measured (mg kg−1) | Certified (mg kg−1) | Recovery (%) | RSD (%) |
| Al | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 ± 300 300 ± 300 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 163 ± 409 163 ± 409 |
93 | 2.3 |
| Ca | 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ± 1300 000 ± 1300 |
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 265 ± 1213 265 ± 1213 |
104 | 2.6 |
| Cr | 95 ± 2 | 103 ± 6.1 | 93 | 1.6 |
| Cu | 410 ± 6 | 403 ± 10 | 102 | 1.4 |
| Fe | 77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 ± 2100 800 ± 2100 |
72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ± 2273 000 ± 2273 |
108 | 2.7 |
| Mg | 9300 ± 200 | 9690 ± 230 | 96 | 2.7 |
| Mn | 700 ± 5 | 737 ± 19 | 95 | 0.7 |
| Ni | 54 ± 1 | 59.2 ± 1.3 | 92 | 1.2 |
| Pb | 720 ± 30 | 764 ± 15 | 95 | 3.6 |
| V | 28 ± 1 | 27.2 ± 1.4 | 104 | 1.6 |
| Zn | 1010 ± 10 | 1114 ± 37 | 91 | 0.7 |
| CRM031-040 | ColdBlock (n = 4) | HotBlock (n = 3)aa EPA method 3050B was applied, data provided by the Niagara Regional Lab. | ||||
|---|---|---|---|---|---|---|
| Element | Certified (mg kg−1) | Measured (mg kg−1) | Recovery (%) | RSD (%) | Recovery (%) | RSD (%) |
| Al | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 ± 874 100 ± 874 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 ± 300 900 ± 300 |
91 | 2.2 | 97.0 | 1.8 |
| Ca | 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 ± 2310 400 ± 2310 |
49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 ± 900 400 ± 900 |
100 | 1.8 | 92.0 | 2.2 |
| Cr | 243 ± 12.3 | 220 ± 5 | 89 | 2.4 | 94.0 | 2.9 |
| Cu | 639 ± 21.3 | 600 ± 20 | 93 | 2.7 | 87.0 | 1.8 |
| Fe | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 ± 999 400 ± 999 |
22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 ± 300 800 ± 300 |
102 | 1.4 | 82.0 | 1.9 |
| Mg | 8920 ± 326 | 9000 ± 200 | 101 | 2.1 | 99.0 | 2.7 |
| Mn | 1240 ± 139 | 1200 ± 30 | 95 | 2.8 | 95.0 | 3.3 |
| Ni | 136 ± 8.8 | 124 ± 3 | 92 | 2.7 | 94.0 | 3.5 |
| Pb | 121 ± 6.3 | 120 ± 2 | 98 | 1.7 | 96.0 | 3.0 |
| V | 133 ± 8.2 | 120 ± 3 | 91 | 2.4 | 95.0 | 2.5 |
| Zn | 908 ± 59 | 960 ± 10 | 106 | 0.8 | 94.0 | 3.1 |
| LPTP12-S2 | ||||||
|---|---|---|---|---|---|---|
| Element | Assigned (mg kg−1) | Measured (mg kg−1) | SDPAa (mg kg−1) | Z | Recovery (%) | RSD (%) |
a Standard deviation for proficiency analysis.
b
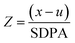 (x: measured score; u: assigned value). (x: measured score; u: assigned value).
|
||||||
| Al | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 ± 200 900 ± 200 |
1750 | −0.83 | 91.0 | 1.8 |
| Ca | 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 ± 1000 600 ± 1000 |
1980 | −1.28 | 96.0 | 0.7 |
| Cr | 129 | 120 ± 2 | 5.78 | −0.29 | 93.0 | 1.3 |
| Cu | 716 | 680 ± 20 | 64.7 | 0.08 | 95.0 | 2.5 |
| Fe | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 ± 500 200 ± 500 |
1900 | 0.04 | 104.0 | 2.1 |
| Mg | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
9500 ± 300 | 475 | −1.29 | 91.0 | 3.1 |
| Mn | 756 | 770 ± 10 | 68.3 | −0.8 | 102.0 | 1.5 |
| Ni | 373 | 370 ± 10 | 42.5 | 0.06 | 99.0 | 1.4 |
| Pb | 192 | 190 ± 5 | 23.9 | −0.07 | 101.0 | 2.4 |
| V | 122 | 100 ± 3 | 7.68 | 0.33 | 96.0 | 3.0 |
| Zn | 833 | 820 ± 20 | 65.7 | −0.71 | 99.0 | 3.0 |
| SS-1a (n = 4) | CRM031-040 (n = 4) | LPTP12-S2 (n = 4) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| As | Sb | Se | As | Sb | Se | As | Sb | Se | |
| a 1 gram of sample used and a reduced dilution factor was applied for the determination of Se. | |||||||||
| Measured (mg kg−1) | 21.9 ± 1.9 | 5.7 ± 0.4 | 0.74 ± 0.06 | 208 ± 6 | 110 ± 4 | 107 ± 3 | 316 ± 8 | 103 ± 2 | 185 ± 4 |
| Certified (mg kg−1) | 20.7 ± 1.0 | 5.5 ± 1.1 | 0.78 ± 0.14 | 217 ± 10 | 107 ± 20 | 120 ± 12 | 330 ± 40 | 110 ± 25 | 200 ± 30 |
| Recovery (%) | 105.8 | 104.4 | 94.9 | 96.3 | 103.3 | 90.1 | 95.1 | 94.0 | 90.0 |
| RSD (%) | 4.4 | 3.4 | 3.9 | 3.0 | 3.3 | 2.9 | 2.6 | 1.5 | 2.0 |
3.4. Other considerations
Whilst the IR radiation is an effective energy source for speedy sample breakdown, and the device shows strong and versatile digestion capabilities towards difficult environmental samples, a number of areas remain that show minor to major influences on digestion efficiency and the quality of analytical results during the validation. Peripheral parts of the ColdBlock™ that do not affect the overall performance of the equipment remain to be optimized, and further optimization of the IR rings continues. The digestion vessel should be of the highest quality quartz (ultra-low-background) to minimize the impacts from the inevitable ablation of the quartz vessel, when hydrofluoric acid is used. Finely divided samples, however, do expose a much larger surface to the HF than does the quartz tube. 100–200 mesh particles will have a surface area in the region of 200 m2 g−1, hence, in a competitive situation between the surface of a quartz tube and the quartz in a finely-divided sample, the powdered quartz outcompetes the quartz tube for reaction with HF. In fact, our in-house digestions of silicate-rich samples employing 0.5 mL of HF recorded an average loss of 0.07 gram in the tube weight. Quartz tubes from different manufacturers may produce rather different backgrounds for several elements, including alkali metals, Al, Ca, Fe, and Ti25 and if these elements are crucial to the analysis, this potential for contamination must be accounted for. Last but not least, the authors perceive that the simplicity effectiveness and particularly the speed of the digestion process of the ‘ColdBlock’ will benefit sample throughput once multi-channel digesters become available. In addition, the potential for the ColdBlock to be further automated to incorporate robotics and automation will allow the integration of automated sample digestion into a production laboratory situation.4. Summary
The ColdBlock™ provides a novel digestion technique that exhibits great potential to be a robust, convenient, flexible, and reliable digestion alternative to the existing digestion methods for environmental samples.Acknowledgements
Financial support for the project initiation by the Ontario Centre of Excellence Technical Problem Solving (TPS) Program (OCE Grant no. 11429) is gratefully acknowledged. This work is also sponsored by the Federal Development Agency for Southern Ontario Applied Research and Commercialization (ARC) program. Mr Ron Emburgh is thanked for his assistance in constructing the prototype of the ColdBlock™ The authors are also grateful to Ms Paula Cheese from the Niagara Regional Laboratory who kindly provided two reference materials. The Canada Foundation for Innovation and the Ontario Research Fund provided funds for the purchase of the SCIEX Elan ICP-MS (DRC II). PerkinElmer generously donated the Optima 3300DV optical instrument.References
- A. Abu-Samra, J. S. Morris and S. R. Koirtyohann, Anal. Chem., 1975, 47, 1475 CrossRef CAS.
- M. Stoeppler, Sampling and Sample Preparation, Springer-Verlag, Heidelberg, 1997 Search PubMed.
- J.-H. Liu, R. E. Sturgeon and S. N. Willie, Analyst, 1995, 120, 1905 RSC.
- J. Sastre, A. Sahuquillo, M. Vidal and G. Rauret, Anal. Chim. Acta, 2002, 462, 59 CrossRef CAS.
- EPA Method 3050.
- K. J. Lamble and S. J. Hill, Analyst, 1998, 123, 103R RSC.
- K. E. Levine, J. D. Batchelor, C. B. Rhoades, Jr and B. T. Jones, J. Anal. At. Spectrom., 1999, 14, 49 RSC.
- Microwave-Enhanced Chemistry: Fundamentals, Sample Preparation, and Applications, ed. H. M. Kingston and S.J. Haswell, American Chemical Society, Washington, DC, 1997 Search PubMed.
- M. Würfels, E. Jackwerth and M. Stoeppler, Fresenius' Z. Anal. Chem., 1987, 329, 459 CrossRef.
- J. Castro, J. C. Spraul and R. K. Marcus, Anal. Methods, 2009, 1, 188 RSC.
- S.-L. Wu, X.-B. Feng and A. Wittmeier, J. Anal. At. Spectrom., 1997, 12, 797 RSC.
- N. Ybáñez, M. L. Cervera, R. Montoro and M. de la Guardia, J. Anal. At. Spectrom., 1991, 6, 379 RSC.
- V. F. Taylor, A. Toms and H. P. Longerich, Anal. Bioanal. Chem., 2002, 372, 360 CrossRef CAS PubMed.
- J. R. Reitz, F. J. Milford, and R. W. Christy, Foundations of Electromagnetic Theory, Pearson/Addison-Wesley, San Francisco, CA, USA, 2009, p. 413 Search PubMed.
- A. N. S. Dantas, W. O. Matos, S. T. Gouveia and G. S. Lopes, Talanta, 2013, 107, 292 CrossRef CAS PubMed.
- D. Harvey, Modern Analytical Chemistry, McGraw-Hill, Boston, USA, 1st edn, 2000, p. 95 Search PubMed.
- W. D. Keller and E. E. Pickett, Am. Mineral., 1949, 34, 855 Search PubMed.
- R. A. Nadkarni, Anal. Chem., 1984, 56, 2233 CrossRef CAS.
- Z. Kowalewska, E. Bulska and A. Hulanicki, Fresenius' J. Anal. Chem., 1998, 362, 125 CrossRef CAS.
- R. Falciani, M. Marchesini and M. Gucciardi, J. Anal. At. Spectrom., 2000, 15, 561 RSC.
- M. V. B. Krishna, K. Chandrasekaran, G. Venkateswarlu and D. Karunasagar, Anal. Methods, 2012, 4, 3290 RSC.
- EPA Method 3015A.
- M. Grotti and R. Frache, J. Anal. At. Spectrom., 2007, 22, 1481–1487 RSC.
- W. Guo, S.-H. Hu, X.-F. Li, J. Zhao, S.-S. Jin, W.-J. Liu and H.-F. Zhang, Talanta, 2011, 84, 887 CrossRef CAS PubMed.
- R. M. Twyman, Encyclopedia of Analytical Science, Elsevier Ltd., 2005, vol. 8, p. 146 Search PubMed.
Footnotes |
| † The quantum energy of microwave photons is generally in 10−6 to 10−4 eV range, whereas the energy state of infrared photons falls in 10−2 to 1.6 eV. |
| ‡ In accordance with the confidence level employed in publishing two certified reference materials. |
| § t(exp) = 1.22, two-tailed t(0.05, 10) = 2.23. |
| This journal is © The Royal Society of Chemistry 2014 |