Highly dispersible polypyrrole nanospheres for advanced nanocomposite ultrafiltration membranes†
Yaozu
Liao
*ab,
Thomas P.
Farrell
a,
Gregory R.
Guillen
c,
Minghua
Li
c,
James A. T.
Temple
c,
Xin-Gui
Li
*b,
Eric M. V.
Hoek
*cd and
Richard B.
Kaner
*a
aDepartment of Chemistry & Biochemistry, California NanoSystems Institute, University of California, Los Angeles, Los Angeles, California 90095, USA. E-mail: kaner@chem.ucla.edu
bCollege of Materials Science & Engineering, Institute of Materials Chemistry, Tongji University, 1239 Si-Ping Road, Shanghai 200092, China. E-mail: yaozu.liao@gmail.com; adamxgli@yahoo.com
cDepartment of Civil & Environmental Engineering, Institute of the Environment & Sustainability, California NanoSystems Institute, University of California, Los Angeles, Los Angeles, California 90095, USA. E-mail: emvhoek@ucla.edu
dDepartment of Applied Chemistry, University of Johannesburg, Johannesburg, South Africa
First published on 3rd September 2013
Abstract
Highly dispersible polypyrrole (PPy) nanospheres were synthesized and used to produce polysulfone (PSf) nanocomposite ultrafiltration membranes by a non-solvent induced phase separation (NIPS) process. The composite networks formed between PPy and PSf lead to higher porosity, hydrophilicity, surface charge, thermal stability, and water permeability, but slightly lower protein rejection. Nanocomposite membranes containing up to 20% PPy nanospheres were >10 times more permeable than pure PSf membranes, while the bovine serum albumin (BSA) rejection decreased from 94 to 82%. With nanoscale pores, high porosity, improved hydrophilicity, and tunable surface charge properties, the PPy/PSf nanocomposite membranes hold great promise for advanced protein separation, dialysis, water filtration and other macro molecular separations.
Introduction
In recent years, ultrafiltration (UF) membranes for separations have been demonstrated as a straightforward, efficient, and scalable alternative to traditional separation and purification processes.1–3 Fundamental advances in membrane technology that improve the efficiency of separations can lower costs, save time, and may someday lead to new devices such as wearable blood dialysis systems.4 In evaluating a membrane for use in a particular separation, there are criteria/figures of merit that determine the utility of a particular membrane: solvent permeability, target solute rejection, fouling resistance, and chemical and mechanical stability.5,6 Typical high-performance synthetic polymers commonly used in the formation of UF membranes include polysulfone, polyethersulfone, and polyacrylonitrile.7–9 These polymers are known to be relatively inexpensive, chemically stable, soluble in common organic solvents, insoluble in water, and mechanically tough. However, UF membranes formed from these polymers by nonsolvent induced phase separation (NIPS) generally show an inverse relationship between permeability and rejection. An important goal is to improve the permeability of a membrane, and thereby reduce the energy input and/or decrease the time needed to achieve separation without sacrificing selectivity. Another major obstacle in membrane separations is fouling by organic and inorganic species.The incorporation of nanomaterials into polymer matrices to form nanocomposite membranes has become an important area of research.10–16 Previous work on adding nanomaterials into membranes has focused on microfiltration, ultrafiltration, nanofiltration and reverse osmosis membranes.17 However, progress in this field has been limited by the types of nanomaterials that can be incorporated into polymer membranes for performance enhancement. This limitation is mainly due to difficulties in ensuring appropriate interactions between filler nanomaterials and polymer in the mixed matrix – either promoting attraction or repulsion to minimize or maximize highly permeable interfacial regions. Much research has focused on compatibilizing zeolites, metal oxides and mesoporous carbon nanoparticles with different polymers, but another approach is to begin with polymeric nanoparticles.
Prior research has produced polypyrrole (PPy) nanomaterials that are sufficiently processable for incorporation into UF membranes.18 Other findings encourage further exploration of PPy as an anti-fouling and charged membrane material due to its anticipated conductivity, biocompatibility, and hydrophilicity.19 Furthermore, incorporation of conductive nanomaterials into membranes may lead to “active transport” separation processes where an electro-stimuli can be used to control the transport of charged compounds through and away from a membrane surface.20–22 Our previous findings indicated that PPy nanoparticles/nanospheres blended with polysulfone (PSf) can be used to fabricate high flux, flexible ultrafiltration membranes.23
In this work, PPy nanospheres were synthesized and blended with polysulfone (PSf) to produce nanocomposite UF membranes by NIPS. The influence of PPy nanosphere loading on the morphology, surface energy, charge characteristics, thermal stability, water permeability, and protein rejection of PPy/PSf nanocomposite membranes was extensively examined. In particular, flushing experiments demonstrate that these membranes can be readily cleaned. With nanoscale pores, high porosity, improved hydrophilicity, tunable surface charge characteristics, and cleanable properties, the PPy/PSf nanocomposite membranes offer great promise for advanced protein separation, dialysis, water filtration, and other macro molecular separations.
Experimental
Materials
Pyrrole, 2,4-diaminodiphenylamine, ferric chloride (FeCl3), hydrochloric acid (HCl), 1-methyl-2-pyrrolidinone (NMP), polysulfone (PSf, average Mn ∼22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), bovine serum albumin (BSA, ∼66 kDa) were purchased from Sigma-Aldrich. Potassium chloride (KCl), sodium hydroxide (NaOH), and sodium chloride (NaCl) were purchased from Fisher Scientific. All the materials were chemical grade and used as received.
000), bovine serum albumin (BSA, ∼66 kDa) were purchased from Sigma-Aldrich. Potassium chloride (KCl), sodium hydroxide (NaOH), and sodium chloride (NaCl) were purchased from Fisher Scientific. All the materials were chemical grade and used as received.
Synthesis of PPy nanospheres
A scaled-up synthesis of PPy nanospheres was carried out as follows: 0.5 g of pyrrole and 0.15 g of 2,4-diaminodiphenylamine (10 mol% relative to pyrrole) as an initiator were dissolved in 0.3 L of methanol, while 1.2 g of FeCl3 as an oxidant was dissolved in 0.3 L of 1.0 mol L−1 of HCl. The two solutions were cooled to 0 °C and rapidly mixed. The reaction was vigorously shaken for 10–15 s by hand and then left undisturbed overnight. The as-synthesized products were purified by centrifugation at a speed of 4500 rpm min−1 using DI water–methanol (90/10) at 15 °C until the supernatant became colorless. A black powder was obtained by drying the above solution at 50 °C for one week to remove all the water. The structure, morphology, and size measurements of the PPy nanospheres are presented elsewhere.18Membrane formation via NIPS
A procedure known as NIPS was used to prepare the UF membranes. In this process, five compositions: 0, 30, 60, 150 and 300 mg of PPy nanospheres were dispersed in 6.83 g of NMP and then 1.5, 1.47, 1.44, 1.35, and 1.2 g of PSf beads were added, respectively. The mixtures consisting of 1.5 g polymers and 6.83 g of NMP were stirred at 50 °C overnight to produce casting solutions at a fixed concentration of 18% with five PPy concentrations (0, 2, 4, 10, and 20%) used for all the polymers. The solutions were cast on a commercial nonwoven polyester support fabric and then immersed in 18 MΩ laboratory deionized water at room temperature to induce precipitation by a solvent/non-solvent (NMP/water) exchange to form homogeneous UF membranes.Measurements and characterization
The morphologies of the pure PSf and PPy/PSf nanocomposite membranes were observed by a JEOL JSM-6700 field emission scanning electron microscopy (SEM) and a Synergy ESPM 3D atomic force microscopy (AFM). Attenuated total reflection/Fourier transform infrared (ATR/FT-IR) spectra of the membranes were recorded on a JASCO-620 spectrometer. Thermogravimetric analysis (TGA) and differential TGA (DTGA) scans of the membranes without the support fabric were carried out on a Perkin Elmer TGA Pyris 1 by heating the samples from room temperature to 1000 °C at a rate of 10 °C min−1. The hydrophilicity of all the UF membranes was determined by the captive bubble technique using a DSA10 Krüss goniometer with at least seven contact angle measurements performed across each membrane coupon at equally spaced intervals. Streaming current was measured using an adjustable gap electrokinetic analyzer (SurPASS Electrokinetic Analyzer, Anton-Paar GmbH). The flow channel gap was set at 100 μm, and a 1 mM KCl solution at 20 °C was used as the background electrolyte. Streaming current was determined in a pH range of 2–10, adjusted using HCl and NaOH. Membrane zeta potential (ζ) was calculated using the Helmholtz–Smoluchowski eqn (1),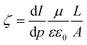 | (1) |
 | (2) |
Results and discussion
Chemical structure and morphology of PPy/PSf nanocomposite membranes
The structure, morphology, and dimensions of PPy nanospheres have been described in great detail elsewhere.18 Membranes of varying PPy and PSf content were formed using the NIPS precipitation technique. Photographs of each membrane in a wet state are shown in Fig. 1a. As the nanosphere loading increases, the membrane color successively changes from white to brown to gray and then to black. When dried, the membranes display a very smooth and shiny surface (Fig. 1b), implying that smooth, thin-skinned ultrafiltration membranes have been formed.The ATR/FT-IR spectra of the pure PSf and PPy/PSf nanocomposite membranes are shown in Fig. 2. The characteristic vibrational bands of PSf occur at 690, 834, 1160, 1240, and 1324 cm−1 due to the C–S–C linkage, the aromatic rings, the symmetric sulfone, the aromatic ether, and an asymmetric sulfone, respectively.26,27 With addition of varying concentrations of PPy nanospheres, the characteristic bands attributed to PSf exhibit no chemical shifts, indicating an interaction typical of physical blending.
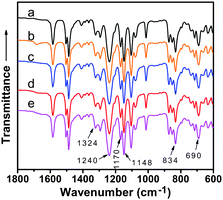 | ||
| Fig. 2 ATR/FT-IR spectra of PPy/PSf nanocomposite membranes prepared with the following concentrations of PPy nanospheres: (a) 0, (b) 2, (c) 4, (d) 10, and (e) 20%. | ||
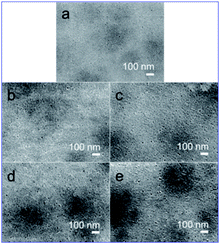
Fig. 3–5 show the cross-sectional and surface morphologies of membranes with different concentrations of PPy nanospheres with diameters of ∼85 nm, as observed by SEM. The cross-sectional morphologies of the pure PSf and nanocomposite membranes consist of a relatively dense, nanoporous top layer (1–2 μm thick) and a porous sublayer with large macrovoids (Fig. 3).
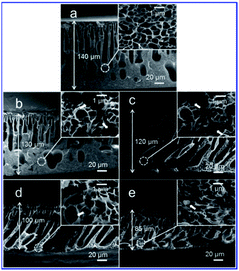 | ||
| Fig. 3 Cross-sectional SEM images of PPy/PSf nanocomposite membranes prepared with the addition of the following concentrations of PPy nanospheres: (a) 0, (b) 2, (c) 4, (d) 10, and (e) 20%. | ||
However, the finger-like structures in the nanocomposite membranes, especially in the membranes containing 10% PPy nanospheres, appear more interconnected than those of the pure PSf membrane, perhaps due to more cavities left by the migration of PPy nanospheres during solvent-exchange. Additionally, it appears that macrovoid formation is reduced in the nanocomposite membranes, producing a more sponge-like morphology (Fig. 3, insets). A pure PSf membrane without the support has a thickness of ∼140 μm, whereas the values decreased to 130 and then to 85 μm with 2 to 20% PPy nanospheres, respectively, as shown in Fig. 3a–e.
With increased PPy nanosphere loading, the apparent membrane surface pore diameters increase and the surface becomes rougher (Fig. 4). The nanocomposite membranes with 10% PPy nanospheres show the highest surface roughness and porosity (5.6%) as determined by analyzing the SEM micrographs using NIH ImageJ software,24 (Fig. S1†). AFM was also used for morphological characterization of the membrane surface to complement SEM. According to the 3D AFM images and histogram analyses of the membranes (Fig. S2 and 3†), the morphological characterization results are presented in Table 1, elucidating a few trends corresponding to the different concentrations of PPy nanospheres.
| Membrane | R a (nm) | RMS (nm) | R max (nm) | SAD (%) | Contact angle (°) | −ΔG13 (mJ m−2) | d p (nm) |
|---|---|---|---|---|---|---|---|
| PSf | 2.6 | 3.3 | 24.5 | 1.8 | 64.7 ± 3.8 | 103.4 | 7.7 |
| 2% PPy | 2.5 | 3.6 | 45.2 | 2.0 | 54.0 ± 5.8 | 114.8 | 7.0 |
| 4% PPy | 5.1 | 7.0 | 64.6 | 1.6 | 42.1 ± 0.7 | 126.0 | 7.2 |
| 10% PPy | 6.5 | 8.5 | 60.9 | 2.9 | 45.6 ± 4.1 | 122.3 | 7.5 |
| 20% PPy | 3.0 | 3.7 | 26.8 | 3.9 | 42.9 ± 2.0 | 124.1 | 8.2 |
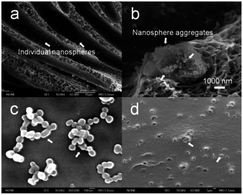
Generally, with the addition of PPy nanospheres, the surface roughness of the nanocomposite membranes appears to be greater than that of the pure PSf membrane. In the range of the scan areas (1 μm × 1 μm), the nanocomposite membranes exhibit the highest root-mean-square (RMS) roughness, average roughness (Ra), and maximum roughness (Rmax) with values of 8.5, 6.5, and 60.9 nm, respectively, at a 10% concentration of PPy nanospheres, compared to 2.6, 3.3, and 24.5 nm for pure PSf membranes. However, with a further increase in the concentration of PPy nanospheres up to 20%, the surface structure starts to become smooth again. The huge change in Ra, RMS, and Rmax, but small change in surface area difference (SAD), is evidence that homogeneous and smooth PPy/PSf nanocomposite membranes have been created. Moreover, PPy nanospheres were observed in both the top and bottom surfaces of the nanocomposite membranes (Fig. 5c and d), indicating that the increased roughness may be caused by the accumulation of hydrophilic PPy nanospheres on the membrane surface. Even the finger-like and sponge-like structures of the nanocomposite membranes show some individual or aggregated nanospheres, as indicated in Fig. 3b–e (insets), and Fig. 5a and b. This implies that the PPy nanospheres have good miscibility with the PSf matrix, leading to improved surface porosity and more interconnected cross-sectional morphologies.
Additionally, different sized PPy nanospheres with diameters of 85, 110, 200, and 220 nm were synthesized using hydrochloric acid (HCl), nitric acid (HNO3), perchloric acid (HClO4), and camphorsulfonic acid (CSA), respectively. When the same concentration of PPy nanospheres (4%) were blended into the PSf membranes, increases in porosity and decreases in thickness of the nanocomposite membranes are observed with the addition of relatively larger particle sizes of PPy nanospheres, as shown in Fig. S4 and 5.† This also suggests that the observed differences in membrane thickness are due to the effects of PPy nanospheres on macrovoid morphology rather than on the decreasing PSf content.
Hydrophilicity, charged surfaces, and thermal stability
The influence of PPy nanosphere loading on the hydrophilicity and surface energy of these composite membranes is presented in Table 1. Note that upon addition of only 2% PPy nanospheres, the contact angles of the resulting nanocomposite membranes (54°) become lower than that of pure PSf membranes (65°). The contact angles further decreased to ∼42° when the concentration of PPy nanospheres is increased to 4%. Then, the contact angles remain relatively constant with values of 42–46° when 10–20% PPy nanospheres were added. On the basis of surface roughness corrected by AFM, solid–liquid interfacial free energies (−ΔG13) of the pure PSf and nanocomposite membranes were calculated from the contact angles and SAD values as previously described.28 These data are presented in Table 1. As expected, the pure PSf membrane is the most hydrophobic, and the membrane surface energies generally increase with increasing PPy concentration.Zeta potential measurements for the pure PSf and nanocomposite membranes are shown in Fig. 6 as a function of streaming pH. The decrease in zeta potential as pH increases is a typical characteristic of polymeric membranes.29,30 The PPy/PSf nanocomposite membranes have amine, amino, and sulfone groups which will be protonated at low pH leading to a positive charge (i.e., a positive zeta potential); the groups will be deprotonated as the pH increases, causing the membrane charge to become more negative (i.e., a negative zeta potential). At the isoelectric point (IEP) pH ≈ 3.4, the pure PSf membrane has a net charge of zero (i.e., zeta potential = 0 mV). The IEP values increased to pH = 4.2–4.8 with the addition of PPy nanospheres. More importantly, either positively charged at low pH due to the protonation or negatively charged at higher pH due to deprotonation, the nanocomposite membranes demonstrate much higher zeta potentials compared to the pure PSf membranes. Additionally, as the PPy nanosphere loading was increased, the nanocomposite membranes became more highly charged. Clearly, the addition of PPy nanospheres has promise for improving separation efficiency of PSf membranes because hydrophilicity and anti-fouling capability generally increase as membrane surfaces become more hydrophilic, energetic, and charged.10,31,32
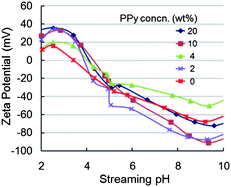 | ||
| Fig. 6 Influence of streaming pH and PPy nanosphere concentration on the zeta potentials of pure PSf and PPy/PSf nanocomposite membranes. | ||
Thermal stabilities of the membranes were measured by TGA and DTGA (Fig. 7). When the membranes were heated to 500 °C, the pure PSf exhibits negligible weight-loss as compared to the nanocomposite which experiences a weight-loss of ∼7%. The acidic dopants of the PPy nanospheres are volatilized upon heating, which explains why the nanocomposite membranes show a slightly lower thermal stability than the pure PSf membrane up to 500 °C. When the membranes were heated to 600 °C, however, the nanocomposite membranes show a slower weight-loss rate than the pure PSf membrane (11.2 vs. 17.1% min−1). Moreover, when the membranes were heated from 600 to 1000 °C, both the pure PSf membrane and the nanocomposite membrane exhibited only ∼5% weight-loss. The nanocomposite membrane left a more massive residue at 1000 °C in comparison to the pure PSf membrane (47 vs. 30%). However, when the weight-loss is calculated by subtraction of the PPy mass, negligible differences between membranes are observed. This implies that PPy has an insignificant influence on the thermal stability of the pure PSf matrix, likely due to the adequate miscibility between the two polymers, which is consistent with the ATR/FT-IR analysis.
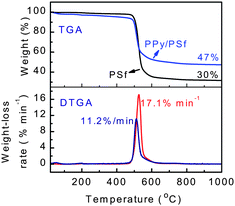 | ||
| Fig. 7 TGA/DTGA scans of pure PSf membrane and PPy/PSf nanocomposite membranes prepared with the addition of 20% PPy nanospheres. | ||
Separation performance
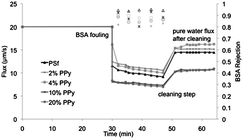
While microscopy and surface energy characterizations indicate that PPy nanospheres produce hydrophilic films resembling UF membranes, meaningful performance data were obtained by UF experiments. The testing procedures employed in this study are described in greater detail in the ESI.†Fig. 8 shows a simple fouling experiment, in which compacted membranes were fouled with BSA, while flux decline and BSA rejection were recorded. After collecting 5 mL of permeate, the membranes were rinsed using deionized water at room temperature in an attempt to clean the membrane surfaces and recover some of the lost permeability. Performance data from these tests are presented in Table 2. We found that the introduction of as little as 2% PPy nanospheres produces UF membranes with markedly improved performance over the pure PSf membrane. All nanocomposite membranes showed improvements in initial, compacted, fouled, and recovered permeability with the most notable improvements in the higher PPy content membranes (46.2 vs. 4.3, 22.2 vs. 4.0, 7.9 vs. 1.9, and 12.3 vs. 2.9 μm s−1 psi−1, respectively). All membranes rejected BSA proteins above 82%, indicating average pore diameters of around 8 nm (see ESI†), consistent with expectations from microscopy.24,25 The pure PSf membrane showed a higher BSA rejection of 94.3%.| Membrane | Initial permeability (μm s−1 psi−1) | Compacted permeability (μm s−1 psi−1) | Fouled permeability (μm s−1 psi−1) | Recovered permeability (μm s−1 psi−1) | Recovered permeability (%) | Flux decline (%) | BSA rejection (%) |
|---|---|---|---|---|---|---|---|
| PSf | 4.3 | 4.0 | 1.9 | 2.9 | 72.4 | 54.1 | 94.3 |
| 2% PPy | 19.5 | 7.6 | 4.0 | 5.8 | 76.1 | 47.2 | 86.5 |
| 4% PPy | 19.7 | 5.8 | 2.9 | 4.7 | 81.2 | 49.9 | 88.3 |
| 10% PPy | 34.0 | 20.2 | 7.4 | 10.9 | 53.7 | 63.4 | 82.5 |
| 20% PPy | 46.2 | 22.5 | 7.9 | 12.3 | 54.6 | 65.1 | 83.2 |
A lower relative flux decline upon BSA fouling was observed in membranes containing 2 and 4% PPy nanospheres. While it is tempting to attribute this observation to the hydrophilicity and surface charge improvements associated with the introduction of nanoparticles, this observation can also be explained by the greater average pore diameters present in the 2 and 4% PPy nanosphere containing membranes. Additionally, while the 10 and 20% PPy membranes showed the largest relative flux decline of 63–65% upon fouling, their fouled permeability was still considerably higher than that of the pure PSf membranes (7.9 vs. 1.9 μm s−1 psi−1).
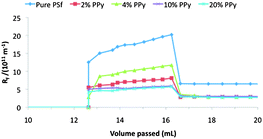
Another way to analyze the data obtained from the fouling experiments is to discuss the total hydraulic resistance present at different stages in the experiment. Initially, prior to introduction of the BSA, the total resistance to water flow through the membrane (Rt) is wholly attributed to the resistance of the membrane (Rm) itself. Upon introduction of BSA, the flux immediately declines due to fouling of the membranes. This fouling adds resistance to the system and we can describe this contribution as Rf. At any point in the experiment the total resistance is a sum of the contributions from the membrane and the fouling layer, simply Rt = Rm + Rf. Fig. 9 shows membrane performance in terms of the hydraulic resistance due to the fouling layer as a function of the volume of water that has passed through the membrane during the experiment. It is clear from the figure that the pure PSf membrane experiences the largest fouling layer resistance due to BSA proteins. The cleaning step, although decidedly unsophisticated, is effective enough to restore nearly two-thirds of the permeability that was lost upon fouling with BSA. The PPy nanosphere-containing membranes also lose permeability upon fouling with BSA, but the effect is not as severe as with the pure PSf membrane. The total irreversible fouling for the nanocomposite membranes is less than half of the corresponding value for PSf, indicating that our new membranes are both resistant to fouling and also efficiently cleaned using only water.
A proposed geometric structure for the PPy/PSf nanocomposite membrane is illustrated in Fig. S6.† The mechanism for separation performance enhancement upon PPy nanosphere introduction is uncertain since many factors come into play. However, change in membrane pore structures is the main contributor to membrane performance. Additionally, we anticipate that the PPy nanospheres behave as porogenic agents. Because the PPy nanospheres used were in a protonated and oxidized form, a unique porosity is induced via dedoping during the NMP/water exchange, leading to void spaces where small molecules such as water can pass through, while larger molecules such as BSA are rejected.
Conclusions
Adjusting the concentration of highly dispersible PPy nanospheres can be used to tailor the hydrophilicity, surface charge, morphology, permeability, and solute rejection of PSf nanocomposite ultrafiltration membranes. High loadings of PPy nanospheres produce significant improvements in membrane permeability, translating into >10 times initial water permeability, >5 times compacted water permeability, >4 times BSA fouled permeability, and >4 times recovered permeability when compared to that of a pure PSf ultrafiltration membrane (46.2 vs. 4.3, 22.2 vs. 4.0, 7.9 vs. 1.9, and 12.3 vs. 2.9 μm s−1 psi−1, respectively); all while retaining relatively high BSA rejection (82–94%). With nanoscale pores, high porosity, improved hydrophilicity, and tunable surface charge properties, the PPy/PSf nanocomposites hold great promise for advanced separation membranes.Acknowledgements
The authors thank Abraxis Bioscience (R.B.K. and E.M.V.H.), the National Natural Science Foundations of China (51203090, Y.Z.L.), the Innovation Program of Shanghai Municipal Education Commission (13YZ074, Y.Z.L.), and the Natural Science Foundation of Shanghai (12ZR1446700, Y.Z.L.) for financial support.Notes and references
- R. Ghosh, J. Chromatogr., A, 2002, 952, 13 CrossRef CAS.
- M. Feins and K. K. Sirkar, Biotechnol. Bioeng., 2004, 86, 603 CrossRef CAS PubMed.
- M. Feins and K. K. Sirkar, J. Membr. Sci., 2005, 248, 137 CrossRef CAS PubMed.
- T. R. Gaborski, J. L. Snyder, C. C. Striemer, D. Z. Fang, M. Hoffman, P. M. Fauchet and J. L. McGrath, ACS Nano, 2010, 4, 6973 CrossRef CAS PubMed.
- W. A. Phillip, B. O'Neill, M. Rodwogin, M. A. Hillmyer and E. L. Cussler, ACS Appl. Mater. Interfaces, 2010, 2, 847 CAS.
- A. Mehta and A. L. Zydney, J. Membr. Sci., 2005, 249, 245 CrossRef CAS PubMed.
- M. Nyström and P. Järvinen, J. Membr. Sci., 1987, 60, 275 CrossRef.
- C. Barth, M. C. Goncalves, A. T. N. Pires, J. Roeder and B. A. Wolf, J. Membr. Sci., 2000, 169, 287 CrossRef CAS.
- Y. L. Su, C. X. Mu, C. Li and Z. Y. Jiang, Ind. Eng. Chem. Res., 2009, 48, 3136 CrossRef CAS.
- M. L. Lind, A. K. Ghosh, A. Jawor, X. F. Huang, W. Hou, Y. Yang and E. M. V. Hoek, Langmuir, 2009, 25, 10139 CrossRef CAS PubMed.
- A. Olubummo, M. Schulz, B.-D. Lechner, P. Scholtysek, K. Bacia, A. Blume, J. Kressler and W. H. Binder, ACS Nano, 2012, 6, 8713 CrossRef CAS PubMed.
- N. Maximous, G. Nakhla, W. Wan and K. Wong, J. Membr. Sci., 2009, 341, 67 CrossRef CAS PubMed.
- C. O. Baker, B. Shedd, R. J. Tseng, A. A. Martinez-Morales, C. S. Ozkan, M. Ozkan, Y. Yang and R. B. Kaner, ACS Nano, 2011, 5, 3469 CrossRef CAS PubMed.
- K. Zodrow, L. Brunet, S. Mahendra, D. Li, A. Zhang, Q. L. Li and P. J. J. Alvarez, Water Res., 2009, 43, 715 CrossRef CAS PubMed.
- Y. N. Yang and P. Wang, Polymer, 2006, 47, 2683 CrossRef CAS PubMed.
- S. Kim, J. R. Jinschek, H. B. Chen, D. S. Sholl and E. Marand, Nano Lett., 2007, 7, 2806 CrossRef CAS PubMed.
- M. M. Pendergast and E. M. V. Hoek, Energy Environ. Sci., 2011, 4, 1946 CAS.
- Y. Z. Liao, X. G. Li and R. B. Kaner, ACS Nano, 2010, 4, 5193 CrossRef CAS PubMed.
- N. K. Guimard, N. Gomeb and C. E. Schmidt, Prog. Polym. Sci., 2007, 32, 876 CrossRef CAS PubMed.
- D. Zhou, C. O. Too, G. G. Wallace, A. M. Hodges and A. W. H. Mau, React. Funct. Polym., 2000, 45, 217 CrossRef CAS.
- X. S. Peng, J. Jin and I. Ichinose, Adv. Funct. Mater., 2007, 17, 1849 CrossRef CAS.
- S. S. Madaeni and S. Molaeipour, Ionics, 2010, 16, 75 CrossRef CAS.
- Y. Z. Liao, X. Wang, W. Qian, Y. Li and X. Y. Li, J. Colloid Interface Sci., 2012, 368, 148 CrossRef PubMed.
- G. R. Guillen, T. P. Farrell, R. B. Kaner and E. M. V. Hoek, J. Mater. Chem., 2010, 20, 4621 RSC.
- Water Treatment: Principles and Design, ed. J. C. Crittenden, R. R. Trussel, D. W. Hand, K. J. Howe and G. Tchobanoglous, John Wiley & Sons, Inc., Hoboken: New Jersey, USA, 2nd edn, 2005 Search PubMed.
- G. S. Sur, H. L. Sun, S. G. Lyu and J. E. Mark, Polymer, 2001, 42, 9783 CrossRef CAS.
- J. M. Yeh, C. L. Chen, Y. C. Chen, C. Y. Ma, H. Y. Huang and Y. H. Yu, J. Appl. Polym. Sci., 2004, 92, 631 CrossRef CAS.
- A. K. Ghosh, B. H. Jeong, X. F. Huang and E. M. V. Hoek, J. Membr. Sci., 2008, 311, 34 CrossRef CAS PubMed.
- A. E. Childress and M. Elimelech, J. Membr. Sci., 1996, 119, 253 CrossRef CAS.
- J. C. Schaep, A. Vandecasteele, W. Mohammad and W. R. Bowen, Sep. Purif. Technol., 2001, 22–23, 169 CrossRef.
- S. Kim and E. M. V. Hoek, Desalination, 2007, 202, 333 CrossRef CAS PubMed.
- A. Subramani and E. M. V. Hoek, J. Membr. Sci., 2008, 319, 111 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Data for contrast adjusted surface SEM images, AFM images, and proposed geometric structure of the PPy/PSf nanocomposite membrane can be found in ESI. See DOI: 10.1039/c3mh00049d |
| This journal is © The Royal Society of Chemistry 2014 |