All-inorganic water-dispersible silicon quantum dots: highly efficient near-infrared luminescence in a wide pH range†
Hiroshi
Sugimoto
a,
Minoru
Fujii
*a,
Yuki
Fukuda
a,
Kenji
Imakita
a and
Kensuke
Akamatsu
b
aDepartment of Electrical and Electronic Engineering, Graduate School of Engineering, Kobe University, Rokkodai, Nada, Kobe 657-8501, Japan. E-mail: fujii@eedept.kobe-u.ac.jp
bDepartment of Nanobiochemistry, Frontiers of Innovative Research in Science and Technology (FIRST), Konan University, 7-1-20 Minatojimaminami, Chuo-ku, Kobe 650-0047, Japan
First published on 10th October 2013
Abstract
We report a novel method to prepare silicon quantum dots (Si-QDs) having excellent stability in water without organic-ligands by simultaneously doping phosphorus and boron. The codoped Si-QDs in water exhibit bright size-tunable luminescence in a biological window. The luminescence of codoped Si-QDs is very stable under continuous photoexcitation in water.
Colloidal semiconductor quantum dots (QDs) have been considered to be a promising alternative to organic dyes for biological fluorescence imaging,1,2 because of the superior properties such as wide tunability of the luminescence wavelength by controlling the size,3 high resistance to degradation in aqueous media,4 high photo-stability during long-term illumination,5etc. The highest quality colloidal QDs commercially available at present are II–IV and IV–VI compound semiconductor QDs. However, these QDs consist of toxic elements such as cadmium (Cd) and lead (Pb), which discourage their extensive applications as contrast agents for not only in vivo but also in vitro biological labeling.6 Compared to compound semiconductor QDs, silicon (Si) QDs have higher compatibility with biological substances7 and are intrinsically more suitable for biomedical applications. Recently, the quality of Si-QDs has been quickly catching up with that of compound semiconductor QDs.8–16
For in vivo biomedical applications, Si-QDs should be hydrophilic and be dispersed in water in a wide pH range. Furthermore, the photoluminescence (PL) wavelength is preferably within the biological window (700–1000 nm).17,18 Unfortunately, the majority of colloidal Si-QDs synthesized so far do not satisfy these criteria. In particular, in many cases, the surface of Si-QDs is hydrophobic,14–16 and only a limited number of studies have been carried out on water-dispersible Si-QDs.19–26 Ruckenstein et al.27 synthesized poly(acrylic acid) grafted water-dispersible Si-QDs with a high PL quantum yield (QY) (24%). However, the PL was in the visible range (∼600 nm). Water-dispersible Si-QDs having PL in a similar range (650 nm) were reported by Zhong et al.28 Their Si-QDs were capped with protein (IgG) and the PL exhibited high pH- and photo-stability.28 Lin et al. realized water-dispersible Si-QDs by the formation of thick oxide shells, although the PL wavelength was very short (450 nm).29 Erogbogbo et al.30 employed a different approach to obtain the dispersibility in water. They synthesized polymer-coated or micelle-encapsulated Si-QD assemblies (50–150 nm), which showed stable PL around 650 nm. However, the large size of the Si-QD assemblies limits the application in specific fields. The development of Si-QDs dispersible in water in a wide pH range and having highly efficient stable PL in the NIR range is still a challenging task.
In previous work,31–34 we reported the preparation of a new type of colloidal Si-QDs, which are dispersed in methanol without a surface functionalization process. The characteristic feature of the colloidal Si-QDs is simultaneous doping of n-(phosphorus (P)) and p-(boron (B)) type dopants.31–34 In the codoped Si-QDs, a majority of doped P and B atoms are accumulated on the surface of the Si-QDs and high P and B concentration layers are formed on the surface.33 The negative surface potential of the layer makes Si-QDs hydrophilic without surface functionalization.31–34 Furthermore, a part of P and B atoms are doped in the substitutional sites of Si-QDs and form donor and acceptor states, respectively, within the band gap. The PL of codoped Si-QDs thus arises from donor to acceptor state transitions. The combination of the quantum size effects and impurity codoping results in PL controllable in a range extended to below bulk Si bandgap (0.85–1.9 eV).34
This work is a direct extension of our previous work on codoped colloidal Si-QDs in alcohol. In general, QDs dispersible in alcohol are not always dispersible in water because of the very high polarity. In fact, maintaining colloidal stability in water in a wide pH range is a challenging task because the negative (positive) charge on the surface of QDs is compensated by cations (anions) under acidic (basic) pH conditions. In this paper, we demonstrate that codoped Si-QDs can be dispersed in water without a functionalization process and exhibit excellent stability in a wide pH range. We show that the colloids exhibit bright size-tunable PL in a suitable range for biological applications (700–1000 nm). The stability of the PL when they are stored in the dark and under light irradiation is also studied.
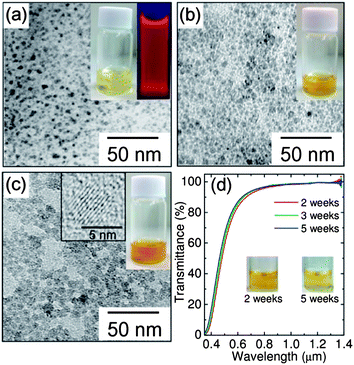
The list of the samples studied in this work is summarized in Table 1. For all the samples (samples A–D) preparation parameters are fixed except for the annealing temperature (see the Experimental section). Fig. 1a–c show photographs of aqueous solutions and transmission electron microscopy (TEM) images of codoped Si-QDs (samples B–D). We can see yellowish optically clear solutions. Fig. 1d shows the optical transmittance spectra of sample D measured 2, 3 and 5 weeks after the preparation. The insets are the photographs after 2 and 5 weeks. The transmittance in the NIR range is almost 100%, indicating that light scattering caused by agglomerates is negligibly small. The solution is very clear and the color density does not change significantly even after 5 weeks. In the TEM images, the dark spots correspond to Si-QDs with the diamond structure as confirmed by electron diffraction patterns (ESI, Fig. S1†). In a high resolution TEM image (the inset of Fig. 1c), lattice fringes corresponding to the {111} planes of the Si crystal can clearly be seen, implying that the codoped Si-QD is a defect-free single crystal. We can confirm from TEM images that Si-QDs are isolated and do not form agglomerates. The average diameters (dave) of Si-QDs in samples B, C and D estimated from the TEM images are 2.8, 3.9 and 5.2 nm, respectively, and the standard deviations of the size distribution (σ) are 0.7, 0.8 and 0.8 nm, respectively. In sample A, Si-QDs were not clearly observed by TEM and the electron diffraction pattern was halo, suggesting that the diamond structure is strongly distorted.
| Sample name | T a (°C) | d ave (nm) | σ (nm) |
|---|---|---|---|
| Sample A | 1000 | — | — |
| Sample B | 1050 | 2.8 | 0.7 |
| Sample C | 1100 | 3.9 | 0.8 |
| Sample D | 1150 | 5.2 | 1.0 |
As shown in Fig. 1a, the solution exhibits bright red luminescence under UV irradiation (352 nm, FL4BLB, TOSHIBA). Fig. 2a shows the PL spectra of codoped colloidal Si-QDs in water (samples A–D). Size dependent shift of the PL from red to NIR regions can clearly be seen. The observed size dependence of the PL wavelength is quantitatively in good agreement with that of codoped colloidal Si-QDs in methanol, despite the large difference in the dielectric constants (εr_water = 78.5 and εr_methanol = 33).35 PL of codoped Si-QDs is thus insensitive to the solvent polarity. In Fig. 2a, the PL-QYs are also shown. The highest PL-QY of 10.2% is obtained for sample B (PL peak: ∼760 nm). This is to the best of our knowledge the highest PL-QY reported for Si-QDs dispersed in water and luminescing in the biological window.
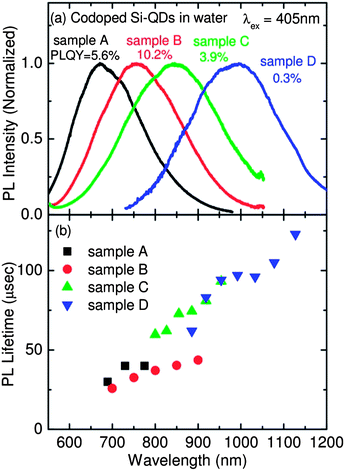 | ||
| Fig. 2 (a) PL spectra of samples A–D. PL quantum yields are shown in the figure. (b) PL lifetimes of samples A–D as a function of detection wavelength. | ||
Fig. 2b shows the PL mean lifetimes defined by 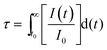 , where I(t) is the PL intensity as a function of time t and I0 is the initial intensity at time t0 of samples A–D as a function of detection wavelength (see Fig. S2 in the ESI† for PL decay curves). The wavelength dependence of the lifetimes is on the same trend for all samples, although the data are scattered. The lifetimes are from several tenths of μs in the red region to over 100 μs in the NIR region. The lifetimes are comparable to those of Si-QDs in nonpolar solvents exhibiting PL in the same wavelength range.15
, where I(t) is the PL intensity as a function of time t and I0 is the initial intensity at time t0 of samples A–D as a function of detection wavelength (see Fig. S2 in the ESI† for PL decay curves). The wavelength dependence of the lifetimes is on the same trend for all samples, although the data are scattered. The lifetimes are from several tenths of μs in the red region to over 100 μs in the NIR region. The lifetimes are comparable to those of Si-QDs in nonpolar solvents exhibiting PL in the same wavelength range.15
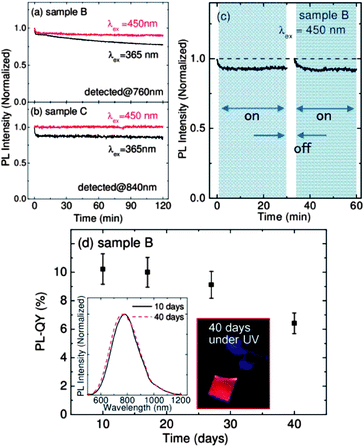
Fig. 3a and b show the evolution of the PL intensities of samples B and C, respectively, in water under continuous irradiation of 450 nm (150 μW cm−2) and 365 nm (180 μW cm−2) light for 2 h. When excited at 365 nm, the PL intensities decrease by about 10% within 100 s for both the samples. After the initial quenching, the PL intensity of sample B slowly decreases to 78% of the initial value in 2 h, while that of sample C is almost constant. When the excitation wavelength is 450 nm, the degradation is much smaller for both the samples. Especially, sample C does not show any degradation of the PL. It should be stressed here that CdSe and CdTe QDs in water, often regarded as photostable materials, degrade more rapidly by continuous irradiation (more than 50% decrease in 100 min).36,37 Therefore, we can conclude that codoped Si-QDs, especially large QDs, possess remarkably high photostability. Hereafter, we focus mainly on sample B to further study the PL stability in different environments because it is relatively more vulnerable than samples with larger QDs and has the highest PL-QY.
To investigate the mechanism of the PL degradation, we introduce a short break in the middle of the continuous excitation. Fig. 3c shows the PL intensity of sample B under continuous excitation at 450 nm for 1 h with a 3 min break in the middle. The PL intensity fully recovers after the 3 min break, indicating that the PL quenching is not due to structural changes such as photo-oxidation commonly observed for colloidal QDs with organic ligands.38,39 A plausible explanation for the temporal decrease under continuous light irradiation is due to charging (ionization) of Si-QDs caused by capturing of either electrons or holes to trap on the surface.40 It is noted here that, although initial fast quenching is fully recovered, gradual quenching observed in Fig. 3a at 365 nm is not recovered after the break. The gradual quenching may be due to defect formation by photo-oxidation.
Fig. 3d shows the PL-QY of sample B as a function of storage time in the dark. The PL-QY starts to decrease after 20 days and reaches 90% of the initial value after 27 days. However, even after 40 days, the QY of codoped Si-QDs is 6.5% (64% of the initial value). A possible mechanism of slight degradation is the formation of defects by oxidation in water. As shown in the inset, the PL spectral shape and the peak wavelength do not change even after 40 days. Compared to previously reported Si-QDs in water, the degree of degradation of codoped Si-QDs is very small. For example, the PL intensity of poly-acrylic acid grafted Si-QDs decreases up to 80% of the initial value within 5 days in the dark.27 In recent work, the intensity of carboxylic acid terminated Si-QDs is reported to decrease by 30% for 16 days.41
The pH stability of codoped colloidal Si-QDs in water is examined. Fig. 4a shows the optical absorbance at 360 nm and 1000 nm (sample B) (transmittance spectra in the ESI, Fig. S3†). The absorbance value at 360 nm, which is roughly proportional to the amount of Si-QDs in water, is almost constant in the wide pH range. The absorbance value at 1000 nm, which represents the scattering loss, is almost 0 in the pH range. These results indicate that no agglomerates and precipitates are formed and almost the same amount of Si-QDs is dispersed in the pH range of 2–10. Fig. 4b shows zeta-potentials of codoped Si-QDs in water (sample D) as a function of pH. In the pH range, zeta-potentials are negative. As the pH value increases, the zeta-potential decreases from −23 mV to −45 mV. This is typical behavior of QDs with negatively charged surface. At lower pH, the negative potential arising from the surface of QDs is cancelled by H+ ions in an aqueous solution. This results in the small absolute value of zeta-potential. On the other hand, at higher pH, OH− ions become the potential-determining ions. In such a pH range, the absolute value of zeta-potential becomes large. In codoped colloidal Si-QDs, the absolute value of the zeta-potential is 23 mV even at pH = 2. This value is usually considered to be high enough for colloidal stability. This is consistent with the pH-independent optical absorbance in Fig. 4a.
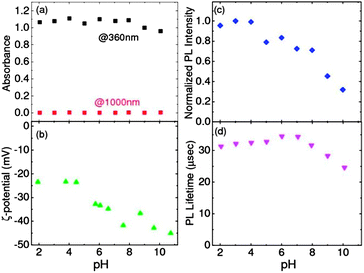 | ||
| Fig. 4 pH dependence of (a) absorbance at 360 and 1000 nm, (b) ζ-potential, (c) normalized PL intensity and (d) PL lifetime detected at 760 nm. | ||
Fig. 4c and d show the PL intensity and PL lifetime detected at 760 nm, respectively, as a function of pH. The PL intensity is stable at low pH (<4) and then decreases by 20–30% in the pH range of 5–8. On the other hand, PL lifetimes are almost constant in the pH range of 2–8. This suggests that the number of Si-QDs contributing to the PL decreases in the pH range of 5–8, although the reason is not clear. In the pH range larger than 8, the intensity decrease is accompanied by the shortening of the lifetime. Nonradiative centers are thus considered to be introduced in or on the surface of Si-QDs in the pH range. A possible origin is defects on the surface generated by etching the surface with alkalized solution.
In summary, we have succeeded in producing Si-QDs having excellent stability in an aqueous solution without organic-ligand passivation by simultaneously doping P and B. The codoped Si-QDs in water exhibit bright size-tunable PL in a biological window (700–1000 nm) with the lifetime of 20–120 μs. The maximum PL-QY reaches 10.2% at 760 nm. The codoped colloidal Si-QDs show high photo- and pH stability in water. The present results suggest that codoped colloidal Si-QDs are a very suitable material for application as contrast agents in biological imaging.
Experimental section
Fabrication of Si-QDs dispersed in water
Si-rich borophosphosilicate glass (BPSG) films were deposited on thin stainless steel plates by cosputtering Si, SiO2, B2O3, and P2O5 in an rf-sputtering apparatus.31–34,42,43 The films were peeled off from the plates and annealed at different temperatures (1000–1150 °C) in a N2 gas atmosphere for 30 min to grow Si-QDs of different sizes in BPSG matrices. During the growth of Si-QDs, a part of P and B atoms were incorporated into Si–NCs from BPSG.31–34,43,44 In order to isolate Si-QDs from matrices, powder samples were dissolved in HF solution (46 wt%). Isolated Si-QDs in HF solution were transferred to methanol, and then the solvent was exchanged with distilled water (see ESI†). At this stage, almost all Si-QDs were dispersed in water. Finally, to make sure that the solutions were precipitation-free, they were subjected to centrifugation (4000 rpm, 2 min) and the supernatant solutions were stored in a vial. All the processes were performed in an ordinary laboratory environment.Characterization
TEM observations (JEM-2010, JEOL) were performed by dropping Si-QD-dispersed water on carbon-coated TEM meshes. P and B concentrations in colloidal SiQDs were determined by inductively coupled plasma atomic emission spectroscopy (ICP-AES), and were 3.0 and 14.5 at%, respectively. Note that a majority of P and B atoms are on the surface of Si-QDs and are not active.32 Only fractions of them are electrically active and are considered to be doped as P–B pairs.32 The zeta potentials were measured with a Zetasizer nano ZS (MalvernInstruments, UK) at 25 °C. The pH values of the samples were controlled by adding NaOH or HCl solutions.Optical measurements
PL spectra of colloidal solutions were obtained by using a single spectrometer equipped with a liquid-N2 cooled InGaAs diode array (OMA-V-SE, Roper Scientific) and a charge coupled device (CCD) (Roper Scientific). The excitation wavelength was 405 nm. The spectral response of the detection system was corrected with the reference spectrum of a standard halogen lamp. PL decay dynamics were measured by using a NIR photomultiplier (R5509-72, Hamamatsu Photonics) and a multi-channel scalar (SR430, Stanford Research). The excitation source was modulated 405 nm light. All the measurements were carried out at room temperature. Although we used UV light for the excitation of Si-QDs, it is not suitable for biological applications because of the small penetration depth in biological tissue. However, PL properties of Si QDs excited at UV wavelength are considered to be almost identical to those excited at longer wavelength suitable for biological applications except for the degree of photo-degradation.PL-QY measurements
The PL-QY was determined by a comparative method.44,45 Rhodamine 6G in water with the QY of 95% was used as a reference solution. PL spectra of reference and colloidal Si-QD samples were obtained in the same condition by using a spectrofluorometer (Fluorolog-3, HORIBA Jovin Yvon). The QY of a sample (Qs) is calculated using Qs = QR (IS × AR × nS2)/(IR × AS × nR2) where Q is the quantum yield, I is the integrated PL intensity, A is the absorbance, and n is the refractive index. The subscript S and R refer to the sample and the reference solutions, respectively. In order to minimize an error due to nonuniform irradiation of solution, the sample solutions were diluted to keep the absorbance below 0.1.Acknowledgements
This work is supported by KAKENHI (23310077, 24651143).Notes and references
- W. B. Cai, D. W. Shin, K. Chen, O. Gheysens, Q. Z. Cao, S. X. Wang, S. S. Gambhir and X. Y. Chen, Nano Lett., 2006, 6, 669 CrossRef CAS PubMed.
- X. Michalet, F. F. Pinaud, L. A. Bentolila, J. M. Tsay, S. Doose, J. J. Li, G. Sundaresan, A. M. Wu, S. S. Gambhir and S. Weiss, Science, 2005, 307, 538–544 CrossRef CAS PubMed.
- M. A. Hines and G. D. Scholes, Adv. Mater, 2003, 15, 1844 CrossRef CAS.
- N. Pradhan, D. M. Battaglia, Y. Liu and X. Peng, Nano Lett., 2007, 7, 312–317 CrossRef CAS PubMed.
- Y. He, H. T. Lu, L. M. Sai, Y. Y. Su, M. Hu, Q. L. Fan, W. Huang and L. H. Wang, Adv. Mater, 2008, 20, 3416–3421 CrossRef CAS.
- K. M. Tsoi, Q. Dai, B. A. Alman and W. C. Chan, Acc. Chem. Res., 2012, 46, 662–671 CrossRef PubMed.
- F. Erogbogbo, K. T. Yong, I. Roy, R. Hu, W. C. Law, W. Zhao, H. Ding, F. Wu, R. Kumar, M. T. Swihart and P. N. Prasad, ACS Nano, 2011, 5, 413–423 CrossRef CAS PubMed.
- A. Gupta, M. Swihart and H. Wiggers, Adv. Funct. Mater., 2009, 19, 696–703 CrossRef CAS.
- L. Mangolini and U. Kortshagen, Adv. Mater, 2007, 19, 2513–2519 CrossRef CAS.
- M. L. Mastronardi, F. Hennrich, E. J. Henderson, F. Maier-Flaig, C. Blum, J. Reichenbach, U. Lemmer, C. Kübel, D. Wang, M. M. Kappes and G. A. Ozin, J. Am. Chem. Soc., 2011, 133, 11928 CrossRef CAS PubMed.
- K. Pettigrew, Q. Liu, P. Philip and S. Kauzlarich, Chem. Mater., 2003, 15, 4005–4011 CrossRef CAS.
- S. Sato and M. Swihart, Chem. Mater., 2006, 18, 4083–4088 CrossRef CAS.
- M. Beard, K. Knutsen, P. Yu, J. Luther, Q. Song, W. Metzger, R. Ellingson and A. Nozik, Nano Lett., 2007, 7, 2506–2512 CrossRef CAS PubMed.
- J. B. Miller, A. R. Van Sickle, R. J. Anthony, D. M. Kroll, U. R. Kortshagen and E. K. Hobbie, ACS Nano, 2012, 6, 7389–7396 CrossRef CAS PubMed.
- M. L. Mastronardi, F. Maier-Flaig, D. Faulkner, E. J. Henderson, C. Kübel, U. Lemmer and G. A. Ozin, Nano Lett., 2011, 12, 337–342 CrossRef PubMed.
- C. M. Hessel, D. Reid, M. Panthani, M. Rasch, B. Goodfellow, J. Wei, H. Fujii, V. Akhavan and B. Korgel, Chem. Mater., 2012, 24, 393 CrossRef CAS.
- W. R. Zipfel, R. M. Williams, R. Christie, A. Nikitin, B. T. Hyman and W. Webb, Proc. Natl. Acad. Sci. U. S. A., 2003, 100(12), 7075–7080 CrossRef CAS PubMed.
- G. B. Sukhorukov, A. L. Rogach, M. Garstka, S. Springer, W. J. Parak, A. Munoz-Javier, O. Kreft, A. G. Skirtach, A. S. Susha, Y. Ramaye, R. Palankar and M. Winterhalter, Small, 2007, 3, 944–955 CrossRef CAS PubMed.
- D. Mariotti, S. Mitra and V. Švrček, Nanoscale, 2013, 5, 1385–1398 RSC.
- R. S. Carter, S. J. Harley, P. P. Power and M. P. Augustine, Chem. Mater., 2005, 17(11), 2932–2939 CrossRef CAS.
- Q. Wang, H. Ni, A. Pietzsch, F. Hennies, Y. Bao and Y. Chao, J. Nanopart. Res., 2011, 13, 405–413 CrossRef CAS.
- Q. Wang, Y. Bao, X. Zhang, P. Coxon, U. Jayasooriya and Y. Chao, Adv. Healthcare Mater., 2012, 1, 189–198 CrossRef CAS PubMed.
- F. M. Dickinson, T. A. Alsop, N. Al-Sharif, C. E. M. Berger, H. K. Datta, L. Siller, Y. Chao, E. M. Tuite, A. Houlton and B. R. Horrocks, Analyst, 2008, 133, 1573–1580 RSC.
- J. H. Ahire, Q. Wang, P. Coxon, G. Malhotra, R. Brydson, R. Chen and Y. Chao, ACS Appl. Mater. Interfaces, 2012, 4, 3285–3292 CAS.
- A. Shiohara, S. Hanada, S. Prabakar, T. H. Lim, K. Fujioka, K. Yamamoto, P. T. Northcote and R. D. Tilley, J. Am. Chem. Soc., 2010, 132, 248–253 CrossRef CAS PubMed.
- E. Froner, E. D'Amato, R. Adamo, N. Prtljaga, S. Larcheri, L. Pavesi, A. Rigo, C. Potrich and M. Scarpa, J. Colloid Interface Sci., 2011, 358, 86–92 CrossRef CAS PubMed.
- Z. F. Li and E. Ruckenstein, Nano Lett., 2004, 4, 1463–1467 CrossRef CAS.
- Y. He, Y. L. Zhong, F. Peng, X. P. Wei, Y. Y. Su, Y. M. Lu, S. Su, W. Gu, L. S. Liao and S. T. Lee, J. Am. Chem. Soc., 2011, 133, 14192–14195 CrossRef CAS PubMed.
- S. W. Lin and D. H. Chen, Small, 2009, 5, 72–76 CrossRef CAS PubMed.
- F. Erogbogbo, K. Yong, I. Roy, G. X. Xu, P. N. Prasad and M. T. Swihart, ACS Nano, 2008, 2, 873 CrossRef CAS PubMed.
- M. Fukuda, M. Fujii, H. Sugimoto, K. Imakita and S. Hayashi, Opt. Lett., 2011, 36, 4026–4028 CrossRef CAS PubMed.
- H. Sugimoto, M. Fujii, K. Imakita, S. Hayashi and K. Akamatsu, J. Phys. Chem. C, 2012, 116, 17969–17974 CAS.
- H. Sugimoto, M. Fujii, K. Imakita, S. Hayashi and K. Akamatsu, J. Phys. Chem. C, 2013, 117, 6807–6813 CAS.
- H. Sugimoto, M. Fujii, K. Imakita, S. Hayashi and K. Akamatsu, J. Phys. Chem. C, 2013, 117, 11850–11857 CAS.
- P. S. Albright and L. J. Gosting, Dielectric constant of the methanol–water system from 5 to 55 °C, J. Am. Chem. Soc., 1946, 68, 1061 CrossRef CAS.
- Y. He, H. T. Lu, Y. Y. Su, L. M. Sai, M. Hu, C. H. Fan and L. Wang, Biomaterials, 2011, 32, 2133–2140 CrossRef CAS PubMed.
- N. Gaponik, D. V. Talapin, A. L. Rogach, K. Hoppe, E. V. Shevchenko, A. Kornowski, A. Eychmüller and H. Weller, J. Phys. Chem. B, 2002, 106, 7177 CrossRef CAS.
- J. Aldana, Y. A. Wang and X. Peng, J. Am. Chem. Soc., 2001, 123, 8844–8850 CrossRef CAS PubMed.
- W. G. J. H. M. van Sark, P. L. T. M. Frederix, D. J. van den Heuvel, A. A. Bol, J. N. J. van Lingen, C. de Mello Donega, H. C. Gerritsen and A. Meijerink, J. Fluoresc., 2002, 12, 69–76 CrossRef CAS.
- K. T. Shimizu, R. G. Neuhauser, C. A. Leatherdale, S. A. Empedocles, W. K. Woo and M. G. Bawendi, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 63, 205316 CrossRef.
- H. Y. Lee, J. J. Lee, J. Park and S. B. Park, Chem.–Eur. J., 2011, 17, 143 CrossRef CAS PubMed.
- M. Fujii, Y. Yamaguchi, Y. Takase, K. Ninomiya and S. Hayashi, Appl. Phys. Lett., 2004, 85, 1158 CrossRef CAS.
- M. Fujii, Y. Yamaguchi, Y. Takase, K. Ninomiya and S. Hayashi, Appl. Phys. Lett., 2005, 87, 211919 CrossRef.
- J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Plenum Press, New York, 1983 Search PubMed.
- J. A. Yvon, Guide to Recording Fluorescence Quantum Yields, http://www.%20jobinyvon.com.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3nr03863g |
| This journal is © The Royal Society of Chemistry 2014 |