Encoding molecular information in plasmonic nanostructures for anti-counterfeiting applications†
Yan
Cui
a,
Ravi S.
Hegde
b,
In Yee
Phang
c,
Hiang Kwee
Lee
ac and
Xing Yi
Ling
*a
aDivision of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, Singapore 637371. E-mail: xyling@ntu.edu.sg
bInstitute of High Performance Computing, 1 Fusionopolis way, Connexis #16-16, Singapore 138632
cInstitute of Materials Research and Engineering, A*STAR, 3 Research Link, Singapore 117602
First published on 14th October 2013
Abstract
We present the next generation covert plasmonic security labels based on Ag nanowire structures and their polarization dependent surface-enhanced Raman scattering (SERS) imaging. The security labels consist of Ag nanowires fabricated by two-photon lithography and thermal evaporation, where molecular probes of choice are deposited. Simulation and experimental results show that the SERS signals from the embedded molecules depend significantly on the polarization of the incident field. The covert molecular information cannot be revealed directly from the physical features, but can only be read-out selectively by polarization-dependent SERS imaging. Our plasmonic security labels exhibit very narrow spectral fingerprint vibration, which is more specific than broadband colorimetry-based systems. The polarization-dependent SERS intensity, molecular fingerprint of SERS spectra, and versatile geometrical design by two-photon lithography have made our plasmonic Ag nanowire structures an ideal candidate as advanced security solutions for anti-counterfeiting application.
Introduction
The proliferation of counterfeiting and forgery is an international problem with multifaceted challenges. It causes significant (financial) damage and posts security threats to individuals, companies, and society.1,2 A lot of security labels have been developed and incorporated into currency notes, identity cards, credit cards, and pharmaceutical product packaging to fight against counterfeiting. Typically, these security labels possess unique physical features that are hard to copy, such as fine prints, security inks,3 watermarks,4 and holograms.5 They can be made from stimuli-responsive molecules, photonic structures, and nanomaterials such as semiconductor nanoparticles, up-conversion nanoparticles and magnetic particles.6–9 A change in their optical or physical properties can be induced by heat,10 light,11,12 and other external stimuli,13–17 which can then be directly visualized and validated using colorimetry and fluorometry.6,18 However, with the development of technology, some of the security labels have become known to counterfeiters and easy to copy. There is an imperative to continue developing new security labels with an increased level of security to better support product authentication and to “outsmart” counterfeiters. Specifically, the new generation of security labels must be equipped with “layered security” solutions, where the first security level is based on simple colored and/or holographic features that can be easily verified by the public. On top of that, an additional security level with a covert security feature, which can only be authenticated by an advanced and sophisticated analytical system, is necessary.Plasmonic nanostructures hold great potential as the next generation security labels.19,20 Yet, there has been limited literature and applications in the anti-counterfeiting field. Plasmonic nanostructures enhance the Raman scattering signals by 4–10 orders of magnitude.21,22 This is due to the coherent oscillation of conduction electrons in metal nanostructures with incident light that enhances the electromagnetic field strength on their surfaces and increases scattering.23 Such surface-enhanced Raman scattering (SERS) is an attractive molecular detection system. It can be readily incorporated into current color- and/or visual-based security labels24 to further increase the security level in authentication. In addition, the SERS signal is strongly dependent on the incident field polarization and wavelength with respect to plasmonic nanostructures.25–29 For example, the SERS scattering of a silver nanowire at its longitudinal mode is much weaker (and nearly negligible) compared to its transverse plasmon owing to the momentum mismatch between the incident photon and the propagating plasmons.30,31 Such polarization-dependent plasmonic and SERS response may be undesirable for general molecular sensing applications, but it is highly promising for anti-counterfeiting purposes because molecular information can be encrypted selectively at different polarizations.24
Here, we introduce a plasmonic security label using Ag nanowire structures and its selective polarization-dependent SERS imaging as an advanced security solution for anti-counterfeiting application. Our strategy enables encryption of molecular information within the plasmonic nanostructures, which cannot be revealed directly from the physical feature of our plasmonic security labels. Owing to the unique enhanced directional optical properties of Ag nanowires, selective molecular Raman imaging can only be read-out spatially and spectroscopically by manipulating the polarization of incident light. Using two probe molecules on separate nanowire structures, we will demonstrate that our plasmonic security labels exhibit very narrow spectral fingerprint vibration, which is more specific than broadband colorimetry-based systems.
Results and discussion
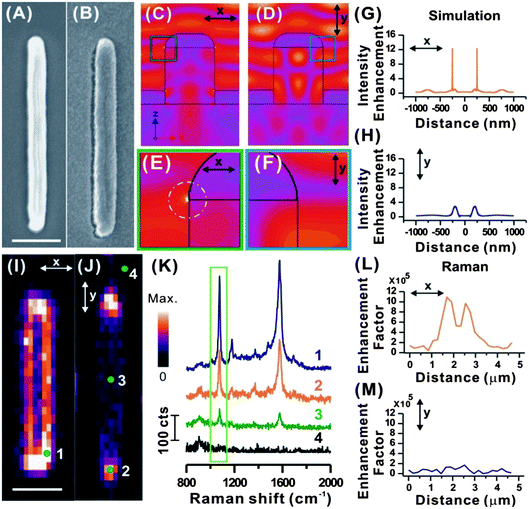
The enhanced directional optical properties of plasmonic nanowires make them an attractive option for advanced security applications. The one-dimensional morphology of plasmonic nanowires, i.e. nanometer-sized diameter and micrometer longitudinal length, has rendered anisotropic surface plasmon resonance and SERS response with incident light at different polarizations.25–27 The polarization dependent SERS response of plasmonic nanowires has never been explored in anti-counterfeiting applications.Our fabrication strategy focuses on using a two-photon lithography technique to construct tailored polymeric nanowires, followed by silver deposition to obtain plasmonic Ag nanowire structures. The structure of our plasmonic nanowire is schematically illustrated in Fig. S1.† The fabricated polymeric nanowires have a diameter of 500 nm and length of 4 μm (Fig. 1A). Subsequently the polymeric nanowires are thermally evaporated with a 2 nm Cr film, followed by another 150 nm Ag film to render them plasmonic for SERS application (Fig. 1B). Molecular information is encrypted onto the plasmonic structures via Ag–thiol coordination functionalization with analyte molecules. Our fabrication technique is highly versatile, flexible, and yet difficult to be duplicated by counterfeiters.
We begin by using simulation to verify the plasmonic responses of the nanowire at x- and y-polarizations, respectively, where x-polarization (θ = 0°, ↔) and y-polarization (θ = 90°, ↕) denote the incident electric (E) field orientations that are perpendicular and parallel to the long axis of the nanowire, respectively (Fig. 1C and D). A distinct polarization related difference in the response of the nanowire to normal incident light is observed. When the E field is polarized in the x-direction, a strong field enhancement is seen at the corners of the silver layers, at the top and the bottom layer (Fig. 1C and E). At the top layer, the field enhancement is related to the excitation of LSPR. A time domain visualization of the fields (ESI, Movie S1†) shows that a surface wave propagates at the interface between the substrate and the deposited silver. In comparison, no localized field enhancement is seen for the electric field polarized along the y-axis (Fig. 1D and F).
A quantitative comparison of the localized field enhancement is provided by looking at the cross-sectional profile of the electric field intensity enhancement (Fig. 1G and H). At x-polarization, the electric field distribution (Fig. 1G) indicates a sharp and intense electric field, with a ∼12× enhancement, at the edges of the Ag nanowire. In comparison, a modest ∼2× electric field enhancement is observed when scanned using y-polarization (Fig. 1H).
Following this, we perform slit-confocal SERS imaging on a single nanowire at x- and y-polarizations to verify our simulation result. All of the plasmonic nanowires are vertically aligned during the SERS measurements, as illustrated in Fig. 1I and J. We have selected 4-methylbenzenethiol (4-MBT) as our analyte molecule for the SERS measurements. It is an aromatic thiol that is known to form a self-assembled monolayer (SAM) on metal via a metal–thiol coordination bond,32 allowing more accurate calculation of the number of molecules on the surface of plasmonic nanowires contributing to the SERS response. For fast and efficient SERS molecular imaging, high speed slit-confocal Raman imaging is performed, where the embedded molecular vibrational information can be rapidly read-out spatially and spectroscopically in a matter of <30 min (Fig. 1I–K). From here onwards, we use the integral SERS intensity at the 1079 cm−1 peak, corresponding to the combination of phenyl ring-breathing mode, CH in-plane bending, and CS stretching33 for the 2D SERS imaging, and refer to the selected peak as the SERS intensity, unless otherwise stated.
The 2D SERS imaging of the single plasmonic nanowire at x-polarization (Fig. 1I) demonstrates that bright areas, which indicate strong SERS signals, are concentrated at the edges and tips of the Ag nanowire. However, at y-polarization (Fig. 1J), only a few pixels at the tips of the Ag nanowire exhibit SERS intensities compared to that of the Ag nanowire body. SERS intensity comparison in Fig. 1K once again indicates that the strongest SERS signals are observed at the edges (at x-polarization) and tips (both x- and y-polarizations) of Ag nanowires.
The SERS cross-sectional profile at the x-polarization also demonstrates two strong SERS signals at the edges of the Ag nanowires (Fig. 1L – Raman), whereas negligible enhancement is observed when polarized at the y-axis (Fig. 1M – Raman). The SERS enhancement factor of 106 has been achieved at the edges, as obtained by the calculation in the ESI.† The peak of SERS enhancement at the edges is broader in the experimental than in the simulation due to the optical diffraction. Nevertheless, the result is in good agreement with our simulated electric field cross-sectional profile in Fig. 1G and H, simulation and other literature.30,31 These results validate our simulation results in Fig. 1C that the edges of the Ag nanowire support localized surface plasmon resonance and localized intense electric fields surrounding them. The tip of an Ag nanowire with an isotropic hemispheric morphology (Fig. 1J and K-2) functions as an antenna27 with identical localized surface plasmon resonance in all polarization directions. Hence, the tips contribute to strong SERS hot spots in both x- and y-polarizations.2 At y-polarization, the SERS signal at the body of the Ag nanowire (Fig. 1J and K-3) is significantly weaker than the tips, indicating minimal excitation of the localized surface plasmon resonance. There is a negligible SERS background from the substrate (Fig. 1J and K-4). Both simulation and experimental results indicate that the polarization-dependent SERS response predominantly originates from the Ag nanowires, and cannot be attributed solely to the surface roughness of the Ag film (Fig. S1A and B†).
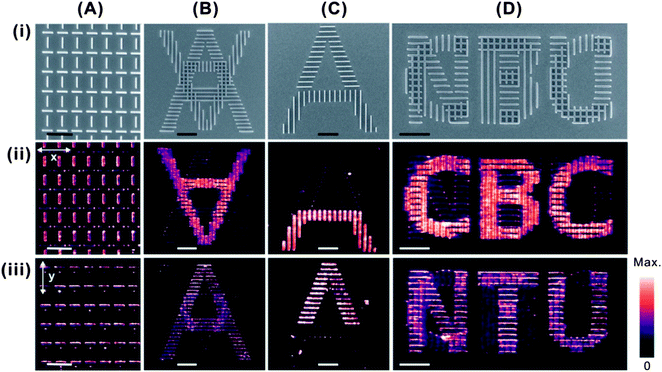
Generally, our simulated and experimental results manifest the advanced covert security feature in Ag nanowires, i.e. their SERS response can be selectively read-out by manipulating the polarization angle of incident light. The molecular information, in the form of Raman vibrational spectrum, is only turned “on” when the plasmonic nanowires are coupled to incident light at the transverse axis, resulting in strong electromagnetic fields and enhanced SERS intensities at the edges of the nanostructures. The ability to tune and prescribe the SERS intensity from “off” to “on” is the foundation for the design of our “nanostructured SERS security label”. We design and fabricate nanostructures with customized polarization-dependent SERS scattering response. Despite being coated with a homogeneous monolayer of molecules over the entire surface, the encrypted molecular information can only be authenticated by polarized SERS molecular imaging, and not by simple optical visualization. Here, four examples of tailor-made plasmonic nanostructures, from a simple orthogonal nanowire pattern to more complicated information encryption, are presented.
In the first example, we design and fabricate horizontal and vertical Ag nanowire arrays in an alternating fashion (Fig. 2A). Under the x-polarization, only the vertical nanowires are coupled to the incident light, hence yield higher SERS intensity. In y-polarization, the effect is reversed, where only the horizontal nanowires exhibit strong SERS intensity (Fig. 2Ai–iii).
In addition to graphical patterns, we further extend our capability by encrypting molecular information within alphabets. Fig. 2B shows a structure superimposed with two alphabets “A”s, with the upright “A” consisting of horizontal nanowires only, and the inverted “A” is written using vertical nanowires only. Using x-polarized SERS imaging, the vertically lined inverted “A” can be clearly read-out, and the upright “A” is invisible. Conversely, when polarized along the y-axis, only the molecular imaging of upright “A” is apparent. The inverted “A”, despite having the same molecular monolayer on its surface, remains “invisible” owing to the selective plasmonic coupling of the Ag nanowire with the polarization angle of incident light. Similarly, an alphabet “A” fabricated using 50% horizontal nanowires and 50% vertical nanowires is shown in Fig. 2C. Despite having the physical features of a complete alphabet “A”, only the polarized SERS imaging is able to reveal the molecular coding embedded underneath. In this case, the covert molecular information is that only half of the “A” is SERS visible under x- or y-polarization.
Based on the above mentioned design, it is possible to have more sophisticated encryption of molecular information in the plasmonic structure. A plasmonic structure that consists of overlaid alphabets “CBC” of vertical nanowires and alphabets “NTU” of horizontal nanowires is constructed (Fig. 2D). “CBC” and “NTU” are the acronyms of the division of Chemistry and Biological Chemistry, and Nanyang Technological University, respectively. From the SEM imaging, the alphabetical information embedded within the structure cannot be distinguished directly. Only via incident polarized SERS imaging, the alphabetical “CBC” and “NTU” can be clearly distinguished by x- and y-polarizations, respectively.
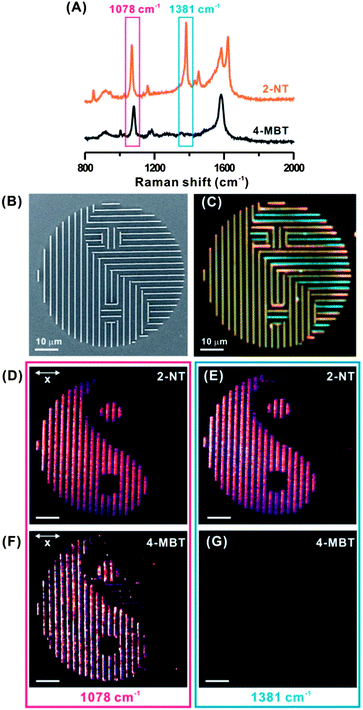
The sharp fingerprint vibration spectrum of SERS can further strengthen the security level of our plasmonic security labels owing to its much narrower spectral resolution than the broadband plasmonic and fluorescent color. Here, we demonstrate that SERS vibrational fingerprint of very narrow Raman shift width (< 2 nm) can be used as an advanced authentication feature for our plasmonic security labels. We prepare two “Taiji” structures (Fig. 3B) that are independently functionalized with 2-naphthalenethiol (2-NT) and 4-MBT molecules, respectively. For 2-NT, the peak at 1068 cm−1 can be assigned to the symmetric C–H bending vibration and that at 1381 cm−1 corresponds to the ring stretching vibration (Fig. 3A).34 The characteristic ring stretching vibrations of 2-NT are observed at 1580 and 1621 cm−1. Both 2-NT and 4-MBT exhibit overlapping Raman spectra at 1040–1120 cm−1 and 1400–1600 cm−1, except an exclusive strong vibration at 1381 cm−1 can only be found in 2-NT molecules.
When an integral 1078 cm−1 Raman shift window is used for SERS imaging, both patterns reveal the intended “Taiji” when polarized at the x-axis owing to the overlapping molecular spectra (Fig. 3D and F). However, when the specific fingerprint of 2-NT at the 1381 cm−1 spectral window is used for SERS imaging, only the 2-NT-functionalized structure exhibits a SERS-active “Taiji” structure (Fig. 3E), whereas the 4-MBT structure is completely invisible under SERS imaging (Fig. 3G). Note that the Raman shift windows for both 1078 cm−1 and 1381 cm−1 are 60 cm−1, i.e. <2 nm in resolution.35 Our demonstration in Fig. 3 emphasizes the SERS fingerprint as a unique identification. Although both molecules have overlapping SERS spectra at 1040–1120 cm−1 and 1400–1600 cm−1, only the ring stretching vibration of 2-NT at 1381 cm−1 gives rise to unique fingerprint for authentication. For security labels encoded with a specific covert molecule, a narrow and exclusive fingerprint spectral window will be able to reveal the difference between an authentic and a fake security label. In comparison, the darkfield optical images of “Taiji” structures functionalized with 2-NT and 4-MBT (Fig. 3C) are identical and unable to decode the molecular information embedded within the structures. This highlights that the spectral specificity of <2 nm in our plasmonic SERS security labels is much more precise in authentication than the commonly used broadband fluorescence and plasmonic colorimetry. Considering there is a vast library of probe molecules that can be implemented into our plasmonic SERS security labels, each with its characteristic unique spectral fingerprint, our plasmonic SERS security labels will be very powerful and extremely difficult to forge.
Conclusions
In summary, we report a proof-of-concept novel plasmonic SERS security label using Ag nanowire structures. Owing to the enhanced directional plasmonic response of the Ag nanowires, selective SERS imaging has been demonstrated spatially and spectroscopically by tuning the polarization of incident light. The SERS imaging specificity can be further enhanced using the extremely narrow spectral fingerprint vibration (<2 nm) of probe molecules, which is more specific than the broadband fluorescence and color-based system. Hence, the molecular information is encrypted within the nanostructure and cannot be revealed directly. Only via polarized SERS imaging in both x- and y-polarizations, the full encrypted molecular image(s) can be authenticated. Such plasmonic SERS security labels can potentially be integrated with the existing fluorescence and/or color-based security systems to form a “layered security” solution. The design features of the plasmonic nanostructures are very flexible yet sophisticated. The highly selective and specific molecular information is read-out in the sub-micrometer or nanometer range. Customer-specific plasmonic SERS security labels can be mixed and matched with different geometrical structures and probe molecules, making such security labels extremely difficult to forge. The authentication of the plasmonic SERS security labels requires highly skilled analytical expertise and access to slit-confocal Raman imaging techniques, which can be a costly investment for counterfeiters. The plasmonic SERS security label is a proof-of-concept demonstration of the potential of plasmonic nanostructures and selective molecular SERS imaging as the next generation advanced security solutions.Experimental section
Materials
IP-L 780 photoresist (Nanoscribe Inc, Germany) that contains pentaerythritol triacrylate (>95%) and 7-(diethylamino)-3-(2-thienylcarbonyl)-2H-1-benzopyran-2-one (<5%) was used as a negative photoresist for two-photon lithography. 4-Methylbenzenethiol (4-MBT, 98%), 2-naphthalenethiol (2-NT, 99%), propylene glycol, monomethyl ether acetate, isopropyl alcohol, and ethanol were purchased from Sigma-Aldrich. All chemicals were used without further purification, unless otherwise stated. Milli-Q water (>18.0 MΩ cm) was purified with a Sartorius Arium® 611 UV ultrapure water system.Fabrication of well-defined plasmonic structures
The fabrication method consists of two parts – the fabrication of a polymeric template, and the deposition of the Ag film. To begin, polymeric nano- and/or microstructures were fabricated using a direct laser writing system (Nanoscribe Inc., Germany). In brief, a droplet of IP-L 780 monomer drop-casted on a glass substrate was polymerized by a computer-assisted femto-second pulsed fiber laser with a center wavelength of 780 nm to form a polymer structure pre-defined by a graphic program. The direct laser writing was performed using an inverted optical microscope with an oil immersion lens (100×, NA 1.4), and a computer-controlled piezoelectric stage. The average laser power was around 12 mW. A writing speed of 30 μm s−1 was used. After writing, unexposed IP-L 780 was removed using propylene glycol and monomethyl ether acetate for 30 min, and then washed with isopropyl alcohol for another 30 min. The polymeric structures were subsequently thermal evaporated with 2 nm Cr and 150 nm Ag using thermal evaporation. Each nanowire was separated by at least 2 μm to eliminate plasmonic coupling.Simulation
Free standing polymer bases on a glass substrate were thermally evaporated with ∼150 nm Ag, resulting in a nanowire like structure and also an aperture beneath. Since the distance between the nanowires, P, and the nanowire length, ly, were large, compared to the illumination wavelength, the simulation of the electromagnetic fields was performed on an equivalent structure that is infinitely extended in the direction parallel to the nanowire axis and periodically extended in the direction perpendicular to it. Although the polymer bases were nearly rectangular in shape, the evaporation process resulted in the deposited silver layer on top of the polymer to assume a rounded shape as seen in the AFM and SEM measurements (Fig. S1C–E†). A rounded shape was assumed for the top silver layer to match closely the experimentally observed morphology (Fig. S1D and E†). A plane wave of free space wavelength 532 nm was incident perpendicularly along the z-axis on the structure. The incident electric field E orientation was changed from being parallel to the nanowire x-polarization (θ = 0°, ↔) to being perpendicular to the long axis y-polarization (θ = 90°, ↕).The structure was simulated with the frequency domain solver of computer simulation technology (CST) microwave studio. Unit cell boundary conditions were assumed along the x- and y-directions and Floquet ports were used along the z direction. The periodicity along x was 2000 nm, and the polymer layer was assumed to be 600 nm tall and 500 nm wide. The glass substrate and the polymer were both assumed to have a refractive index of n = 1.45 and fully dispersive permittivity data were used for Ag.36
High speed slit-scanning confocal Raman spectroscopy measurements
The plasmonic structures were incubated in either 10 mM 4-MBT or 10 mM 2-NT in ethanol solution overnight. After that, the samples were removed and rinsed with a copious amount of ethanol, and dried in nitrogen gas. Owing to a strong Ag–S coordination bond, 4-MBT/2-NT molecules form a SAM on the Ag nanostructures. SERS spectra and SERS imaging were obtained with the sample mounted on a Ramantouch microspectrometer (Nanophoton Inc, Osaka, Japan). A linearly polarized 532 nm laser was used as an excitation laser, with an excitation power of 0.09 mW on the sample plane. The excitation laser light was focused into a line on the sample through a cylindrical lens and an air objective lens (LU Plan Fluor 100×, NA 0.9). The back-scattered SERS signal from the line-illuminated site was collected with the same objective lens, and a one-dimensional SERS image (1D space and SERS spectra) was obtained with a two-dimensional image sensor (pixels 400 BR, −70 °C, 1340 × 400 pixels) at once. The exposure time for each line was 3 s for SERS imaging. A half wave plate and a polarizer were used to change the polarization direction.At a single acquisition, line-shaped illumination is shone on the sample, where 400 SERS scattering points are then collected simultaneously in the x-direction. To complete the SERS imaging, the laser confocal is shifted in the y-direction (resolution: 300 nm) by scanning mirror, where another line scan is performed. The line illumination drastically reduces the acquisition time for x–y axis SERS imaging to less than half an hour for a 6400 μm2 area, as compared to the few hours required when using a conventional Raman system. This makes the use of such an ultrafast Raman system sufficiently efficient as an advanced analytical tool, while offering much richer (molecular) information than simple fluorescence and/or colorimetry.33
Characterization
Scanning electron microscopy (SEM) was performed using a JEOL-JSM-7600F with an accelerated voltage of 5 kV. 10 nm Pt was sputtered onto substrates to increase their conductivity for SEM imaging. The morphology and height profiles of the structures were measured using JPK Nanowizard 3 BioScience atomic force microscopy (AFM) (Berlin, Germany) on a Nikon inverted microscope. The system was equipped with Ultra scanner head (max. scan size is 30 × 30 μm2 with a z-range of 6.5 μm) and 2-axes TAO stage with a scan range of 100 × 100 μm2. Silicon cantilevers from Nanosensors (Neuchatel, Switzerland) were used for AC mode (non-contact mode) operation. The typical free amplitude set-point of the cantilever was around 2 V, which is roughly around 40–50 nm. The scan rate is varied from 0.3–1 Hz.Acknowledgements
X.Y.L. and C.Y. thank the support from National Research Foundation, Singapore (NRF-NRFF2012-04) and Nanyang Technological University's start-up grant (M4080758). H.K.L. thanks the A*STAR Graduate Scholarship support from A*STAR, Singapore.Notes and references
- B. Yoon, J. Lee, I. S. Park, S. Jeon and J. M. Kim, J. Mater. Chem. C, 2013, 1, 2388–2403 RSC
.
- J. Rodriguez-Fernandez, A. M. Funston, J. Perez-Juste, R. A. Alvarez-Puebla, L. M. Liz-Marzan and P. Mulvaney, Phys. Chem. Chem. Phys., 2009, 11, 5909–5914 RSC
.
- H. Kim, J. P. Ge, J. Kim, S. Choi, H. Lee, W. Park, Y. Yin and S. Kwon, Nat. Photonics, 2009, 3, 534–540 CrossRef CAS
.
- S. Huang and J. K. Wu, IEEE Trans. Inf. Forensic Secur., 2007, 2, 164–173 CrossRef
.
- Y. T. Lu and S. Chi, Optik, 2003, 114, 161–167 CrossRef
.
- Z. S. Lu, Y. S. Liu, W. Hu, X. W. Lou and C. M. Li, Chem. Commun., 2011, 47, 9609–9611 RSC
.
- Y. L. Liu, K. L. Ai and L. H. Lu, Nanoscale, 2011, 3, 4804–4810 RSC
.
- T. Blumenthal, J. Meruga, P. S. May, J. Kellar, W. Cross, K. Ankireddy, S. Vunnam and Q. N. Luu, Nanotechnology, 2011, 23, 8 Search PubMed
.
- L. He, M. S. Wang, J. P. Ge and Y. D. Yin, Acc. Chem. Res., 2012, 45, 1431–1440 CrossRef CAS PubMed
.
- R. R. Chance, R. H. Baughman, H. Muller and C. J. Eckhardt, J. Chem. Phys., 1977, 67, 3616–3618 CrossRef CAS
.
- H. H. Pham, I. Gourevich, J. K. Oh, J. E. N. Jonkman and E. Kumacheva, Adv. Mater., 2004, 16, 516–520 CrossRef CAS
.
- H. H. Pham, I. Gourevich, J. E. N. Jonkman and E. Kumacheva, J. Mater. Chem., 2007, 17, 523–526 RSC
.
- X. Chen, S. Kang, M. J. Kim, J. Kim, Y. S. Kim, H. Kim, B. Chi, S. J. Kim, J. Y. Lee and J. Yoon, Angew. Chem., Int. Ed., 2010, 49, 1422–1425 CrossRef CAS PubMed
.
- X. L. Chen, L. Li, X. M. Sun, Y. P. Liu, B. Luo, C. C. Wang, Y. P. Bao, H. Xu and H. S. Peng, Angew. Chem., Int. Ed., 2011, 50, 5486–5489 CrossRef CAS PubMed
.
- U. Jonas, K. Shah, S. Norvez and D. H. Charych, J. Am. Chem. Soc., 1999, 121, 4580–4588 CrossRef CAS
.
- R. W. Carpick, D. Y. Sasaki and A. R. Burns, Langmuir, 2000, 16, 1270–1278 CrossRef CAS
.
- J. Yoon, S. K. Chae and J. M. Kim, J. Am. Chem. Soc., 2007, 129, 3038–3039 CrossRef CAS PubMed
.
- B. Yoon, H. Shin, O. Yarimaga, D. Y. Ham, J. Kim, I. S. Park and J. M. Kim, J. Mater. Chem., 2012, 22, 8680–8686 RSC
.
- K. Kumar, H. G. Duan, R. S. Hegde, S. C. W. Koh, J. N. Wei and J. K. W. Yang, Nat. Nanotechnol., 2012, 7, 557–561 CrossRef CAS PubMed
.
- T. Xu, Y. K. Wu, X. G. Luo and L. J. Guo, Nat. Commun., 2010, 1, 5 Search PubMed
.
- M. Moskovits, Phys. Chem. Chem. Phys., 2013, 15, 5301–5311 RSC
.
- J. N. Anker, W. P. Hall, O. Lyandres, N. C. Shah, J. Zhao and R. P. Van Duyne, Nat. Mater., 2008, 7, 442–453 CrossRef CAS PubMed
.
- L. L. Zhao, L. Jensen and G. C. Schatz, J. Am. Chem. Soc., 2006, 128, 2911–2919 CrossRef CAS PubMed
.
- C. Kuemin, L. Nowack, L. Bozano, N. D. Spencer and H. Wolf, Adv. Funct. Mater., 2012, 22, 702–708 CrossRef CAS
.
- A. R. Tao and P. D. Yang, J. Phys. Chem. B, 2005, 109, 15687–15690 CrossRef CAS PubMed
.
- S. J. Lee, J. M. Baik and M. Moskovits, Nano Lett., 2008, 8, 3244–3247 CrossRef CAS PubMed
.
- H. Wei, F. Hao, Y. Z. Huang, W. Z. Wang, P. Nordlander and H. X. Xu, Nano Lett., 2008, 8, 2497–2502 CrossRef CAS PubMed
.
- T. Ellenbogen, K. Seo and K. B. Crozier, Nano Lett., 2012, 12, 1026–1031 CrossRef CAS PubMed
.
- S. Chang, H. Ko, R. Gunawidjaja and V. V. Tsukruk, J. Phys. Chem. C, 2011, 115, 4387–4394 CAS
.
- M. S. Goh, Y. H. Lee, S. Pedireddy, I. Y. Phang, W. W. Tjiu, J. M. R. Tan and X. Y. Ling, Langmuir, 2012, 28, 14441–14449 CrossRef CAS PubMed
.
- M. S. Chen, I. Y. Pang, M. R. Lee, J. K. W. Yang and X. Y. Ling, Langmuir, 2013, 29, 7061–7069 CrossRef CAS PubMed
.
- A. Ulman, Chem. Rev., 1996, 96, 1533–1554 CrossRef CAS PubMed
.
- W. Y. Li, P. H. C. Camargo, X. M. Lu and Y. N. Xia, Nano Lett., 2009, 9, 485–490 CrossRef CAS PubMed
.
- R. A. Alvarez-Puebla, D. S. Dos Santos and R. F. Aroca, Analyst, 2004, 129, 1251–1256 RSC
.
- T. Shegai, B. Brian, V. D. Miljkovic and M. Kall, ACS Nano, 2011, 5, 2036–2041 CrossRef CAS PubMed
.
- P. B. Johnson and R. W. Christy, Phys. Rev. B: Solid State, 1972, 6, 4370–4379 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: AFM and tilted SEM images, and simulation structure of a silver nanowire (Fig. S1), 2D Raman imaging “Taiji” patterns functionalized with 2-NT and 4-MBT molecules (Fig. S2), darkfield optical images of silver “Taiji” structures functionalized with 2-NT and 4-MBT molecules (Fig. S3), and calculation of the SERS enhancement factor. See DOI: 10.1039/c3nr04375d |
| This journal is © The Royal Society of Chemistry 2014 |