Synthesis and internal electric field dependent photoreactivity of Bi3O4Cl single-crystalline nanosheets with high {001} facet exposure percentages†
Jie
Li
ab,
Lizhi
Zhang
*a,
Yujie
Li
b and
Ying
Yu
b
aKey Laboratory of Pesticide & Chemical Biology of Ministry of Education, Institute of Environmental Chemistry, College of Chemistry, Central China Normal University, Wuhan 430079, P.R. China. E-mail: zhanglz@mail.ccnu.edu.cn; Fax: +86-27-6786 7535; Tel: +86-27-6786 7535
bInstitute of Nanoscience and Nanotechnology, College of Physical Science and Technology, Central China Normal University, Wuhan 430079, P.R. China
First published on 23rd October 2013
Abstract
We prepared Bi3O4Cl single-crystalline nanosheets with high {001} facet exposure percentages and demonstrated that their photoreactivity strongly depended on the magnitude of the internal electric field (IEF), which was correlated with the {001} facet exposure percentage. More {001} facet exposure could induce the generation of stronger IEF, which favored the photogenerated charge separation and transfer, and thus enhancing the photoreactivity.
Since the first report of anatase TiO2 single-crystals with exposed highly reactive {001} facets in 2008,1 crystal facet engineering of semiconductors has attracted worldwide attention, and has become a pivotal strategy to finely tailor the physicochemical properties and enhance the reactivity of photocatalysts.2 Generally, the conventional understanding of the crystal facet effect on the photoreactivity mainly relies on facet-related surface properties: plenty of atomic steps, dangling bonds, kinks and ledges on reactive facets with high surface energy can act as active sites to facilitate the adsorption of reactant molecules, the surface transfer between photoexcited electrons and reactant molecules, and the desorption of product molecules.3 However, this theory became ambiguous because some recent studies demonstrated that the facet-dependent band-structure and/or the charge transfer orientation played significant roles in determining the photoreactivity, even more important than those played by the surface energy.4 On the basis of a systematic literature study, we think that the previous conflicting phenomena of crystal facet effects on the photoreactivity might have arisen due to the following three reasons. (1) The exposure degree of the targeted facets was still low and the effect of coexisting facets could not be ruled out; (2) most previous studies mainly focused on surface properties, while the corresponding bulk properties on the photoreactivity were usually neglected; (3) many conclusions were drawn by comparing the photoreactivity of the samples with different facet exposure percentages, but how an individual facet would affect the photoreactivity was still elusive. Therefore, it is of great importance but a challenge to clarify the origin of the facet effect of semiconductors on their photoreactivity.
In this communication, we first demonstrate that the photoreactivity of Bi3O4Cl single-crystalline nanosheets with high {001} facet exposure percentages strongly depends on the magnitude of the internal electric field (IEF), which is correlated with the percentage of {001} facets. More exposure of {001} facets can induce the generation of a stronger internal electric field, which favors the photogenerated charge separation and transfer, and thus enhances the photoreactivity.
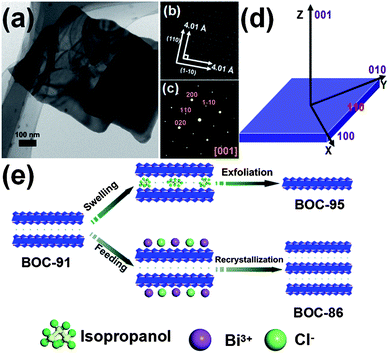
Bi3O4Cl nanosheets were hydrothermally synthesized with bismuth nitrate pentahydrate and sodium chloride. The detailed synthesis procedure was provided in the ESI.† The as-synthesized product was of a pure monoclinic Bi3O4Cl crystal phase (space group I2/a, JCPDS no. 86-2221) with layered structure according to the X-ray diffraction pattern and X-ray photoelectron spectroscopy analysis (Fig. S1 and S2, ESI†). SEM images revealed that the as-synthesized product consisted of large numbers of sheets with 60 nm thickness and micrometer-sized width and length (Fig. S3, ESI†). The individual nanosheet was transparent under the electron-beam illumination during transmission electron microscopy (TEM) measurements (Fig. 1a), confirming that the as-synthesized nanosheet was very thin. High-resolution TEM (HRTEM) analysis revealed the high crystalline nature of the nanosheets with two sets of lattices perpendicular to each other with an equal fringe spacing of 4.01 Å, corresponding to (110) and (1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) planes of the monoclinic Bi3O4Cl crystal (Fig. 1b). The corresponding selected-area electron diffraction (SAED) pattern could be indexed as the diffraction spots of the [001] zone of a Bi3O4Cl single crystal (Fig. 1c). On the basis of the above results and the symmetry of Bi3O4Cl, we conclude that the as-synthesized Bi3O4Cl nanosheets are exposed with {001} facets on both the top and the bottom surfaces and four {110} facets on the lateral surfaces (Fig. 1d). A detailed statistical analysis revealed that the estimated percentage of {001} facets of the as-synthesized Bi3O4Cl nanosheet was as high as 91% (Fig. S3 and S4, ESI†), which we called BOC-91. Subsequently, we employed a liquid phase exfoliation and hydrothermal recrystallization method to finely tune the {001} facet exposure percentage of Bi3O4Cl nanosheets from 91 to 95 and 86% (Fig. 1e), respectively. The two new samples were correspondingly called BOC-95 and BOC-86.
0) planes of the monoclinic Bi3O4Cl crystal (Fig. 1b). The corresponding selected-area electron diffraction (SAED) pattern could be indexed as the diffraction spots of the [001] zone of a Bi3O4Cl single crystal (Fig. 1c). On the basis of the above results and the symmetry of Bi3O4Cl, we conclude that the as-synthesized Bi3O4Cl nanosheets are exposed with {001} facets on both the top and the bottom surfaces and four {110} facets on the lateral surfaces (Fig. 1d). A detailed statistical analysis revealed that the estimated percentage of {001} facets of the as-synthesized Bi3O4Cl nanosheet was as high as 91% (Fig. S3 and S4, ESI†), which we called BOC-91. Subsequently, we employed a liquid phase exfoliation and hydrothermal recrystallization method to finely tune the {001} facet exposure percentage of Bi3O4Cl nanosheets from 91 to 95 and 86% (Fig. 1e), respectively. The two new samples were correspondingly called BOC-95 and BOC-86.
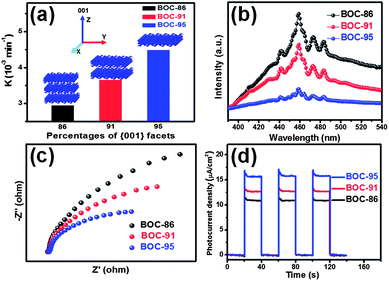
The success in controlled synthesis of Bi3O4Cl nanosheets with high and different {001} facet exposure percentages allowed us to investigate the origin of the facet effect on the photoreactivity by minimizing the influence of coexisting facets as low as possible. The photocatalytic performances of the three samples were evaluated by using salicylic acid (SA) as the probe molecule under visible light irradiation (λ > 420 nm). As expected, the photoreactivity of Bi3O4Cl nanosheets increased monotonously with the increased percentages of exposed {001} facets (Fig. 2a), indicating that the exposed {001} facets contribute positively to the photocatalytic degradation of SA over Bi3O4Cl nanosheets under visible light. In general, the surface properties of the semiconductor are crucial for their photoreactivity because a high density of surface unsaturated-coordinated atoms would cause a high surface energy of the crystal facet and a crystal with a higher percentage of the reactive facet is usually more reactive in heterogeneous reactions.2a,3c,4a Therefore, we normalized photocatalytic degradation rate constants of BOC-86, BOC-91 and BOC-95 simultaneously with their {001} facet percentages and surface areas to check if the surface property of Bi3O4Cl nanosheets is the major factor to determine their photoreactivity, and surprisingly found that the normalized rate constants still increased monotonously with increasing {001} facet percentages (Table S1 and Fig. S10, ESI†). This result suggests that the photoreactivity of Bi3O4Cl nanosheets with high {001} facet exposure percentages is not mainly dominated by the surface properties of {001} facets in this study, but possibly by some other {001} facet-related bulk properties.
It is well known that the photocatalytic process with semiconductor materials involves light photoexcitation, the subsequent separation and transfer of photogenerated charge carriers, and the final redox reactions between the charge carriers and adsorbate on the semiconductor surface, and thus the charge dynamics property plays an important role in determining the ultimate photoreactivity.5 We therefore investigated the separation and transfer of photogenerated charge carriers in the Bi3O4Cl nanosheets by photoluminescence (PL) emission and electrochemical impedance spectroscopy (EIS) as well as transient photocurrent responses.6 The PL intensity and the Nyquist plot diameter were found to be inversely proportional to the percentages of exposed {001} facets (Fig. 2b and c), indicating the separation and transfer efficiency of photogenerated electron–hole pairs highly depends on the percentages of exposed {001} facets and more {001} facet exposure favors the separation and transfer efficiency of photogenerated electron–hole pairs, which was further confirmed by transient photocurrent responses (Fig. 2d). It is highly possible that the charge dynamic property is the primary factor to govern the {001} facet-dependent photoreactivity of Bi3O4Cl nanosheets in this study.
As mentioned previously, high {001} facet exposure percentages of Bi3O4Cl nanosheets might minimize the influence of coexisting facets as little as possible. If the contribution of coexisting facets on the separation and transfer efficiency of photogenerated electron–hole pairs was negligible, how could the exposed {001} facets of Bi3O4Cl nanosheets affect the separation and transfer efficiency of photogenerated electron–hole pairs and thus influence the photoreactivity without considerably changing the exposure percentages (86% → 91% → 95%)?
To answer this question, we need to recall our recent discovery: BiOCl single-crystalline nanosheets with exposed {001} facets were more capable of efficient photoinduced charge separation and transfer, and thus exhibited higher activity for direct semiconductor photoexcitation pollutant degradation under UV light because of the cooperative effect between the surface atomic structure and suitable internal electric fields.7 Similar to BiOCl, Bi3O4Cl also possesses a layered crystal structure with the [Bi3O4] and [Cl] slices stacked together by the nonbonding interaction through the Cl atoms along the c-axis to form the unique layered structure (Fig. 3a), indicative of the existence of the self-induced electric field in Bi3O4Cl.8 As aforementioned, the photoreactivity of Bi3O4Cl with high {001} facet exposure percentages is not dominated by the surface atomic structure of {001} facets, we therefore employed density functional theory (DFT) to calculate charge density in the {110} and {1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0} lateral surfaces of Bi3O4Cl exposed with {001} facets on the top and the bottom surfaces to check how {001} facet exposure could influence the IEF magnitude of Bi3O4Cl. From the charge density contour plots viewed from either {110} or {1
0} lateral surfaces of Bi3O4Cl exposed with {001} facets on the top and the bottom surfaces to check how {001} facet exposure could influence the IEF magnitude of Bi3O4Cl. From the charge density contour plots viewed from either {110} or {1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0} facets of Bi3O4Cl (Fig. 3b), it can be seen clearly that the charge density surrounding Bi atoms is higher than that of Cl atoms. The non-uniform charge distribution between [Bi3O4] and [Cl] slices in the nanosheets would polarize the related atoms and orbitals to form permanent static electric fields along the [001] orientation perpendicular to the [Cl] and [Bi3O4] layers, indicating that the emergence of IEF is strongly related to the preferential {001} facet exposure of Bi3O4Cl. It was well established that the IEF in materials could afford fast separation, reduced diffusion resistance, and improved accessibility of the photogenerated charge carriers to reactants, which consequently promote the photocatalytic activity.9 Hence, we propose that the IEF magnitude of Bi3O4Cl nanosheets might be the right important factor to affect {001} facet-dependent charge dynamic properties and thus influence their photoreactivity. Then, we calculated the variation of the IEF magnitude with the {001} facet exposure percentages of Bi3O4Cl nanosheets by using the model (eqn (1)) developed by Kanata et al.10
0} facets of Bi3O4Cl (Fig. 3b), it can be seen clearly that the charge density surrounding Bi atoms is higher than that of Cl atoms. The non-uniform charge distribution between [Bi3O4] and [Cl] slices in the nanosheets would polarize the related atoms and orbitals to form permanent static electric fields along the [001] orientation perpendicular to the [Cl] and [Bi3O4] layers, indicating that the emergence of IEF is strongly related to the preferential {001} facet exposure of Bi3O4Cl. It was well established that the IEF in materials could afford fast separation, reduced diffusion resistance, and improved accessibility of the photogenerated charge carriers to reactants, which consequently promote the photocatalytic activity.9 Hence, we propose that the IEF magnitude of Bi3O4Cl nanosheets might be the right important factor to affect {001} facet-dependent charge dynamic properties and thus influence their photoreactivity. Then, we calculated the variation of the IEF magnitude with the {001} facet exposure percentages of Bi3O4Cl nanosheets by using the model (eqn (1)) developed by Kanata et al.10
| Fs = (−2Vsρ/εε0)1/2 | (1) |
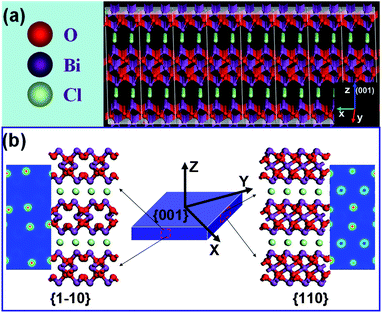 | ||
Fig. 3 (a) Crystal structure of Bi3O4Cl nanosheets; (b) charge density contour plots viewed from {110} and {![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 10} facets of Bi3O4Cl nanosheets. 10} facets of Bi3O4Cl nanosheets. | ||
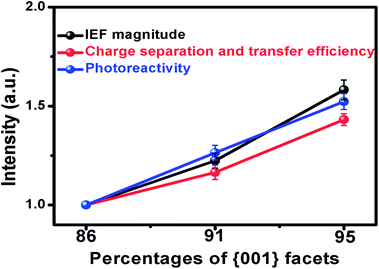
To further clarify the effect of IEF on the photoreactivity, the IEF magnitude, the efficiency of charge separation and transfer, and the photoreactivity versus the {001} facet percentages of Bi3O4Cl nanosheets are qualitatively correlated and shown in Fig. 4. Herein the charge separation and transfer efficiency was expressed by the photocurrent intensity qualitatively because the transient photocurrent response could reflect the amount of surviving electrons during the competitive processes of separation and recombination of photogenerated electrons and holes.6 We set all the IEF magnitude, the efficiency of charge separation and transfer and the photoreactivity values of BOC-86 as “1”, the normalized IEF magnitude, the charge separation and transfer efficiency, and the photoreactivity of BOC-91 were calculated to be 1.22, 1.17 and 1.26, respectively, and the corresponding values of BOC-95 were 1.58, 1.43 and 1.52, respectively. Obviously, with the increase of the {001} facet exposure percentage of Bi3O4Cl nanosheets, the IEF magnitude, the efficiency of charge separation and transfer, and the photoreactivity increased. The enhancement times of the IEF magnitude, the efficiency of charge separation, and transfer and the photoreactivity for either BOC-91 or BOC-95 are almost identical in comparison with those of BOC-86, strongly indicating that the {001} facet exposure percentage change would induce the IEF variation, then determine the efficiency of charge carrier separation and transfer, and finally affect the photoreactivity. It can be seen that the slight increase (9%) of the {001} facet exposure percentage from 86% to 95% would strengthen the IEF magnitude more remarkably by 58%, thus speed up the efficiency of charge separation and transfer by 43%, and finally improve the photoreactivity of Bi3O4Cl nanosheets by 52%. Therefore, we conclude that it is not the surface atomic structure of {001} facets, but the IEF magnitude change induced by the {001} facet exposure is the main factor to determine the photoreactivity of Bi3O4Cl nanosheets with high {001} facet exposure percentages.
In light of the above results, we would like to explain the {001} facet dependent photoreactivity of Bi3O4Cl nanosheets with high {001} facet exposure percentages as follows. Upon visible-light excitation of Bi3O4Cl nanosheets by adsorbing photons with energy equal to or greater than the band gap of the semiconductor, electrons are excited from the valence band to the conduction band, leaving holes in the valence band. The polarization-induced IEF along the [001] crystal orientation of nanosheets would drive the photoinduced charge separation and transfer from the bulk to the surface for the subsequent photocatalytic reactions on the surface. Higher exposure of {001} facets would significantly increase the IEF magnitude and thus further improve the separation and transfer efficiency of photogenerated electron–hole pairs to enhance the photoreactivity of Bi3O4Cl nanosheets with high {001} facet exposure percentages.
Besides the IEF magnitude, the thickness of the nanosheets might also influence the charge separation rate and the photocatalytic activity of the Bi3O4Cl nanosheets, but we think the influence might be tiny. This is because only these photogenerated charge carriers near the surface of photocatalysts could reach the surface to initiate the subsequent redox reactions.13 Meanwhile, the transfer orientation of photogenerated electrons and holes highly depended on the facet exposure. However the transfer of photogenerated electrons and holes might not be reverse. For instance, the transfer of photogenerated electrons in BiVO4 is inclined to the {010} facets, but the corresponding holes prefer to migrate to the {110} facets, not to the {0![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0} facets.6a This means that the photogenerated electrons and holes need not traverse the full distance of the Bi3O4Cl nanosheets along the [001] direction. We therefore conclude that the distinct migration paths caused by the different thicknesses of the Bi3O4Cl nanosheets would not strongly affect the charge separation rate and the photocatalytic activity.
0} facets.6a This means that the photogenerated electrons and holes need not traverse the full distance of the Bi3O4Cl nanosheets along the [001] direction. We therefore conclude that the distinct migration paths caused by the different thicknesses of the Bi3O4Cl nanosheets would not strongly affect the charge separation rate and the photocatalytic activity.
Conclusions
In conclusion, we have prepared Bi3O4Cl single-crystalline nanosheets with high {001} facet exposure percentages and demonstrated that their photoreactivity strongly depends on the IEF magnitude, other than the surface atomic structure. The IEF magnitude is found to be correlated with the {001} facet exposure percentage. More {001} facet exposure can induce the generation of stronger IEF, which favors the photogenerated charge separation and transfer and thus enhances the photoreactivity. This interesting finding not only provides new insight into the origin of the crystal facet effect on the photoreactivity of semiconductors, but also highlights the importance of the IEF magnitude in the photoreactivity of layered materials. To our knowledge, this is the first report on tuning the reactivity of photocatalysts with the IEF magnitude. We believe that this strategy maybe applicable to other layered photocatalysts also.Acknowledgements
This work was supported by National Basic Research Program of China (973 Program) (Grant 2013CB632402), and National Science Foundation of China (Grants 21073069, 91023010, 21177048, and 21377044).Notes and references
- H. G. Yang, C. H. Sun, S. Z. Qiao, J. Zou, G. Liu, S. C. Smith, H. M. Cheng and G. Q. Lu, Nature, 2008, 453, 638 CrossRef CAS PubMed.
- (a) H. G. Yang, G. Liu, S. Z. Qiao, C. H. Sun, Y. G. Jin, S. C. Smith, J. Zou, H. M. Cheng and G. Q. Lu, J. Am. Chem. Soc., 2009, 131, 4078 CrossRef CAS PubMed; (b) G. Liu, J. C. Yu, G. Q. Lu and H. M. Cheng, Chem. Commun., 2011, 47, 6763 RSC.
- (a) H. B. Jiang, Q. Cuan, C. Z. Wen, J. Xing, D. Wu, X. Q. Gong, C. Z. Li and H. G. Yang, Angew. Chem., Int. Ed., 2011, 50, 3764 CrossRef CAS PubMed; (b) Y. P. Bi, S. X. Ouyang, N. Umezawa, J. Y. Cao and J. H. Ye, J. Am. Chem. Soc., 2011, 133, 6490 CrossRef CAS PubMed; (c) D. E. Wang, H. F. Jiang, X. Zong, Q. Xu, Y. Ma, G. L. Li and C. Li, Chem.–Eur. J., 2011, 17, 1275 CrossRef CAS PubMed.
- (a) J. Pan, G. Liu, G. Q. Lu and H. M. Cheng, Angew. Chem., Int. Ed., 2011, 50, 2133 CrossRef CAS PubMed; (b) Z. K. Zheng, B. B. Huang, J. B. Lu, X. Y. Qin, X. Y. Zhang and Y. Dai, Chem.–Eur. J., 2011, 17, 15032 CrossRef CAS PubMed; (c) C. Liu, X. G. Han, S. F. Xie, Q. Kuang, X. Wang, M. S. Jin, Z. X. Xie and L. S. Zheng, Chem.–Asian. J., 2013, 8, 282 CrossRef CAS PubMed; (d) Q. J. Xiang, K. L. Lv and J. G. Yu, Appl. Catal., B, 2010, 96, 557 CrossRef CAS PubMed; (e) T. Tachikawa, S. Yamashita and T. Majima, J. Am. Chem. Soc., 2011, 133, 7197 CrossRef CAS PubMed.
- M. R. Hoffmann, S. T. Martin, W. D. Choi and W. Bahnemann, Chem. Rev., 1995, 95, 69 CrossRef CAS.
- (a) R. G. Li, F. X. Zhang, D. E. Wang, J. X. Yang, M. R. Li, J. Zhu, X. Zhou, H. X. Han and C. Li, Nat. Commun., 2013, 4, 1432 CrossRef PubMed; (b) J. S. Zhang, G. G. Zhang, X. F. Chen, S. Lin, L. Mohlmann, G. Dotega, G. Lipner, M. Antonietti, S. Blechert and X. C. Wang, Angew. Chem., Int. Ed., 2012, 51, 3183 CrossRef CAS PubMed; (c) M. Kong, Y. Z. Li, X. Chen, T. T. Tian, P. F. Fang, F. Zheng and X. J. Zhao, J. Am. Chem. Soc., 2011, 133, 16414 CrossRef CAS PubMed.
- J. Jiang, K. Zhao, X. Y. Xiao and L. Z. Zhang, J. Am. Chem. Soc., 2012, 134, 4473 CrossRef CAS PubMed.
- (a) X. P. Lin, T. Huang, F. Q. Huang, W. D. Wang and J. L. Shi, J. Phys. Chem. B, 2006, 110, 24629 CrossRef CAS PubMed; (b) A. K. Chakraborty and M. A. Kebede, React. Kinet., Mech. Catal., 2012, 106, 83 CrossRef CAS.
- (a) K. L. Zhang, C. M. Liu, F. Q. Huang, C. Zheng and W. D. Wang, Appl. Catal., B, 2006, 68, 125 CrossRef CAS PubMed; (b) X. P. Lin, T. Huang, F. Q. Huang, W. D. Wang and J. L. Shi, J. Mater. Chem., 2007, 17, 2145 RSC; (c) L. Q. Ye, L. H. Tian, T. Y. Peng and L. Zan, J. Mater. Chem., 2011, 21, 12479 RSC.
- (a) T. Kanata, M. Matsunaga, H. Takakura, Y. Hamakawa and T. Nishino, Proc. SPIE, 1990, 56, 1286 Search PubMed; (b) P. Lefebvre, J. Allegre, B. Gil and H. Mathieu, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 15363 CrossRef CAS; (c) G. Morello, F. D. Sala, L. Carbone, L. Manna, G. Maruccio, R. Cingolani and M. D. Giorgi, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 78, 195313 CrossRef.
- S. V. Yanina and K. M. Rosso, Science, 2008, 320, 218 CrossRef CAS PubMed.
- (a) A. G. S. Prado, L. B. Bolzon, C. P. Pedroso, A. O. Moura and L. L. Costa, Appl. Catal., B, 2008, 82, 219 CrossRef CAS PubMed; (b) A. G. S. Prado and L. L. Costa, J. Hazard. Mater., 2003, 169, 297 CrossRef PubMed.
- W. B. Hou and S. B. Cronin, Adv. Funct. Mater., 2012, 23, 1612 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3nr05246j |
| This journal is © The Royal Society of Chemistry 2014 |