Reversibly crosslinked thermo- and redox-responsive nanogels for controlled drug release†
Ji
Liu
ac,
Christophe
Detrembleur
*a,
Marie
Hurtgen
a,
Antoine
Debuigne
a,
Marie-Claire
De Pauw-Gillet
b,
Stéphane
Mornet
c,
Etienne
Duguet
c and
Christine
Jérôme
*a
aCenter for Education and Research on Macromolecules (CERM), University of Liege, B6 Sart Tilman, B-4000 Liège, Belgium. E-mail: c.jerome@ulg.ac.be; christophe.detrembleur@ulg.ac.be; Fax: +32 4-36663497; Tel: +32 4-3663565
bLaboratory of Mammalian Cell Culture (GIGA-R), University of Liège, B6 Sart Tilman, B-4000 Liege, Belgium
cCNRS, Univ. Bordeaux, ICMCB, UPR 9048, F-33600 Pessac, France
First published on 31st July 2013
Abstract
Reversibly crosslinked poly(vinyl alcohol)-b-poly(N-vinylcaprolactam) PVOH-b-PNVCL nanogels were prepared by using a redox-responsive crosslinking agent, 3,3′-dithiodipropionic acid (DPA), to crosslink the PVOH corona, above the lower critical solution temperature (LCST) of the PNVCL block. The stability of the as-prepared nanogels against heating and diluting with water was studied by dynamic light scattering (DLS) to follow the evolution of the hydrodynamic diameter and size distribution. Stability under reductive conditions was also studied by DLS and transmission electron microscopy (TEM) after exposure to dithiothreitol (DTT) buffer solutions at different pH. The reversibility of the crosslinking was evaluated by treating the de-crosslinked nanogels with hydrogen peroxide (H2O2) above the LCST. As a hydrophobic drug model, Nile red (NR) was loaded into the nanogels, and triggered release behaviours were studied after exposure to the same DTT buffer solutions. Moreover, two PVOH-b-PNVCL copolymers with different compositions and LCST were used to evaluate the effect of the LCST on the release behaviours of the nanogels. The cytotoxicity of the nanogels against a mouse fibroblast-like L929 cell line was assessed via the MTS assay, and preliminary studies on cellular uptake of the nanogels within human melanoma MEL-5 cells were also carried out by fluorescence microscopy and fluorescence-activated cell sorting.
Introduction
Recently, considerable efforts have been devoted to the development of novel drug delivery systems (DDS), which are expected to overcome the key therapeutical issues emerging in current clinical practices, such as poor intracellular delivery, lack of control over the release behaviours, significant side effects, etc.1,2 In the past few years, nano-sized polymer micelles have been suggested as promising vehicles for DDS due to their outstanding advantages in drug loading capacity and cellular uptake efficiency, as well as their nano-scaled dimensions which can accomplish an enhanced permeability and retention (EPR) effect at tumour sites.2–5 Generally, self-assembled micelles could be obtained from unimers above the critical micelle concentration (CMC), and when stimuli-responsive materials are used, release of the drug payloads could be triggered by external stimuli, such as light or magnetic field application, reductive reagent activation, variation in pH, temperature or ionic strength, etc.4–7However, the main drawback of these physically assembled micelles should be the incapacity to avoid premature release during the in vivo delivery routine.2,8 To circumvent this limitation, crosslinking strategies have been attempted. The covalently stabilized micelles, particularly shell crosslinked micelles, are attracting increasing attention.9 Various chemical reactions were attempted to crosslink the shell of those physically assembled micelles, such as carbodiimide coupling of carboxylic acid groups with diamines,10 coupling of hydroxyl groups with divinyl sulfone,11 and UV-induced coupling of cinnamoyl groups,12 to cite only a few.
These traditional covalently crosslinked structures aimed for DDS application are always limited by a number of drawbacks, including poor solubility, low reactivity, toxicity and lack of control over the release behaviours due to the irreversible crosslinking structures, etc.4,6,13 An alternative approach involves the reversible crosslinking with disulfide bonds, which are redox-responsive and can be easily cleaved when exposed to some reductive reagents, such as dithiothreitol (DTT) or glutathione. It was reported that the concentration of glutathione in the cytosol (10 mM) is ca. 2–3 orders higher than that in the extracellular fluids (ca. 2–20 μM).14,15 Thus, this specific feature might favour the cleavage of disulfide bonds responding to the intracellular glutathione level, and makes this kind of disulfide-crosslinked micelles more appealing for DDS. Some reversibly crosslinked polymer micelles based on disulfide bonds have been reported for DDS and also triggered drug release.13,16–24
As far as we know, poly(N-vinylcaprolactam) (PNVCL) is one of the most recently studied thermo-responsive macromolecules with a lower critical solution temperature (LCST) of ca. 32 °C. And a change in temperature can cause the phase transition between the hydrophobic and hydrophilic states.5 When involved in a block copolymer with another hydrophilic block, it results in the formation or dissociation of core/corona-structured micelles, driven by the change in temperatures.3–5 On the other hand, poly(vinyl alcohol) (PVOH)-based micelles or hydrogels have also been extensively investigated as good candidates for DDS application, due to the hydrophilic essence and also excellent biocompatibility of the PVOH block.25–28 Recently, we have reported the synthesis of well-defined PVOH-b-PNVCL copolymers with different compositions via the cobalt-mediated radical polymerization (CMRP) strategy,27,29,30 and the dependence of the LCST on the chemical composition of the copolymers was also properly investigated.30 Thermo-induced micelles from poly(N-isopropylacrylamide) (PNIPAm) or PNVCL-based amphiphilic copolymers, with a hydrophobic PNIPAm or PNVCL core and another hydrophilic block as the corona above the LCST, have been widely reported.18,31–34 In the case of PVOH-b-PNVCL copolymers, thermo-induced formation of micelles with a PVOH corona was reported in our recent report.30 However, due to their inferior stability against heating, inter-particle agglomeration occurred upon heating above the LCST.
In this work, we report the synthesis and loading–release properties of the reversibly crosslinked thermo- and redox-responsive nanogels based on PVOH-b-PNVCL copolymers (Scheme 1). 3,3′-Dithiodipropionic acid (DPA) was used to crosslink the PVOH corona above the LCST of the PNVCL blocks; then crosslinked nanogels were obtained after cooling down below the volume phase transition temperature (VPTT) of the crosslinked structures. The stability of the crosslinked nanogels against heating and diluting with de-ionized water was evaluated using the dynamic light scattering (DLS) technique. The stability of the crosslinked nanogels against reductive reagents was also studied by DLS after exposure to DTT buffer solution (10 mM) with different pH values, while an oxidizing reagent (H2O2) was used to evaluate the reversibility of the crosslinking. Nile red (NR) was used as a hydrophobic drug model to study the triggered release behaviours under different conditions. The cytotoxicity of the crosslinked nanogels against the mouse fibroblast-like L929 cells was also evaluated via the MTS assay, and preliminary studies on cellular uptake of the crosslinked nanogels within human melanoma MEL-5 cells were finally investigated.
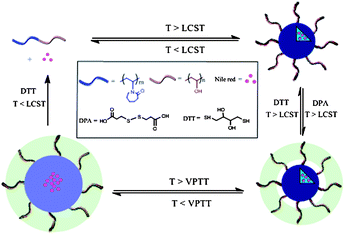 | ||
| Scheme 1 Schematic illustration of the preparation, dissociation of PVOH-b-PNVCL crosslinked nanogels, loading of Nile red and redox-triggered release behaviours (LCST: lower critical solution temperature, VPTT: volume phase transition temperature). | ||
Experimental details
Materials
3,3′-Dithiodipropionic acid (DPA, 99%), 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC, 98%), Nile red (NR, 98%), dithiothreitol (DTT, 99%), 4-dimethylaminopyridine (DMAP, 98%), sodium phosphate dibasic (Na2HPO4, 98.5%), potassium phosphate monobasic (KH2PO4, 98%), sodium acetate trihydrate (NaAc·3H2O, 99.0%), acetic acid (HAc, 99%), sodium chloride (NaCl, 98%), potassium chloride (KCl, 99%), 4,6-diamidino-2-phenylindole (DAPI, 98%) and hydrogen peroxide solution (H2O2, 30 wt%) were purchased from Aldrich. DMEM (1 g L−1glucose), L-glutamine, PBS buffer solution (Ca2+ and Mg2+ free), fetal bovine serum (FBS) and trypsin were obtained from BioWhittaker (Walkersville, MD). 3-(4,5-Dimethylthiazol-2-yl-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) was purchased from Promega (Madison, USA). PBS buffer solution (with Ca2+ and Mg2+), penicillin G and streptomycin were purchased from GIBCO BRL (Gaithersburg, MD).PVOH-b-PNVCL copolymers were obtained by hydrolysis of poly(vinyl acetate)-b-poly(N-vinylcaprolactam) copolymers (PVAc-b-PNVCL), prepared via a CMRP strategy according to a previously reported procedure.30 The physico-chemical features of the PVOH-b-PNVCL copolymers used in this work are summarized in Table 1.
| Sample | PVOH-b-PNVCL | Crosslinked nanogels at 25 °C | NR@crosslinked nanogels at 25 °C | ||||
|---|---|---|---|---|---|---|---|
| Structure | PDI | LCSTa/°C | D h/nm | PDI | D h/nm | PDI | |
| a LCST was detected via the turbidimetry measurement (1 °C min−1, 0.05 wt%), and defined as the temperature where transmittance started to decrease. | |||||||
| AM01 | PVOH180-b-PNVCL110 | 1.06 | 41 | 280 | 0.17 | 300 | 0.22 |
| AM02 | PVOH226-b-PNVCL494 | 1.10 | 37 | 460 | 0.21 | 490 | 0.26 |
Methods
Preparation of PVOH-b-PNVCL crosslinked nanogels
PVOH-b-PNVCL shell crosslinked micelles were first prepared from the aqueous polymer solution above its LCST, and after the crosslinking reaction, the crosslinked (CL) nanogels were obtained upon cooling down below the VPTT of the crosslinked structures. Typically, the PVOH180-b-PNVCL110copolymer was dissolved in 20 mL of de-ionized water to obtain a polymer concentration of 0.05 wt% (i.e. 7.8 × 10−5 mol VOH monomer units) before stirring and heating to 50 °C. Immediately, milky-white colloidal aggregates were detected upon heating, indicating the formation of thermo-induced micelles. After equilibration at 50 °C for 10 min, DPA (8.4 mg, 3.9 × 10−5 mol, molar ratio DPA/VOH = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), EDC (12.0 mg, 7.8 × 10−5 mol) and DMAP (11.4 mg, 9.5 × 10−5 mol) were added. After degassing by bubbling nitrogen for 20 min, the mixture was stirred at 50 °C for another 48 h. Upon cooling down to room temperature, the mixture was dialyzed (cut-off: 3
2), EDC (12.0 mg, 7.8 × 10−5 mol) and DMAP (11.4 mg, 9.5 × 10−5 mol) were added. After degassing by bubbling nitrogen for 20 min, the mixture was stirred at 50 °C for another 48 h. Upon cooling down to room temperature, the mixture was dialyzed (cut-off: 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 g mol−1) against de-ionized water for another 48 h, in order to remove the free DPA, catalysts and by-products. White powders were obtained after freeze-drying.
500 g mol−1) against de-ionized water for another 48 h, in order to remove the free DPA, catalysts and by-products. White powders were obtained after freeze-drying.
Stability of the PVOH-b-PNVCL crosslinked nanogels and reversibility of the crosslinking
The stability of the PVOH-b-PNVCL crosslinked nanogels against swelling was investigated by diluting the crosslinked nanogel solution (0.05 wt%) with de-ionized water at 25 °C. DLS measurement (25 °C) was used to follow the change in hydrodynamic diameters (Dh) and size distribution (PDI) after dilution. For each dilution period, the solution was kept for at least 1 h to reach the swelling equilibrium before characterization.The stability of the PVOH-b-PNVCL crosslinked nanogels against heating was also studied by DLS with the crosslinked nanogel solution (0.05 wt%) under gradual heating from 25 to 50 °C.
The stability of the PVOH-b-PNVCL crosslinked nanogels against reductive reagents was studied by exposing the crosslinked nanogel solution (0.05 wt%) to 10 mM DTT solution in different degassed buffers, such as PBS (pH 7.4), PBS (pH 8.5) and NaAc/HAc (pH 4.5) buffers at 37 °C. At each predetermined interval, 1 mL nanogel solution was collected with a syringe for DLS measurement (25 °C), in order to monitor the change in Dh and PDI. After 24 h treatment, the mixture was cooled down and dialyzed (cut-off: 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 g mol−1) against de-ionized water at room temperature for another 48 h. The purified de-crosslinked (DCL) nanogel solution was freeze-dried and white powders were obtained.
500 g mol−1) against de-ionized water at room temperature for another 48 h. The purified de-crosslinked (DCL) nanogel solution was freeze-dried and white powders were obtained.
Re-crosslinking (RCL) of the PVOH-b-PNVCL DCL nanogels was conducted by adding 1 mL of hydrogen peroxide solution (30 wt%) into 10 mL of DCL nanogel solution (0.05 wt%) at 50 °C and then stirring overnight. RCL nanogels' white powders were obtained after freeze-drying.
Model studies of guest loading and triggered release
Nile red, a hydrophobic drug model, was loaded into the PVOH-b-PNVCL crosslinked nanogels in order to monitor the redox-triggered release behaviours with a fluorometer. Herein, after the thermo-induced formation of PVOH-b-PNVCL micelles at 50 °C, 2 mL of NR solution in THF (0.2 mg mL−1) were added, and then the mixture was stirred for another 30 min in the dark, at the end of which the THF solvent was supposed to be fully evaporated. The crosslinking reaction was carried out according to the above-mentioned protocol. The non-encapsulated NR was removed by filtration (0.8 μm), and other free DPA and by-products were removed viadialysis against de-ionized water in the dark.To study the release profiles, 10 mL of the NR-loaded PVOH-b-PNVCL crosslinked nanogel solution (0.05 wt%) were put in a dialysis bag (cut-off: 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 g mol−1), and then dialyzed against 200 mL of DTT-contained (10 mM) buffer solutions with different pH values, under gentle stirring at 37 °C. The release behaviours in buffer solutions (no DTT) with different pH values were taken for comparison. At each predetermined interval, 0.2 mL of the supernatant nanogel solution was withdrawn from the dialysis bag (released NR precipitated into the bottom of the dialysis bag) for fluorescence measurement at 37 °C, in order to estimate the retained amount of NR. Cumulative release of NR was calculated according to the formula:
500 g mol−1), and then dialyzed against 200 mL of DTT-contained (10 mM) buffer solutions with different pH values, under gentle stirring at 37 °C. The release behaviours in buffer solutions (no DTT) with different pH values were taken for comparison. At each predetermined interval, 0.2 mL of the supernatant nanogel solution was withdrawn from the dialysis bag (released NR precipitated into the bottom of the dialysis bag) for fluorescence measurement at 37 °C, in order to estimate the retained amount of NR. Cumulative release of NR was calculated according to the formula:
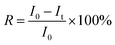 | (1) |
Cell culture and cytotoxicity against fibroblast-like L929 cells
The mouse fibroblast-like L929 cells (ATCC CCL-1) were grown for 24 h at 37 °C under humidified air (5 vol% of CO2) in DMEM medium, which was supplemented with 5 vol% of FBS, 1 vol% of glutamax, 1 vol% of penicillin/streptomycin (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 units of penicillin (base) and 10
000 units of penicillin (base) and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 units of streptomycin (base) per mL) before use (DMEM complete medium). The human melanoma MEL-5 cell line (originated from a non-pigmented clone 32, gift from Dr G. Degiovanni, University of Liège) was also cultured in DMEM complete medium for 24 h. After rinsing with PBS (Ca2+/Mg2+ free) buffer solution, the cells were detached with trypsin (0.2 vol%)/PBS (Ca2+/Mg2+ free) buffer solution.
000 units of streptomycin (base) per mL) before use (DMEM complete medium). The human melanoma MEL-5 cell line (originated from a non-pigmented clone 32, gift from Dr G. Degiovanni, University of Liège) was also cultured in DMEM complete medium for 24 h. After rinsing with PBS (Ca2+/Mg2+ free) buffer solution, the cells were detached with trypsin (0.2 vol%)/PBS (Ca2+/Mg2+ free) buffer solution.
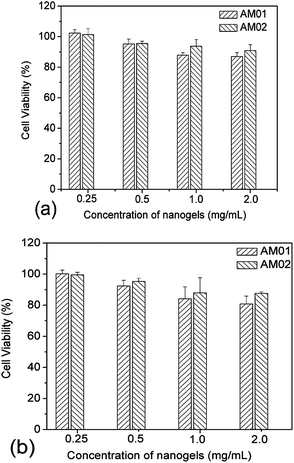
Cytotoxicity measurements were carried out with the L929 cell line. The cells were first seeded in 96-well plates at a density of 5 × 103 cells per well and grown in DMEM complete medium for 24 h. Cells were then treated with PVOH-b-PNVCL crosslinked nanogels in DMEM complete medium with different concentrations (2, 1, 0.5 and 0.25 mg mL−1) for 24 and 48 h. For each concentration, five parallel measurements were carried out at the same time. After each incubation period, the cells were rinsed with PBS (with Ca2+/Mg2+) buffer solution, and cell viabilities were evaluated via the MTS assay. Specifically, 20 μL of MTS and 100 μL of PBS (with Ca2+/Mg2+) buffer solutions were added into each well, and then the plates were incubated at 37 °C for 30 min. The absorbance at 490 nm was measured by using a Power wave X (Biotek instrument Inc.) micro-plate reader. Percentage cell viabilities were determined relative to the untreated cells (control, 100% viability).
Cellular uptake into human melanoma MEL-5 cells
Qualitative studies of the cellular uptake using fluorescence microscopy were carried out with the treated MEL-5 cells after internalizing with NR-uploaded PVOH-b-PNVCL crosslinked nanogels. MEL-5 cells (3.8 × 105) were seeded in a 12-well plate within 2 mL of DMEM complete medium. After 24 h incubation, the culture medium was replaced with 2 mL of fresh DMEM complete medium (blank) or NR-loaded crosslinked nanogels in DMEM complete medium (1.0, 0.5, 0.25 or 0.1 mg mL−1), respectively. After incubating for a predetermined period (3, 6, 15 or 24 h), the medium was removed and the cells were rinsed twice with PBS buffer (Ca2+/Mg2+ free) to remove the free nanogels. Then the cells were treated with paraformaldehyde (4 vol%)/DAPI (1 vol%)/PBS buffer solution (with Ca2+/Mg2+) at room temperature for 15 min in the dark. After rinsing with PBS buffer solution (with Ca2+/Mg2+) two times, 2 mL of PBS buffer solution (with Ca2+/Mg2+) were added. Analysis of the treated cells was performed with an Olympus IX81 inverted fluorescence microscope.Quantitative studies on the cellular uptake of NR-uploaded PVOH-b-PNVCL crosslinked nanogels within MEL-5 cells were conducted with a FACScan fluorescence-activated cell sorter (FACS canto II, Becton-Dickinson). MEL-5 cells internalized with the NR-uploaded crosslinked nanogels were obtained according to the above-mentioned protocol. Then the cells were detached with trypsin (0.2 vol%)/PBS (Ca2+/Mg2+ free) buffer solution, centrifuged (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 rpm, 5 min), and re-dispersed in fresh PBS (Ca2+/Mg2+ free) buffer solution. 400 μL of the treated MEL-5 cell suspension (5 × 105 cells per mL) were used for the FACS measurement. Fluorescence intensities and the percentage of cell-associated fluorescence were determined by using the CellQuest software. The ratio of the fluorescence intensity per 10
300 rpm, 5 min), and re-dispersed in fresh PBS (Ca2+/Mg2+ free) buffer solution. 400 μL of the treated MEL-5 cell suspension (5 × 105 cells per mL) were used for the FACS measurement. Fluorescence intensities and the percentage of cell-associated fluorescence were determined by using the CellQuest software. The ratio of the fluorescence intensity per 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 treated cells to that of 10
000 treated cells to that of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 untreated cells was expressed as mean fluorescence intensity (MFI). The bar graphs in the figures represent mean values (±standard deviation) from three independent experiments.
000 untreated cells was expressed as mean fluorescence intensity (MFI). The bar graphs in the figures represent mean values (±standard deviation) from three independent experiments.
Statistical analysis
Cell culture experiments were performed in triplicate. Results were all presented as mean value (±standard deviation). Statistical analyses of the data were performed using the unpaired and two-tailed Student's t-test. Statistical significance was determined at p < 0.05.Characterization
Raman spectra of the PVOH-b-PNVCL crosslinked nanogel samples and the parent PVOH-b-PNVCL block copolymers after freeze-drying were recorded using a LabRam spectrometer, equipped with an Olympus confocal microscope and a liquid nitrogen-cooled open-electrodedetector (1024 × 256 CCD). An argon-ion laser with emission wavelength at 514.5 nm and a power of 100 mW were used. All measurements were integrated within 50 s.Dynamic light scattering (DLS) measurements were carried out with a Malvern Instrument Nano-ZS, which was equipped with a He–Ne laser (λ = 663 nm) and a scattering angle of 90°. The correlation function was analysed via the CONTIN method, and Dh was determined by using the Stokes-Einstein equation. The averaged Dh was obtained from three different runs. Standard deviation was used to evaluate the size polydispersity index (PDI). Every measurement was carried out with a PVOH-b-PNVCL crosslinked nanogel or the parent PVOH-b-PNVCL polymer solution with a fixed concentration of 0.05 wt%.
Transmission electron microscopy (TEM) was used to measure the size of the PVOH-b-PNVCL crosslinked nanogels and analyse their morphology with a Philips CM-100 microscope. A drop of the nanogel solution was placed onto a carbon-coated copper grid and left to dry under air before observation. The size distribution and average size were obtained by sampling ca. 100 particles on the TEM images.
Scanning electron microscopy (SEM) was used to study the surface of the PVOH-b-PNVCL crosslinked nanogels with a JSM 840A microscope at 4 kV under high vacuum conditions. A drop of nanogel solution (0.05 wt%) was deposited on a glass substrate, and dried overnight at RT under air. Then the sample was sputtered with gold in a cathode evaporator under an argon atmosphere before observation.
Turbidimetry measurement of the PVOH-b-PNVCL crosslinked nanogels or the parent PVOH-b-PNVCL polymer solutions (0.05 wt%) was carried out with a Hitachi U-3300 spectrophotometer from 25 to 50 °C at a heating rate of 1 °C min−1. Transmittance at 700 nm was plotted against the temperature, and the LCST was defined as the temperature where transmittance started to decrease.
1 H Nuclear magnetic resonance (NMR) spectra of the PVOH-b-PNVCL DCL nanogels or the parent PVOH-b-PNVCL polymer were recorded with a 250 MHz Bruker spectrometer in DMSO-d6 at 353 K.
Fluorescence spectra of the NR-loaded PVOH-b-PNVCL crosslinked nanogel solution or NR solutions were recorded at 37 °C using a LS-55 Perkin Elmer fluorometer at an emission wavelength of 595 nm (excitation wavelength of 485 nm). For each measurement, the nanogel concentration was fixed at 0.05 wt%.
Results and discussion
Preparation of the crosslinked nanogels
Similarly to PNIPAm, the PNVCL homopolymer could also dissolve in cold and dilute aqueous medium; however, it becomes insoluble above ca. 32 °C due to its thermo-induced hydrophilic–hydrophobic phase transition around its LCST.5 The value of this LCST is highly dependent on the concentration and chain length of PNVCL. In the case of copolymers, the nature, content and block length of the second co-monomer might also affect the LCST. Generally, the lower the content, the shorter length of the thermo-responsive block, the higher the LCST.35–37 In order to tune the LCST of the PNVCL block above the human body temperature (37 °C), a hydrophilic PVOH block was chosen, and block copolymers of PVOH-b-PNVCL were prepared via cobalt-mediated radical polymerization29,38 following our previous report.30 Briefly, a poly(vinyl acetate) macroinitiator end-capped with Co(acac)2 was prepared in the bulk using an alkyl-Co(acac)2 initiator at 40 °C. Then, the polymerization of N-vinylcaprolactam (NVCL) monomers was initiated by this macroinitiator in DMF at 40 °C to synthesize PVAc-b-PNVCL copolymers. Methanolysis of the PVAc block in the presence of potassium hydroxide led to the corresponding PVOH-b-PNVCL block copolymers.As reported in our previous work,30 above the LCST of the PNVCL block, the collapsed hydrophobic PNVCL block formed the core of the thermo-induced micelles, while the PVOH block formed the hydrophilic corona. However, these physically assembled micelles were not stable against heating, and inter-micellar agglomeration was observed when the temperature was further increased above the LCST. Furthermore, dissociation into unimers occurred when the temperature was decreased below the LCST. Herein, 3,3-dithiodipropionic acid (DPA; Scheme 1) is used to crosslink the PVOH corona above the LCST. And after cooling down below the VPTT, the PNVCL block becomes hydrophilic, and nanogels are obtained due to the presence of crosslinking. Additionally, the presence of disulfide bonds in the crosslinker also makes it possible to de-crosslink the nanogels with a reducing agent, such as dithiothreitol (DTT). Thus, if some drugs are loaded within the crosslinked nanogels, upon the DTT-treatment below the LCST of PNVCL blocks, the crosslinked nanogels could dissociate into unimers, thereby a triggered release is achieved (Scheme 1). However, upon the DTT-treatment above the LCST of PNVCL blocks, the micellar structures would remain intact, and the drugs could be still retained within the micellar structure, even when the crosslinking structures are dissociated. Here, two different PVOH-b-PNVCL copolymers, one with the LCST at human body temperature (37 °C) and the other above it (41 °C), are used (parameters listed in Table. 1), in order to exploit the effect of the LCST of the PVOH-b-PNVCL copolymers on the subsequent redox-triggered release behaviours.
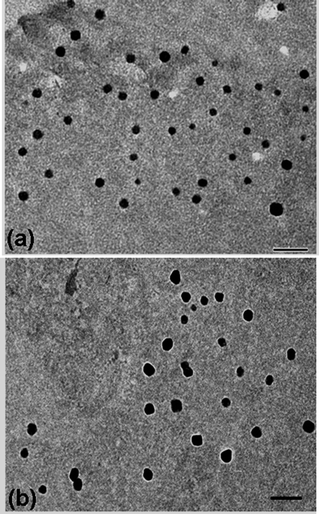
After purification of the PVOH-b-PNVCL nanogels viadialysis, Raman spectroscopy was used to confirm the crosslinking reaction with the presence of the S–S stretching vibration at 514 cm−1, which is typical for the disulfide bonds in the crosslinker (Fig. 1). The morphology of the crosslinked nanogels was studied using TEM, and approximately spherical crosslinked nanogels were observed, within the size range of 45–95 and 70–110 nm for AM01 (Fig. 2a) and AM02 (Fig. 2b), respectively. The morphology was also confirmed using the SEM technique, as shown in the representative SEM images of sample AM01 (see ESI, Fig. S1a and b†). Dynamic light scattering (DLS) measurements allowed us to determine the hydrodynamic diameters of the nanogels at 25 °C, which are 280 nm (PDI = 0.17) and 460 nm (DPI = 0.21) for AM01 and AM02, respectively. The larger size observed for AM02 than AM01 could be attributed to the longer chain length, especially for the PNVCL block in their parent polymers. The large difference between sizes from DLS and TEM analyses results from the fact that TEM images were taken when the crosslinked nanogels were in a dry and collapsed state, while aqueous and swelled states for DLS analysis.
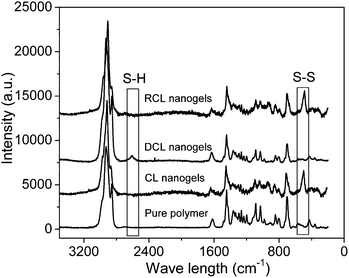 | ||
| Fig. 1 Raman spectra of the PVOH180-b-PNVCL110copolymer, crosslinked (CL) nanogels AM01, de-crosslinked (DCL) nanogels and re-crosslinked (RCL) nanogels. | ||
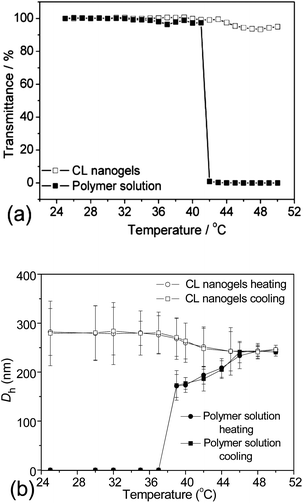
The thermo-responsiveness of the PVOH-b-PNVCL crosslinked nanogels and the parent block copolymers was evaluated and compared by turbidimetry. In Fig. 3a, the transmittance of the two aqueous solutions (0.05 wt% of PVOH180-b-PNVCL110 or of the corresponding crosslinked nanogel AM01) at 700 nm was plotted as a function of temperature from 25 to 50 °C. In the case of PVOH180-b-PNVCL110, the aqueous solution became milky-white and the transmittance dropped sharply to zero when the temperature was increased to ca. 41 °C, higher than the LCST of the PNVCL homopolymer of a similar molar mass (ca. 32 °C), in agreement with the theory that introduction of another hydrophilic block results in a higher LCST.28,29 However, for the corresponding crosslinked nanogel AM01 solution (0.05 wt%), a negligible decrease in transmittance was observed even above 44 °C. This could be attributed to the restricted mobility of the PNVCL block after crosslinking. The same phenomenon was also detected for crosslinked nanogel AM02 made of the PVOH226-b-PNVCL494copolymer with an LCST of 37 °C (see ESI, Fig. S2a†).
DLS was used as another method to study the thermo-responsiveness and stability against heating of the PVOH-b-PNVCL crosslinked nanogels. Here, Dh and PDI of the PVOH180-b-PNVCL110polymer solution and the corresponding AM01 nanogel solution (0.05 wt%) were measured at different temperatures. As shown in Fig. 3b, particles were detected below the VPTT in the nanogel solution, but none for the polymer solution. Upon further heating, thermo-induced micelles could be detected above 39 °C for the polymer solution. However, unlike the polymer solution, neither an inter-micellar aggregation nor an increase in Dh, but a slight decrease in Dh was observed for the crosslinked nanogels when the temperature increased further, indicating a superior resistance of the crosslinked nanogels to inter-particle agglomeration above the VPTT after crosslinking. Moreover, the same behaviour was observed for the aqueous solution of PVOH226-b-PNVCL494 and its corresponding crosslinked nanogel AM02 (see ESI, Fig. S2b†).
It is important to note that the average sizes of the non-crosslinked and crosslinked nanogels (AM01) at 50 °C were found to be similar: 240 nm (PDI = 0.04) against 250 nm (PDI = 0.06) in the size distribution diagrams in Fig. 4. The same observation was made on the scattering intensities, which are proportional to the concentration of crosslinked nanogels. Moreover, for the crosslinked nanogels, after cooling down to 25 °C from the shell crosslinked micelles (240 nm) at 50 °C, the size distribution diagram slightly shifted to the right (290 nm), due to the phase transition and re-arrangement of the PNVCL block below the VPTT. The presence of nanogels below the LCST of the PNVCL blocks could evidence a robust crosslinking within the as-prepared nanogels, compared with unimers obtained from the polymer solution upon cooling from 50 to 25 °C. On the other hand, for an idealistic nanogel-based vehicle targeted for DDS application, it is indispensable to possess a short-term stability but also a long-term stability, and a slight change in size distribution diagram was observed after 3 month storage at room temperature for the AM01 sample (see ESI, Fig. S3†).
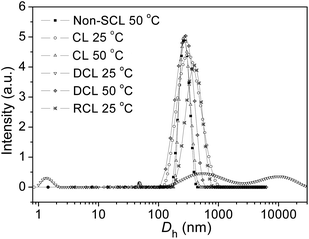 | ||
| Fig. 4 Size distribution diagrams of the crosslinked nanogel AM01 aqueous solution (0.05 wt%) under different conditions from DLS analysis: non-crosslinked nanogels (polymer solution) at 50 °C, crosslinked (CL) nanogels at 25 and 50 °C, de-crosslinked (DCL) nanogels at 25 °C, and re-crosslinked (RCL) nanogels at 25 °C. The solid lines just serve as eye guidance. | ||
Stability of the nanogels and reversibility of the crosslinking
It is of great importance for drug delivery vehicles to remain stable during the delivery so that they can efficiently trap the drug payloads until they arrive at the desirable sites. Studies on the stability of crosslinked micelles or nanogels against different solvents were previously reported to evaluate their stability as DDS.19,39–41 Herein, the stability of the crosslinked nanogel AM01 against dilution was studied by following the change in Dh when diluted with de-ionized water below the VPTT; while studies on the swelling process were conducted by mixing equal volumes of the crosslinked nanogel aqueous solution (0.05 wt%) and de-ionized water. The change in Dh and PDI was measured by DLS and plotted against the swelling period (Fig. 5a). Similar to other crosslinked systems,39,40 slight and lagged swelling was observed against water dilution, indicating a good stability against swelling due to the presence of crosslinking. Additionally, the effect of dilution times on the swelling behaviour of AM01 was also investigated with the size distribution diagrams recorded in Fig. 5b. For each dilution time, the mixture was kept for another one hour to ensure that the swelling equilibrium was reached. A slight right-shift of the size distribution patterns was observed with an increase in the average size from 290 to 360 nm (1/4 vol dilution), indicating that the crosslinked nanogels experienced a relatively mild swelling. Moreover, the size distribution becomes broader, and the swelling was restrained upon further dilution. All these results indicate that neither compact nor rigid crosslinking is accomplished for the as-prepared nanogels; and this slight swelling against dilution is critical to the success of zero premature drug release upon intravenous injection, as compared to those of non-crosslinked micelle systems.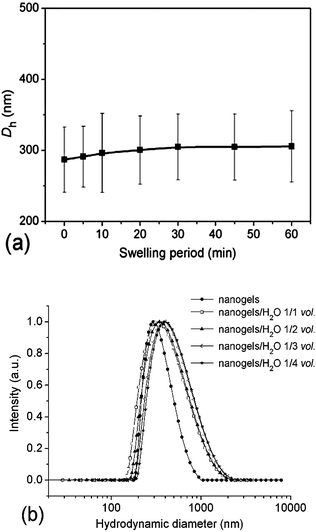 | ||
| Fig. 5 Dependence of Dh and PDI of the crosslinked nanogel AM01 aqueous solution (0.05 wt%) on the swelling period after an equal-volume of water was added (a), normalized size distribution diagrams of the nanogel AM01 aqueous solution (0.05 wt%) at different dilution times (nanogel solution/H2O, vol.) at 25 °C (b). The solid lines just serve as eye guidance. | ||
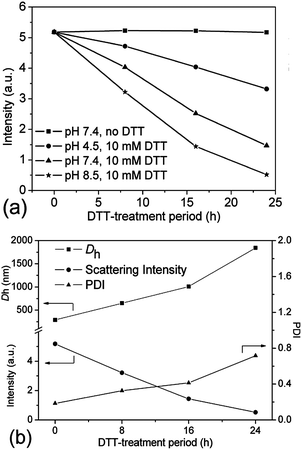
As far as we know, the disulfide bonds could be easily dissociated upon exposure to some reductive reagents, such as glutathione or dithiothreitol (DTT) under mild basic conditions, through the well-established thiol–disulfide exchange reaction.4,6 Although the concentration of glutathione, which is naturally present in the cytoplasm, is very low (mM-scale), it is still high enough to cleave the disulfide bonds.17,42 As previously reported, the most favourable reductive environment for DTT is under basic conditions.43 Herein, the stabilities of the crosslinked nanogel AM01 against different DTT (10 mM) buffer solutions at pH values of 7.4 (serum condition), 8.5 (pancreatic condition) and 4.5 (lysosome condition) were also studied by DLS and are summarized in Fig. 6a. As expected, the amount of nanogels (qualitatively probed by the intensity of the scattered light) decreased much more rapidly at a pH of 8.5 than under acidic conditions. In contrast, in the absence of DTT, nanogels remained stable over the experiment period (see ESI, Fig. S4a and b†). Fig. 6b summarizes the variation of Dh, PDI and scattering intensity at different periods of the DTT-treatment (10 mM DTT, pH 8.5 and 37 °C). It was found that Dh increased from 290 to 1850 nm (size distribution diagram in Fig. 4) while the scattering intensity decreased from 5.2 to 0.5 after 24 h DTT treatment. Very few nanogels might be left after the DTT-treatment, which may be explained by the fact that disulfide bonds were not completely cleaved and/or the re-oxidation of some thiol groups into disulfide bonds occurred during the sampling or dialysis process. Furthermore, similar to the report of Kataoka,17,44 due to the non-uniform cleavage of disulfide bonds, the crosslinked nanogels might experience a dramatic swelling, and here an increase in PDI was also observed from 0.2 to 0.8, which was also evidenced in the size distribution diagram in Fig. 4. Moreover, the presence of the peak in the range of 1–2 nm for DCL nanogels at 25 °C could be ascribed to the free unimers after de-crosslinking. The dissociation of the crosslinked nanogels was also confirmed by the TEM image of DCL nanogel samples after 24 h DTT treatment. In fact, it took us a long time to find nanogel particles on the TEM grid, and a particle with a large size of ca. 500 nm is presented in ESI Fig. S5a,† in agreement with the size distribution of DCL nanogels in Fig. 4 and Dh value in Fig. 6b. However, when taking the number-averaged distribution diagrams into consideration, as shown in ESI Fig. S5b,† a strong signal in the range of 1–2 nm was detected for DCL nanogels, which could be assigned to the presence of free macromolecules, compared with the signals appearing in the size range of 100–300 nm for the crosslinked nanogels. These results would corroborate that almost all the nanogels were disintegrated into unimers, even with very few swollen nanogels left. The DCL nanogels were then analysed with a 1H NMR spectrometer. As shown in ESI Fig. S5c,† compared with the parent PVOH-b-PNVCL copolymer, new proton signals from the pendant –(O)COCH2CH2SH group could be detected, and a DPA conversion of ca. 10% was roughly estimated.
Additionally, it is of interest to note that DCL nanogels containing thiol groups still retain the thermo-responsive character. As shown in Fig. 4, upon heating, thermo-induced micelles were observed again within the DCL nanogel solution (0.05 wt%) at 50 °C, and a slightly larger Dh (280 nm, PDI 0.11) was observed, compared with that for the physically assembled micelles (non-CL nanogels, 240 nm, PDI 0.05). This difference might be attributed to the presence of thiol side-groups along the PVOH block after DTT-treatment, which is expected to affect the macromolecular chain hydrophilicity and configuration of the PVOH block, according to a previous report.45 Moreover, thiol groups were reported to be easily oxidized into disulfide bonds by some oxidative reagents, such as Fe3+, hydrogen peroxide, oxygen, etc.46,47 Herein, the reversibility of the crosslinking was also tested by adding H2O2 solution (30 wt%) into the solution of DCL nanogels at 50 °C (0.05 wt%). Nanogel particles were detected again by DLS analysis with an average Dh of 380 nm (PDI 0.28) at 25 °C (Fig. 4), a little bit larger than the former crosslinked nanogels (Dh 280 nm, PDI 0.17), which might be attributed to the lower crosslinking degree upon re-crosslinking, thereby higher swelling after cooling down below the VPTT. Furthermore, the TEM image (see ESI, Fig. S5d†) and Raman spectra (Fig. 1, S–S bonds) also evidenced the formation of re-crosslinked (RCL) nanogels, suggesting the reversibility of the crosslinking.
Model studies on guest loading of Nile red and triggered release behaviours
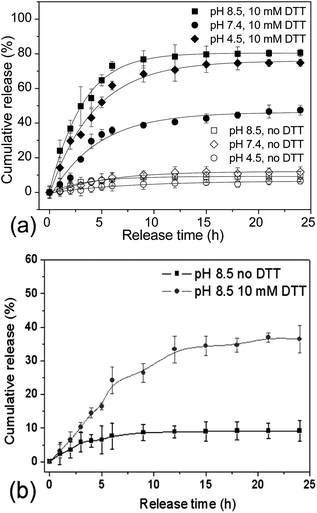
The reversibly crosslinked nanostructures could impart the micelles or nanogels with great potential as DDS, since they are capable of effectively trapping the drug payloads within the microenvironment, while triggered release could be accomplished by the external stimuli.7,8,36 To explore the potential of PVOH-b-PNVCL reversibly crosslinked nanogels as DDS, Nile red, a hydrophobic dye, was chosen as a model drug and loaded into the hydrophobic PNVCL domain above the LCST (Scheme 1). It has been reported that fluorescence emission of NR is negligible in aqueous solution due to its weak solubility (<1 μg mL−1, 25 °C); however, it increases substantially in a hydrophobic environment.48 Stable nanogel aqueous dispersion with red luminance was obtained after the loading of NR (see ESI, Fig. S6b†), while a transparent solution without NR loaded was observed for crosslinked nanogel solution (see ESI, Fig. S6a†). Spherical nanogels were observed again from the TEM image of the NR-loaded crosslinked nanogel AM01 (see ESI, Fig. S6c†), and a slight increase in Dh was observed from 290 to 300 nm for NR-loaded AM01, due to the presence of NR (see ESI, Fig. S7†). The drug loading capacity (mass ratio of NR loaded to nanogels) was spectrophotometrically estimated to be ca. 1.9 wt% and drug loading efficiency (mass ratio of NR loaded to NR fed) of 48%, comparable to the loading capability of other disulfide-crosslinked nanogels as DDS, as reported by Du.22Release profiles of NR from the NR-loaded PVOH-b-PNVCL nanogels were traced by following the change in NR fluorescence intensity over a period of 24 h under different conditions. Fig. 7a shows the typical release profiles of NR from AM01 at 37 °C with or without 10 mM DTT at different pH values. It was found that, without DTT, release below 8% was detected over this period in spite of different pH values, indicating a stable trapping of NR within the crosslinked nanogels. However, in the presence of 10 mM DTT, a release of ca. 72% at pH 7.4 and ca. 80% at pH 8.5 can be accomplished after 24 h release, while only ca. 50% at pH 4.5 was obtained. This is in agreement with preferable cleavage of disulfide bonds under basic conditions, as revealed in Fig. 6a. It might, however, be conjectured that the different release behaviours result from the different solubility of NR in those buffers. To justify or deny this hypothesis, the solubility of NR in different buffers was checked by dispersing 1 mg of NR in 1 mL of buffer solutions with a pH of 4.5 (NaAc/HAc), 7.4 (PBS) and 8.5 (PBS), respectively. After removing the precipitated NR, the supernatant solution was checked with a fluorometer, and the fluorescence emission intensity at 595 nm (ex: 485 nm) was 237, 251 and 266, respectively, while for the NR-loaded crosslinked nanogel AM01, the fluorescence intensity was higher than 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. This confirmed that the solubility of NR in water was not affected by pH, in agreement with the previous report.49 This time-dependent release behaviour strongly supports that the drug release indeed resulted from the cleavage of disulfide bonds, rather than due to the effect of diffusion, osmotic pressure, or even improved solubility at different pH values, etc. Furthermore, the incomplete release even under basic conditions might be explained by the incomplete dissociation of the nanogels and unavoidable oxidation of thiol groups to disulfide bonds by ambient O2, as some nanogels could still be detected in Fig. 4 and ESI Fig. S5a.†
000. This confirmed that the solubility of NR in water was not affected by pH, in agreement with the previous report.49 This time-dependent release behaviour strongly supports that the drug release indeed resulted from the cleavage of disulfide bonds, rather than due to the effect of diffusion, osmotic pressure, or even improved solubility at different pH values, etc. Furthermore, the incomplete release even under basic conditions might be explained by the incomplete dissociation of the nanogels and unavoidable oxidation of thiol groups to disulfide bonds by ambient O2, as some nanogels could still be detected in Fig. 4 and ESI Fig. S5a.†
To elucidate the effect of the composition as well as LCST of the PVOH-b-PNVCL copolymers on the release behaviours, NR was also attempted to be loaded within the nanogel AM02, which were prepared from the PVOH226-b-PNVCL494copolymer with a LCST of 37 °C. A higher loading capacity (2.5 wt%) and efficiency (62%) of NR were obtained above the LCST, due to the larger hydrophobic PNVCL domains, as well as larger size (Dh of 460 nm). The release behaviours were also followed in the presence or absence of DTT (10 mM, pH 8.5). As shown in Fig. 7b, similarly to the release behaviours of AM01, a very small amount of NR was released in the absence of DTT. However, as expected, the release was accelerated when exposed to DTT. It should be noted that this release was very low (36%) after 24 h DTT treatment, compared to 80% for AM01 in Fig. 7a under the same conditions. To explain the difference between these two release profiles, DLS was used to check the size distribution of the NR-loaded crosslinked nanogel solutions at 37 °C after 24 h DTT treatment, with the released NR removed. As shown in ESI Fig. S7a,† a sharp decrease in the scattering intensity (proportional to the amount of nanogels) and wide size distribution was detected for AM01 after 24 h DTT treatment, similar to the size distribution profile of AM01 DCL nanogels (Fig. 4) after 24 h 10 mM DTT treatment. However, the amount of nanogels seems to be not affected too much for AM02 (see ESI, Fig. S7b†). It is probably because of the lower LCST of 37 °C for the PVOH226-b-PNVCL494copolymer, which resulted in the intact micellar structure at 37 °C, even after the cleavage of crosslinking structures. The micellar structures at 37 °C were also evidenced by DLS in the thermo-responsiveness studies of PVOH226-b-PNVCL494 (see ESI, Fig. S2†). Therefore, for the nanogels with LCST below or near 37 °C, the remaining NR was still trapped within the nanogels, resulting in a retarded release even after the de-crosslinking (Scheme 1).
Cytotoxicity assessment and cellular uptake of the crosslinked nanogels
Covalently crosslinked micelles or nanogels exhibit improved stability compared to those non-crosslinked micelles during the in vivo delivery, leading to minimal premature release if used for DDS.8 However, the chemical crosslinking also raises increasing concerns on the potential toxicity of those covalent crosslinking residues or the unexpected side effects with drugs loaded during the chemical crosslinking reaction. The cytotoxicity of the PVOH-b-PNVCL crosslinked nanogels against a mouse fibroblast-like L929 cell line was evaluated via the MTS assay. To exclude the interference of PVOH-b-PNVCL nanogels themselves on the absorbance of the formazan product at 490 nm, we recorded the UV/vis absorption spectra of the parent copolymer and the resultant nanogel solution (0.1 wt%) in ESI Fig. S8,† and the absorbance at 490 nm is both below 0.005 (a.u.). Furthermore, we also measured the UV/vis absorbance of the mixture of 100 μL of nanogel solution in PBS buffer (0.1 wt%) and 20 μL of MTS at 490 nm, and an averaged value of ca. 0.008 from five independent samples, compared to the value ca. 0.554 for those untreated cells. Thus, it justifies that the use of the MTS assay to evaluate the cytotoxicity of the PVOH-b-PNVCL nanogels was feasible and the presence of PVOH-b-PNVCL nanogels will not disturb the absorbance of the final formazan product. The cells were treated with crosslinked nanogels AM01 and AM02 with different incubation concentrations for different periods, while non-treated cells were taken as a control (100% viability). As shown in Fig. 8a and b, both nanogels exhibit high cell viability over 85%, even after 48 h incubation even with a concentrated nanogel solution (2 mg mL−1). The slight effect on the cell viability and cell proliferation capability might suggest low cytotoxicity for these crosslinked nanogels, as well as the crosslinking nanostructures.Generally, an enhanced efficiency of disease treatment could be achieved via higher drug release efficiency and/or efficient uptake of the drug-loaded DDS. Here, the cellular uptake of the NR-loaded crosslinked nanogel AM01 into the human melanoma MEL-5 cells could be directly visualized by fluorescence microscopy (Fig. 9a), with NR as a fluorescence probe. Red fluorescence could be observed only in the cytoplasm of MEL-5 cells other than nuclei (blue) after 24 h incubation with the NR-loaded PVOH-b-PNVCL crosslinked nanogel (AM01, 0.25 mg mL−1), while the non-treated MEL-5 cells did not exhibit any red fluorescence of Nile red (see ESI, Fig. S9†). The fluorescence-activated cell sorting (FACS) technique was also used to characterize the treated MEL-5 cells, while un-treated cells were taken as blank. It is obvious to see two totally different cell populations in Fig. 9b-1 and b-2, with a big shift in the histograms in Fig. 9b-3, and also a sharp increase in the fluorescence intensity, as listed in Fig. 9b-4. These FACS results also corroborate the cellular uptake of the crosslinked nanogels, which is a prerequisite for the intracellular DDS. Furthermore, quantitative studies on the cellular uptake were carried out with the FACS results. The mean fluorescence intensity (MFI), which was denoted as the ratio of fluorescence intensity per 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 treated cells to that of 10
000 treated cells to that of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 un-treated cells, was used as an indirect measure to evaluate the cellular uptake efficiency. This approach has also been used to assess the uptake of some drugs50 and gene delivery vehicles,51etc. Here, MFI was plotted against the incubation period as well as incubation concentration in Fig. 9c-1 and c-2, respectively. As shown, a faster increase in MFI was observed within the first 15 h, while a slight increase afterward. A dose-dependent cellular uptake was also detected when the incubation concentration of crosslinked nanogels was increased, suggesting the possibility of manipulation of cellular uptake efficiency of the PVOH-b-PNVCL crosslinked nanogels via control over the incubation concentration or/and incubation period.
000 un-treated cells, was used as an indirect measure to evaluate the cellular uptake efficiency. This approach has also been used to assess the uptake of some drugs50 and gene delivery vehicles,51etc. Here, MFI was plotted against the incubation period as well as incubation concentration in Fig. 9c-1 and c-2, respectively. As shown, a faster increase in MFI was observed within the first 15 h, while a slight increase afterward. A dose-dependent cellular uptake was also detected when the incubation concentration of crosslinked nanogels was increased, suggesting the possibility of manipulation of cellular uptake efficiency of the PVOH-b-PNVCL crosslinked nanogels via control over the incubation concentration or/and incubation period.
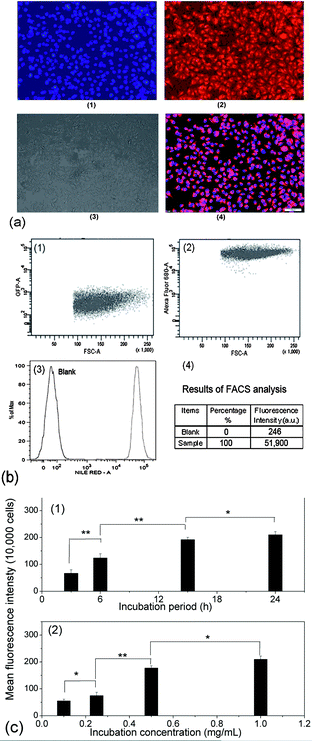 | ||
| Fig. 9 (a): Fluorescence microscopy images of the treated MEL-5 cells after 24 h incubation with the NR-loaded PVOH-b-PNVCL crosslinked nanogel AM01 (0.25 mg mL−1): (1) nuclei stained with DAPI (blue), (2) fluorescence pattern of NR (red), (3) contrast field pattern, (4) merged images of (1), (2) and (3), scale bar: 100 μm; (b) FACS diagrams of (1) untreated MEL-5 cells, (2) treated MEL-5 cells after 24 h incubation with NR-loaded crosslinked nanogel AM01 (0.25 mg mL−1), (3) plotting of the number of cells (counts on y-axis) against the log of fluorescence intensity (Nile Red-A on the x-axis) and (4) FACS analysis results; (c): (c-1) dependence of the MFI of the treated MEL-5 cells on the incubation period after incubation with NR-loaded crosslinked nanogel AM01 (3, 6, 15 and 24 h, 0.25 mg mL−1), and (c-2) dependence of the MFI of the treated MEL-5 cells on the incubation concentration after 15 h incubation with NR-loaded crosslinked nanogel AM01 (0.1, 0.25, 0.5 and 1.0 g L−1) (* = P < 0.05, ** = P < 0.01 by the Student' t-test, n = 3). | ||
Conclusions
In this study, reversibly crosslinked thermo- and redox-responsive PVOH-b-PNVCL nanogels were fabricated with the disulfide-bearing crosslinking reagent 3,3′-dithiodipropionic acid. Their spherical shape was confirmed by TEM, with a Dh of ca. 280 nm (PDI 0.17). Good colloidal stability against heating was confirmed above the VPTT, with a slight shrinkage rather than inter-particle agglomeration. Compared to non-crosslinked micelles, only a slight swelling of the crosslinked nanogels was observed against water dilution below the VPTT, which is critical to the success of zero premature drug release upon intravenous injection. However, the crosslinked nanogels can be easily dissociated in a reductive environment thanks to the redox-sensitive crosslinking structures, so that the drug release could be triggered intracellularly. Interestingly, the de-crosslinked copolymers (bearing free thiols along the PVOH block) can be crosslinked again by exposure to an oxidative agent, such as H2O2, above the LCST. The hydrophobic model drug (NR) could be easily loaded into the crosslinked nanogels, while cleavage of the disulfide bonds can effectively trigger the drug release (80% drug release in the presence of 10 mM DTT while only 8% without DTT in 24 h). This specific redox-responsive property might also facilitate the release of the loaded drugs within the cytoplasm in response to glutathione (10 mM). In addition, we also disclosed that the use of the PVOH-b-PNVCL copolymer exhibiting a LCST above 37 °C was preferable, with a fast release upon exposure to DTT-treatment under physiological conditions. Cytotoxicity studies disclosed a low toxicity and good biocompatibility with a mouse fibroblast-like L929 cell line for the crosslinked nanogels. Moreover, the crosslinked nanogels can easily be internalized within the human melanoma MEL-5 cells. Incubation concentration- and period-dependent cellular uptake was also detected for these PVOH-b-PNVCL crosslinked nanogels. This kind of crosslinked nanogel is thus promising for the triggered delivery of hydrophobic drugs for intracellular release.Acknowledgements
The authors thank the Belgian National Funds for Scientific Research (F.R.S.-FNRS), the European Community in the frame of the Erasmus Mundus International doctoral school IDS-FunMat and the Science Policy Office of the Belgian Federal Government (PAI VII-05) for their financial support. The authors are also grateful to the GIGA-imaging and flow cytometry platform in University of Liège for their help with CLSM and flow cytometry measurement. C.D. and A.D. are Research Director and Research Associate by F.R.S.-FNRS, respectively.Notes and references
- J. C. Leroux and A. Vintiloiu, J. Controlled Release, 2008, 125, 179–192 CrossRef PubMed.
- S. Kim, Y. Shi, J. Y. Kim, K. Park and J. X. Cheng, Expert Opin. Drug Delivery, 2010, 7, 49–62 CrossRef CAS PubMed.
- X. B. Xiong, A. Falamarzian, S. M. Garg and A. Lavasanifar, J. Controlled Release, 2011, 155, 248–261 CrossRef CAS PubMed.
- C. Deng, Y. J. Jiang, R. Cheng, F. H. Meng and Z. Y. Zhong, Nano Today, 2012, 7, 467–480 CrossRef CAS PubMed.
- M. Talelli and W. E. Hennink, Nanomedicine, 2011, 6, 1245–1255 CrossRef CAS PubMed.
- Y. Shao, W. Huang, C. Shi, S. T. Atkinson and J. Luo, Ther. Delivery, 2012, 3, 1409–1427 CrossRef CAS PubMed.
- M. Amiji, S. Ganta, H. Devalapally and A. Shahiwala, J. Controlled Release, 2008, 126, 187–204 CrossRef PubMed.
- R. K. O'Reilly, C. J. Hawker and K. L. Wooley, Chem. Soc. Rev., 2006, 35, 1068–1083 RSC.
- S. P. Armes and E. S. Read, Chem. Commun., 2007, 3021–3035 Search PubMed.
- J. O. You, M. Rafat and D. T. Auguste, Langmuir, 2011, 27, 11282–11286 CrossRef CAS PubMed.
- H. Gao, M. C. Jones, J. Chen, R. E. Prud'homme and J. C. Leroux, Chem. Mater., 2008, 20, 3063–3067 CrossRef CAS.
- Z. Zhou, G. Liu and L. Hong, Biomacromolecules, 2011, 12, 813–823 CrossRef CAS PubMed.
- M. H. Stenze, L. Zhang, W. G. Liu, L. Lin and D. Y. Chen, Biomacromolecules, 2008, 9, 3321–3331 CrossRef PubMed.
- K. Kataoka, Y. Kakizawa and A. Harada, J. Am. Chem. Soc., 1999, 121, 11247–11248 CrossRef.
- R. Cheng, F. Feng, F. Meng, C. Deng, J. Feijen and Z. Zhong, J. Controlled Release, 2011, 152, 2–12 CrossRef CAS PubMed.
- A. Gopferich, S. Bauhuber, C. Hozsa and M. Breunig, Adv. Mater., 2009, 21, 3286–3306 CrossRef PubMed.
- W. F. Dong, A. Kishimura, Y. Anraku, S. Chuanoi and K. Kataoka, J. Am. Chem. Soc., 2009, 131, 3804–3805 CrossRef CAS PubMed.
- S. Y. Liu, J. Y. Zhang, X. Jiang, Y. F. Zhang and Y. T. Li, Macromolecules, 2007, 40, 9125–9132 CrossRef.
- S. Cajot, N. Lautram, C. Passirani and C. Jerome, J. Controlled Release, 2011, 152, 30–36 CrossRef CAS PubMed.
- Y. Li, K. Xiao, J. Luo, W. Xiao, J. S. Lee, A. M. Gonik, J. Kato, T. A. Dong and K. S. Lam, Biomaterials, 2011, 32, 6633–6645 CrossRef CAS PubMed.
- A. P. Zhang, Z. Zhang, F. H. Shi, J. X. Ding, C. S. Xiao, X. L. Zhuang, C. L. He, L. Chen and X. S. Chen, Soft Matter, 2013, 9, 2224–2233 RSC.
- Z. Y. Qiao, R. Zhang, F. S. Du, D. H. Liang and Z. C. Li, J. Controlled Release, 2011, 152, 57–66 CrossRef CAS PubMed.
- J. Kato, Y. Li, K. Xiao, J. S. Lee, J. Luo, J. M. Tuscano, R. T. O'Donnell and K. S. Lam, Mol. Pharmaceutics, 2012, 9, 1727–1735 CrossRef CAS PubMed.
- H. Sun, B. Guo, X. Li, R. Cheng, F. Meng, H. Liu and Z. Zhong, Biomacromolecules, 2010, 11, 848–854 CrossRef CAS PubMed.
- C. Detrembleur, R. Bryaskova, N. Willet, A. S. Duwez, A. Debuigne, L. Lepot, B. Gilbert, C. Jerome and R. Jerome, Chem.–Asian J., 2009, 4, 1338–1345 CrossRef PubMed.
- R. Jerome, R. Bryaskova, N. Willet, A. Debuigne and C. Detrembleur, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 81–89 CrossRef.
- R. Bryaskova, D. Pencheva, M. Kyulavska, D. Bozukova, A. Debuigne and C. Detrembleur, J. Colloid Interface Sci., 2010, 344, 424–428 CrossRef CAS PubMed.
- C. Detrembleur, A. Debuigne, N. Willet and R. Jerome, Macromolecules, 2007, 40, 7111–7118 CrossRef.
- M. Hurtgen, C. Detrembleur, C. Jerome and A. Debuigne, Polym. Rev., 2011, 51, 188–213 CrossRef CAS.
- M. Hurtgen, J. Liu, A. Debuigne, C. Jerome and C. Detrembleur, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 400–408 CrossRef CAS.
- X. Z. Zhang, H. Wei, C. Y. Quan, C. Chang and R. X. Zhuo, J. Phys. Chem. B, 2010, 114, 5309–5314 CrossRef PubMed.
- X. Z. Zhang, H. Wei, D. Q. Wu, Q. Li, C. Chang, J. P. Zhou and R. X. Zhuo, J. Phys. Chem. C, 2008, 112, 15329–15334 Search PubMed.
- S. Y. Liu, Y. M. Zhou, K. Q. Jiang and Y. Q. Chen, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 6518–6531 CrossRef.
- X. Z. Zhang, H. Wei, C. Cheng, C. Chang, W. Q. Chen, S. Cheng and R. X. Zhuo, Langmuir, 2008, 24, 4564–4570 CrossRef PubMed.
- R. Hoogenboom, H. M. Thijs, M. J. Jochems, B. M. van Lankvelt, M. W. Fijten and U. S. Schubert, Chem. Commun., 2008, 5758–5760 RSC.
- H. Wei, S.-X. Cheng, X.-Z. Zhang and R.-X. Zhuo, Prog. Polym. Sci., 2009, 34, 893–910 CrossRef CAS PubMed.
- A. E. Smith, X. Xu and C. L. McCormick, Prog. Polym. Sci., 2010, 35, 45–93 CrossRef CAS PubMed.
- A. Debuigne, R. Poli, C. Jerome, R. Jerome and C. Detrembleur, Prog. Polym. Sci., 2009, 34, 211–239 CrossRef CAS PubMed.
- T. Kissel, X. T. Shuai, T. Merdan, A. K. Schaper and F. Xi, Bioconjugate Chem., 2004, 15, 441–448 CrossRef PubMed.
- Q. Huo, J. Liu, L. Q. Wang, Y. Jiang, T. N. Lambert and E. Fang, J. Am. Chem. Soc., 2006, 128, 6447–6453 CrossRef CAS PubMed.
- G. Gaucher, M. H. Dufresne, V. P. Sant, N. Kang, D. Maysinger and J. C. Leroux, J. Controlled Release, 2005, 109, 169–188 CrossRef CAS PubMed.
- A. L. Weikel, S. G. Owens, T. Fushimi and H. R. Allcock, Macromolecules, 2010, 43, 5205–5210 CrossRef CAS.
- X. Zhang, J. Niu, F. Shi, Z. Liu and Z. Q. Wang, Langmuir, 2007, 23, 6377–6384 CrossRef PubMed.
- S. Matsumoto, R. J. Christie, N. Nishiyama, K. Miyata, A. Ishii, M. Oba, H. Koyama, Y. Yamasaki and K. Kataoka, Biomacromolecules, 2009, 10, 119–127 CrossRef CAS PubMed.
- A. Eisenberg and T. Azzam, Angew. Chem., Int. Ed., 2006, 45, 7443–7447 CrossRef PubMed.
- J. Y. Zhang, X. Jiang, Y. F. Zhang, Y. T. Li and S. Y. Liu, Macromolecules, 2007, 40, 9125–9132 CrossRef CAS.
- C. L. McCormick, X. W. Xu and A. E. Smith, Aust. J. Chem., 2009, 62, 1520–1527 CrossRef.
- M. C. A. Stuart, J. C. van de Pas and J. B. F. N. Engberts, J. Phys. Org. Chem., 2005, 18, 929–934 CrossRef CAS.
- D. L. Sackett and J. Wolff, Anal. Biochem., 1987, 167, 228–234 CrossRef CAS.
- J. M. Rosenholm, A. Meinander, E. Peuhu, R. Niemi, J. E. Eriksson, C. Sahlgren and M. Linden, ACS Nano, 2009, 3, 197–206 CrossRef CAS PubMed.
- P. Vader, L. J. van der Aa, J. F. Engbersen, G. Storm and R. M. Schiffelers, J. Controlled Release, 2010, 148, 106–109 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: SEM image of the crosslinked nanogel AM01, TEM image of the de-crosslinked and re-crosslinked nanogel AM01, picture of the crosslinked nanogel AM01 aqueous solution (0.05 wt%) with or without NR loaded, size distribution diagrams of the NR-loaded crosslinked nanogel AM01 before and after DTT-treatment, UV/vis spectra of the crosslinked nanogel solution (0.1 wt%) and parent PVOH-b-PNVCL copolymer solution (0.1 wt%), etc. See DOI: 10.1039/c3py00839h |
| This journal is © The Royal Society of Chemistry 2014 |