Nanosized zinc peroxide (ZnO2): a novel inorganic oxidant for the oxidation of aromatic alcohols to carbonyl compounds
Sanny
Verma
and
Suman L.
Jain
*
Chemical Sciences Division, CSIR-Indian Institute of Petroleum, Dehradun-248005, India. E-mail: suman@iip.res.in; Fax: +91-135-2660202; Tel: +91-135-2525788
First published on 16th June 2014
Abstract
Zinc peroxide (ZnO2) nanoparticles were readily synthesized from the oxidation of zinc acetate with hydrogen peroxide at room temperature. The synthesized material was fully characterized by FTIR, XRD, TG-DSC, FE-SEM, TEM and XPS analysis. As obtained nanosized zinc peroxide was found to be an efficient oxidant for the oxidation of aromatic alcohols to the corresponding carbonyl compounds selectively in excellent yields using dimethyl carbonate as an environmentally benign solvent.
The development of novel materials with dimensions on the nanoscale has become a rising star in the field of materials science.1 These nanocrystalline materials often have novel physical and chemical properties differing from those of the corresponding bulk materials and therefore open up new avenues for their wide applications.2 Inorganic peroxy compounds because of their exceptional reactivity and oxidative capacity are widely being used in industry as oxidizing agents, as catalysts and oxygen source in organic synthesis and as precursors for the synthesis of metal oxides.3 Most of these peroxy salts reversibly add oxygen and upon heating release oxygen and thus can be considered as “oxygen batteries”.4 Sodium peroxide, sodium perborate, and sodium persulfate are commonly known inorganic peroxy compounds, which are considered to be inorganic salts of the hydrogen peroxide and react with water to produce H2O2.5 The oxidation of organic compounds with these peroxy salts in acetic acid using KBr and transition metal based catalysts is well reported in the literature.6 The metal peroxides such as CaO2, MgO2 and BaO2 are highly stable and have the potential to promote the oxidation of organic substrates only at higher reaction temperatures.7,8
Selective oxidation of aromatic alcohols to aryl aldehydes and ketones is a synthetically important transformation because of the wide applications of these products in synthetic organic chemistry.9 Furthermore, it is difficult to control the over-oxidation of aldehydes to the corresponding acids. Conventionally the oxidation of alcohols is carried out using stoichiometric inorganic oxidants such as permanganate,10 bromate11 or Cr(VI) based reagents12 which generates a large amount of heavy metal waste. Furthermore, these oxidants are expensive, hazardous and require toxic solvents to dissolve in. Thus, the development of cost effective environmentally friendly oxidants is highly desired from economical and environmental viewpoints. In recent years the oxidation reactions using O2 or hydrogen peroxide are gaining particular interest as they are cheap, readily available and give water as the only byproduct.13 In this regard, several methods employing precious metals such as Ru,14 Ru-modified catalysts,15 Pd-modified materials,16 supported gold nanoparticles17 have been used for the oxidation of various alcohols. However, the higher cost of the precious metals, and toxic and volatile solvents are the major limitations of these catalytic systems. Recently nanocrystalline materials such as nano NiO2 have been used for the oxidation of aromatic alcohols.18 However, the use of a large excess of nano NiO2 (4 times than alcohol) and acetic acid, and n-hexane as reaction media makes this method less attractive. We have chosen nanoparticles of ZnO2 for the oxidation of alcohols in the present work because of certain advantages such as ZnO2 nanoparticles decompose at relatively lower temperatures,19 well below the decomposition temperatures of stable metal peroxides, such as MgO2 and BaO2, to give oxygen and the metal oxide. Furthermore, the surface of zinc peroxide is well known to contain reactive species, such as peroxy or hydroperoxy radicals, with the potential to initiate and activate the alcohols for oxidation. In addition, easy synthesis, the non-toxic nature of zinc and lower decomposition temperature fascinated us towards its use.
Accordingly, herein, we report a facile and efficient methodology for the oxidation of aromatic alcohols to the corresponding aldehydes and ketones using nanocrystalline zinc peroxide (ZnO2) as an oxidant and dimethyl carbonate as a reaction medium as shown in Scheme 1
Synthesis and characterization of nanocrystalline ZnO2
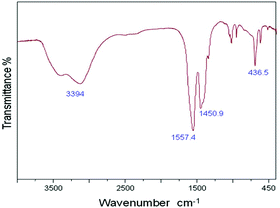
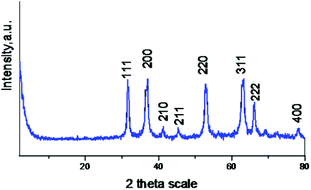
During the present study, nanocrystalline zinc peroxide was obtained through a hydrothermal method using zinc acetate as the precursor and hydrogen peroxide as the oxidant. The BET surface area of the synthesized nanosized ZnO2 was found to be 52 m2 g−1. The synthesized nanocrystalline zinc peroxide was characterized with various techniques. In the FT-IR spectrum of ZnO2 (Fig. 1), the absorption peaks at about 3431 and 1590 cm−1 are attributed to the stretching vibration of the O–H bond and the bending vibration of H–O–H from water molecules, respectively. The peak positions of the synthesized ZnO2 nanoparticles were found to be consistent with previous reports.20 The peak positions centered at 1040, 1334, and 1450 cm−1 may be due to the O–O bands corresponding to the peroxide (O22−) ions of ZnO2 nanoparticles. Another sharp absorption band of the ZnO2 is observed to be located at 436 cm−1 which is corresponding to Zn–O vibration.The XRD pattern of the synthesized nanocrystalline zinc peroxide is shown in Fig. 2 which is in good agreement with that of the typical cubic structure of nano ZnO2 as described in the literature.20,21 The plane 111 is characteristic of the ZnO2 cubic structure. The sharp diffraction peaks apparent in Fig. 2 indicated the good crystallinity of the ZnO2 nanoparticles. A definite line broadening of the diffraction peaks indicated that the synthesized ZnO2 particles are in the nanometer range. Diffraction peaks corresponding to the impurity were not found in the XRD patterns, confirming the high purity of the synthesized material. The average crystallite sizes of the synthesized material were calculated by Scherrer's equation using the full length at half maximum of 111, 200 and 220 of the X-ray diffraction peaks. The average crystallite size of ZnO2 was obtained as 151 nm, confirming the nanocrystalline nature of the synthesized ZnO2.
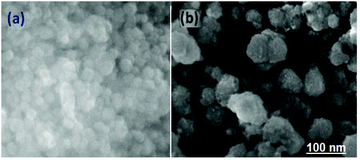
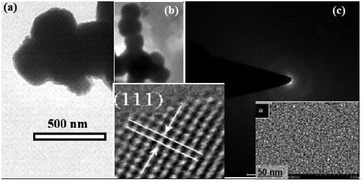
Fig. 3a and b show the SEM image of the as-prepared ZnO2 nanoparticles. As shown in Fig. 3b, the diameters of the spheres are about 100–200 nm and some spheres are apparently bigger, probably due to the joining of several adjacent small spheres.21
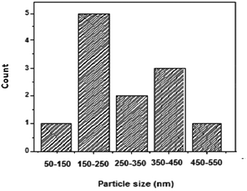
Further insight into the morphology of the synthesized ZnO2 was gained by HR-TEM as shown in Fig. 4. The TEM image shows the consistency with the SEM image with the diameters of around 50–500 nm. The selected area electron diffraction (SAED) pattern of the ZnO2 sample confirms that it is multi-crystalline, which is consistent with the image of Fig. 4b. The atomic structures of ZnO2 were also characterized by HR-TEM, showing that the ZnO2 submicron meter sized spheres are multi-crystalline having (111) planes in the ZnO2 crystal lattice. The particle size distribution based on TEM analysis in the synthesized material is given in Fig. 5, indicating that the size of the ZnO2 nanoparticles is in the range of 50–550 nm.
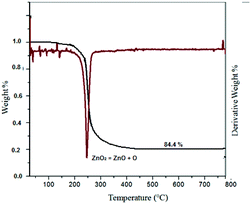
Fig. 6 shows the TG curve and its derivative for ZnO2 nanoparticles. It revealed an exothermic peak (∼235 °C) for oxygen release, according to the following reaction:
| 2ZnO2 = 2ZnO + O2 | (1) |
The temperature of the oxygen release (∼235 °C) was found to be in good agreement with the value from the literature.20 According to the curve the weight loss was 84.4%. After reaching the decomposition temperature, a hexagonal ZnO phase was observed, which is stable up to 800 °C.20
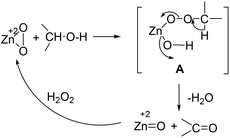
The oxidation state of the synthesized ZnO2 was determined by XPS analysis by calculating the binding energies of O (1s) as shown in Fig. 7. The XPS analysis of ZnO2 (Fig. 7a) showed a characteristic peak of O (1s) of peroxide ions (O2−2) at 532.8 eV. However, after the oxidation reaction, ZnO2 converted into ZnO, which is evidenced by the XPS analysis as shown in Fig. 7b. The peak position on the low binding energy side of the O (1s) spectrum at 530.5 eV is attributed to the O2− ions on the wurzite structure of the ZnO.22 As indicated in Fig. 7a & b, the peak position moved from O2−2 (ZnO2) to O−2 (ZnO) indicating that nanocrystalline ZnO2 led the oxidation of alcohols by the oxygen transfer mechanism as shown in Scheme 2.
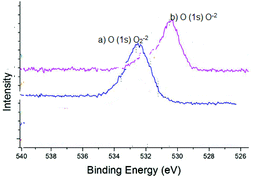 | ||
| Fig. 7 XPS spectra (a) O (1s) of as-synthesized nanocrystalline ZnO2; (b) ZnO as obtained after the oxidation reaction. | ||
Oxidation of alcohols
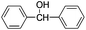
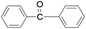
The synthesized nanocrystalline zinc peroxide was used for the oxidation of aromatic alcohols using dimethyl carbonate (DMC) under refluxing conditions. To start with, oxidation of benzhydrol was selected as a representative example. The mixture of benzhydrol (1.0 mmol) and ZnO2 (1.2 mmol) was suspended in DMC (5 mL) and the mixture was stirred for 8 h at 100 °C. The progress of the reaction was monitored by TLC. As expected, ZnO2 exhibited excellent activity and afforded complete conversion with nearly quantitative yield of the benzophenone (Table 1, entry 1). However, the reaction of benzhydrol with DMC and aq. hydrogen peroxide without adding zinc peroxide under identical experimental conditions afforded the desired oxidized product benzophenone in traces even after prolonged reaction time (12 h). Similarly, the oxidation of benzhydrol in DMC with nanosized ZnO and H2O2 was found to be very slow and afforded only 30% yield of the benzophenone. Furthermore, no oxidation was observed when aqueous hydrogen peroxide was used alone under described experimental conditions (Table 1, entry 1).| Entry | Substrate | Product | Time (h) | Conv.b (%) | Yieldc (%) |
|---|---|---|---|---|---|
| a Reaction conditions: alcohol (1.0 mmol), ZnO2 (1.2 mmol), DMC (2.0 mL) at 100 °C. b Determined by GC; remaining is the unreacted substrate. c Isolated yields. d In the absence of ZnO2. e Using ZnO and H2O2. f Using CH3CN. g Using dichloroethane. h Using water as the solvent. i Using bulk sized ZnO2 as the oxidant. | |||||
| 1 |
|
|
8 | 100 | 95 |
| — | Traced | ||||
| 32 | 30e | ||||
| 2 |
|
|
8 | — | 82f, 68g |
| 85h | |||||
| 3 |
|
|
8 | 98 | 92 |
| 12 | 94 | 90i | |||
| 4 |
|
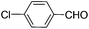
|
8 | 95 | 93 |
| 5 |
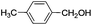
|
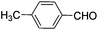
|
8 | 99 | 96 |
| 6 |
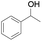
|
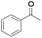
|
8 | 97 | 94 |
| 7 |
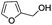
|
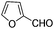
|
8 | 93 | 90 |
| 8 |
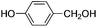
|
|
8 | 92 | 89 |
| 9 |
|
|
8 | 92 | 90 |
| 10 |
|
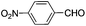
|
8 | 84 | 79 |
| 11 |
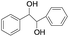
|
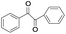
|
5 | 100 | 98 |
| 12 |
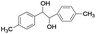
|
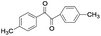
|
5 | 100 | 98 |
| 13 |
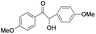
|
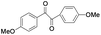
|
5 | 99 | 96 |
| 14 |
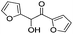
|
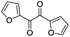
|
6 | 97 | 95 |
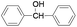
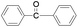
Furthermore we studied the effect of various solvents on the oxidation of benzhydrol under described reaction conditions. Among the various solvents such as acetonitrile, dichloroethane, water and DMC studied (Table 1, entry 2), DMC was found to be best for the present transformation. Due to its classification as a “green solvent”,23 dimethyl carbonate has grown in popularity and applications as a replacement for the toxic and volatile organic solvents for various important organic reactions.
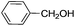
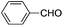
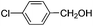
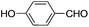
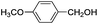
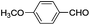
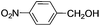
This led us to extend the scope of the reaction and consequently we performed the oxidation of a variety of aromatic alcohols under described reaction conditions. These results are summarized in Table 1. As summarized in Table 1, all the substrates were efficiently converted to their corresponding carbonyl compounds and afforded high to excellent yields of the desired products. Benzyl alcohol and its derivatives containing either electron donating or withdrawing groups in the aromatic ring, i.e. 4-methyl-, 4-methoxy, 4-chloro- and 4-nitro, were selectively oxidized to the corresponding benzaldehydes without any evidence for the formation of the corresponding acids as over-oxidation products. In the case of secondary alcohols, those having carbonyl groups at the adjacent position such as benzoins were found to be more reactive than the secondary alcohols containing aromatic groups (Table 1, entries 11–14). All the products were confirmed by comparing their physical and spectral data with those of reported compounds. After the oxidation reaction, spent zinc oxide could easily be recovered from the reaction mixture by filtration, washed with water and acetone and dried. The recovered ZnO was treated with an excess of aqueous hydrogen peroxide at 150 °C to give zinc peroxide, which can be reused for the recycling experiments. The regenerated ZnO2 was characterized by FTIR and XRD analysis. The results of these analyses were found to be consistent with the freshly prepared ZnO2 nanoparticles. The DMC was found to be stable under the reaction conditions. After the reaction, the product from DMC could easily be extracted with diethyl ether and the recovered DMC was reused for the subsequent runs.
The recycling of the regenerated ZnO2 and recovered DMC was tested for five runs. The almost similar yield of the desired product in all cases confirmed that the oxidant ZnO2 and solvent DMC can be recycled efficiently.
For the comparison purpose, we performed the oxidation of benzyl alcohol with bulk sized ZnO2 under identical experimental conditions. The reaction was found to be slow and required longer reaction time (12 h) for completion (Table 1, entry 2). Similarly, the oxidation of benzaldehyde did not occur under described experimental conditions and the original substrate was observed in the GC analysis.
We also studied the oxidation of aliphatic alcohols such as 1-hexyl, 1-heptyl and 2-octyl alcohol under described experimental conditions. The oxidation occurred but the corresponding acids were obtained as the major products. The main reason for the over-oxidation of aliphatic alcohols may be their higher reactivity as compared to the aromatic ones. Although the exact mechanism of the reaction is not known at this stage, a probable mechanistic pathway of the reaction is depicted in Scheme 2. In analogy to the metal peroxide mediated oxidation,24 we assume the formation of intermediate peroxide species A, which subsequently loses water and cleave to give the corresponding carbonyl compound and zinc oxide as shown in Scheme 2. To confirm the formation of ZnO we analyzed the product by XRD and XPS analyses. The absence of the characteristic plane (111) in the XRD and shifting of the peak position of O (1s) from 532.5 to 530.5 eV in XPS validated the formation of zinc oxide (ZnO) after the oxidation (Fig. 7). The obtained ZnO can be treated with hydrogen peroxide to convert back into zinc peroxide, which can be used for recycling experiments. The exact role of DMC is not clear. We assume that DMC might be enhancing the reaction by hydrogen bonding with alcohols which provide facile activation to the O–H bond.
Conclusions
In summary, adopting a simple, mild and cheap hydrothermal method, we have synthesized ZnO2 nanocrystals of 50–150 nm size that showed excellent efficiency for the selective oxidation of a variety of aromatic alcohols to give the corresponding carbonyl compounds in high to excellent yields using dimethyl carbonate as a green reaction medium. There was no sign of over-oxidation of the alcohol in our system and we have used inexpensive and easily accessible zinc peroxide as the oxidant. Analytical studies indicated the conversion of ZnO2 to ZnO after the oxidation reaction. The obtained zinc oxide can be easily converted into zinc peroxide by treating it with hydrogen peroxide. The regenerated zinc peroxide showed efficient recycling and reusability for the oxidation of benzhydrol, which is of particular interest to chemists for large scale synthesis.Experimental section
All the substrates and solvents were purchased from Acros Organics and used as received. Zinc acetate was purchased from Aldrich. Nanocrystalline zinc peroxide was prepared by adopting a low temperature hydrothermal process using zinc acetate as the precursor. The vibrational spectrum of the synthesized nanomaterial was recorded using a Thermo Scientific Nicolet 8700 Research FT-IR spectrophotometer with a 4 cm−1 resolution using their KBr pellets. Thermal decomposition of the as-synthesized nanocrystalline ZnO2 was determined using a Diamond TG-DTA analyzer (Perkin-Elmer). All samples were analyzed in the temperature range of 50 to 900 °C under nitrogen flow. The morphology and structural features of the zinc peroxide nanoparticles were characterized by FE-SEM. Furthermore, nano-structural features of the as-synthesized material were examined using a transmission electron microscope (TEM, JEOL 3010) at an accelerating voltage of 300 kV. X-ray photoelectron (XPS) analysis was carried out on an ESCALAB 250 (Thermo-VG Scientific), using AlKα as the excitation source. The 1H and 13C NMR spectra were recorded on a Bruker Avance 500 spectrometer in CDCl3 with CHCl3 (7.27 ppm for 1H, 77 ppm for 13C) as the standard and the chemical shifts are expressed in δ parts per million relative to tetramethylsilane (TMS) as the internal standard. GC-MS (HP 5890, series II) analysis was carried out using a mass selective detector (MSD) (30 m × 0.30 mm); 50–250 °C, 8 °C min−1.Synthesis of nanocrystalline zinc peroxide
To a solution of Zn(CH3COO)2·2H2O (1 g) in deionized water (50 mL) was added H2O2 (5 mL, 30 wt%). The resulting mixture was stirred overnight at room temperature and then separated by filtration. The resulting fine powder was washed with deionized water and dried at 60 °C for 3 days to afford nanocrystalline ZnO2. The BET surface area of the as synthesized ZnO2 was found to be 52 m2 g−1.Regeneration of zinc peroxide
Recovered ZnO (1 g) was added to distilled water (15 mL) in a glass beaker and ultrasonically dispersed to obtain a uniform mixture. Then 30 wt% aqueous hydrogen peroxide (60 mL) was added and the resulting mixture was transferred to a Teflon coated autoclave, and heated at 150 °C for 20 h. Then the reaction mixture was cooled to room temperature and the precipitated ZnO2 was washed with ethanol and dried at 60 °C under vacuum.General procedure for oxidation of aromatic alcohols
To a stirred solution of the aromatic alcohol (1.0 mmol) in dimethyl carbonate (2 mL), nanocrystalline zinc peroxide (1.2 mmol) was added and the resulting mixture was stirred under reflux for the time reported in Table 1. Progress of the reaction was monitored by TLC. After completion, the reaction mixture was filtered to recover the spent zinc peroxide. The organic layer was concentrated under reduced pressure and thus the obtained crude product was purified by column chromatography (SiO2) using ethyl acetate–hexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) as the eluent. The selectivity and conversion were determined by high resolution GC-MS analysis; however, the identity of the products was confirmed by comparing their physical and spectral data with the known compounds.
9) as the eluent. The selectivity and conversion were determined by high resolution GC-MS analysis; however, the identity of the products was confirmed by comparing their physical and spectral data with the known compounds.
Acknowledgements
We are thankful to the Director, CSIR-IIP for his kind permission to publish these results. SV acknowledges CSIR, New Delhi, for his Research Fellowship.Notes and references
- (a) C. Buzea, I. I. P. Blandino and K. Robbie, Biointerphases, 2007, 2, MR17–MR172 CrossRef; (b) N. Lubick and K. Betts, Environ. Sci. Technol., 2008, 42, 3910–3910 CrossRef CAS.
- P. Holister, C. Roman Vas and T. Harper, Cientifica, 2003 Search PubMed.
- J. A. Connor and E. A. V. Ebsworth, Adv. Inorg. Chem. RadioChem., 1964, 6, 279–381 CrossRef CAS.
- (a) A. Kraytsberg and Y. Ein-Eli, J. Power Sources, 2011, 196, 886–893 CrossRef CAS PubMed; (b) J. Read, J. Electrochem. Soc., 2002, 149, 1190–1195 CrossRef PubMed.
- H. Jakob, S. Leininger, T. Lehmann, S. Jacobi and S. Gutewort, Peroxo Compounds, Inorganic, in Ullmann's Encyclopedia of Industrial Chemistry, 2007 Search PubMed.
- (a) A. McKillop and W. R. Sanderson, J. Chem. Soc., Perkin Trans. 1, 2000, 471–476 RSC; (b) S. L. Jain, V. B. Sharma and B. Sain, Tetrahedron, 2006, 62, 6841–6847 CrossRef CAS PubMed; (c) A. R. Vaino, J. Org. Chem., 2000, 65, 4210–4212 CrossRef CAS.
- (a) S. Ahmad, M. Kharkwal, G. Gupta and R. Nagarajan, J. Phys. Chem. C, 2011, 115, 10131–10139 CrossRef CAS; (b) X. Han, R. Liu, Z. Xu, W. Chen and Y. Zheng, Electrochem. Commun., 2005, 7, 1195–1198 CrossRef CAS PubMed.
- (a) M. J. Tribelhorn and M. E. Brown, Thermochim. Acta, 1995, 255, 143–154 CrossRef CAS; (b) A. A. Rywak, J. M. Burlitch and T. M. Loehr, Chem. Mater., 1995, 7, 2028–2038 CrossRef CAS.
- (a) M. P. Doyle and V. Bagheri, J. Org. Chem., 1981, 46, 4806–4809 CrossRef CAS; (b) D. R. Williams, F. D. Klingler, E. E. Allen and F. W. Lichtenthaler, Tetrahedron Lett., 1988, 29, 5087–5090 CrossRef CAS; (c) T. Tanaka, K. Murakami, O. Okuda, T. Kuroda, T. Inoue, K. Kamei, T. Murata, H. Yoshino, T. Imanishi and C. Iwata, Chem. Pharm. Bull., 1994, 42, 1756–1759 CrossRef CAS; (d) J.-I. Matsuo, A. Kawana, H. Yamanaka and T. Mukaiyama, Chem. Lett., 2003, 32, 182–183 CrossRef CAS.
- F. M. Menger and C. Lee, Tetrahedron Lett., 1981, 22, 1655–1656 CrossRef CAS.
- C. K. Lee, B. S. Koo, Y. S. Lee, H. K. Cho and K.-J. Lee, Bull. Korean Chem. Soc., 2002, 23, 1667–1670 CrossRef CAS.
- For a general review on chromium oxidations, see for example: (a) G. Cainelli and G. Cardillo, Chromium Oxidants in Organic Chemistry, Springer, Berlin, 1984 Search PubMed; (b) S. V. Ley and A. Madin, in Comprehensive Organic Synthesis, ed. B. M. Trost, I. Fleming and S. V. Ley, Pergamon, Oxford, 1991, vol. 7, p. 251 Search PubMed.
- (a) Z. Shi, C. Zhang, C. Tang and N. Jiao, Chem. Soc. Rev., 2012, 41, 3381–3430 RSC; (b) Catalytic Oxidations with Hydrogen Peroxide as Oxidant, ed. G. Strukul, Kluwer Academic, Dordrecht, 1992 Search PubMed; (c) C. W. Jones, Applications of Hydrogen Peroxide and Derivatives, Royal Society of Chemistry, Cambridge, 1999 Search PubMed; (d) W. R. Sanderson, Pure Appl. Chem., 2000, 72, 1289–1304 CrossRef CAS.
- (a) H. B. Ji, K. Ebitani, T. Mizugaki and K. Kaneda, Catal. Commun., 2002, 3, 511–517 CrossRef CAS; (b) H. B. Ji, K. Ebitani, T. Mizugaki and K. Kaneda, React. Kinet. Catal. Lett., 2003, 78, 73–80 CrossRef CAS.
- (a) K. Yamaguchi and N. Mizuno, Angew. Chem., Int. Ed., 2002, 41, 4538–4542 CrossRef CAS; (b) M. Musawir, P. N. Davey, G. Kelly and I. V. Kozhevnikov, Chem. Commun., 2003, 1414–1415 RSC.
- (a) B. Z. Zhan, M. A. White, T. K. Sham, J. A. Pincock, R. J. Doucet, K. V. R. Rao, K. N. Robertson and T. S. Cameron, J. Am. Chem. Soc., 2003, 125, 2195–2199 CrossRef CAS PubMed; (b) K. Mori, K. Yamaguchi, T. Hara, T. Mizugaki, K. Ebitani and K. Kaneda, J. Am. Chem. Soc., 2002, 124, 11572–11573 CrossRef CAS PubMed.
- (a) V. R. Choudhary, A. Dhar, P. Jana, R. Jha and B. S. Uphade, Green Chem., 2005, 7, 768–770 RSC; (b) V. R. Choudhary, R. Jha and P. Jana, Green Chem., 2007, 9, 267–272 RSC; (c) D. I. Enache, J. K. Edwards, P. Landon, B. Solsona-Espriu, A. F. Carley, A. A. Herzing, M. Watanabe, C. J. Kiely, D. W. Knight and G. J. Hutchings, Science, 2006, 311, 362–365 CrossRef CAS PubMed.
- (a) L. Ibarra and M. Alzorriz, Polym. Int., 1996, 48, 550–586 Search PubMed; (b) L. Ibarra and M. Alzorriz, J. Appl. Polym. Sci., 2002, 84, 605–615 CrossRef CAS; (c) L. Ibarra and M. Alzorriz, Polymer, 2002, 43, 1649–1655 CrossRef CAS.
- M. Kooti and M. E. Jorfi, J. Chem., 2008, 5, 365–369 CAS.
- S. Cheng, D. Yan, J. T. Chen, R. F. Zhuo, J. J. Feng, H. J. Li, H. T. Feng and P. X. Yan, J. Phys. Chem. C, 2009, 113, 13630–13635 CAS.
- W. Chen, Y. H. Lu, M. Wang, L. Kroner, H. Paul, H.-J. Fecht, J. Bednarcik, K. Stahl, Z. L. Zhang, U. Wiedwald, U. Kaiser, P. Ziemann, T. Kikegawa, O. C. D. Wu and J. Z. Jiang, J. Phys. Chem. C, 2009, 113, 1320–1324 CAS.
- J.-C. Dupin, D. Gonbeau, P. Vinatier and A. Levasseur, Phys. Chem. Chem. Phys., 2000, 2, 1319–1324 RSC.
- (a) P. Tundo, F. Aricò, A. E. Rosamilia, S. Grego and L. Rossi, Green Chemical Reactions NATO Science for Peace and Security Series, 2008, pp. 213–232 Search PubMed; (b) C. B. Kreutzberger, Chloroformates and Carbonates, Kirk-Othmer Encyclopedia of Chemical Technology, 2001 Search PubMed.
- W. A. Herrmann, J. D. G. Correia, F. E. Kuhn, G. R. J. Artus and C. C. Romao, Chem. – Eur. J., 1996, 2, 168–173 CrossRef CAS.
| This journal is © the Partner Organisations 2014 |