Transmetalation of Ar1ZnX with [Ar2–Pd–X] is the rate-limiting step: kinetic insights from a live Pd-catalyzed Negishi coupling†
Jing
Li
a,
Liqun
Jin
a,
Chao
Liu
a and
Aiwen
Lei
*ab
aCollege of Chemistry and Molecular Sciences, Wuhan University, Wuhan 430072, Hubei, P. R. China. E-mail: aiwenlei@whu.edu.cn
bNational Research Center for Carbohydrate Synthesis, Jiangxi Normal University, Nanchang 330022, Jiangxi, P. R. China
First published on 19th December 2013
Abstract
Transmetalation is the rate-limiting step in Negishi coupling: in a live “Pd-catalyzed Negishi coupling of ArI with Ar′ZnX”, the transmetalation was assigned as the rate-limiting step through kinetic investigation. Quantitative measurements of the transmetalation of ArZnX with [Ar–Pd–X] from this live Pd-catalyzed coupling reaction were obtained and the activation enthalpy is 11.3 kcal mol−1.
Negishi coupling, as one of the most powerful synthetic tools for C–C bond construction, has been widely applied in chemical synthesis.1–6 However, the mechanistic proposals on Negishi coupling are relatively speculative and the kinetic aspects remain obscure.7
The Pd-catalyzed Negishi coupling is generally believed to follow three fundamental steps: oxidative addition, transmetalation and reductive elimination.8 Among these three steps, the transmetalation occurs between an organozinc reagent and [RPdX] species, which is one of the most important ways to introduce σ-bonded carbon atoms into the coordination sphere of palladium and furnish the [R–Pd–R′] species.2,9 Obviously, revealing mechanistic insights into the transmetalation step of Negishi coupling is significant and urgent for better conducting these transformations in chemical synthesis.10–13
However, mechanistic studies on the transmetalation of Negishi coupling are relatively less reported compared with Suzuki coupling and Stille coupling,14–30 especially the transmetalation of the Ar1ZnX reagent with Ar2PdX species from “live” Pd-catalyzed biaryl-syntheses (Ar1–Ar2). In recent years, mechanistic studies on transmetalation of organozinc reagents have drawn more and more attention. For example, Espinet and coworkers continually reported their understanding of the transmetalation step of [Ralkyl–Pd–X] with MeZnX/Me2Zn through stoichiometric experiments under low temperature with special ligands and substrates.7,31,32 However, the stoichiometric experiments would become problematic as the transmetalation of [R–Pd–X] with an organozinc reagent is too rapid to be probed under “live” Negishi coupling reaction conditions. Therefore, the challenge remains in gaining mechanistic insights into the transmetalation of Negishi coupling under live catalytic reaction conditions. Herein, we document our recent results in quantitative measurements of the transmetalation of Ar1ZnX with [Ar2–Pd–X] through kinetic investigations from a live Pd-catalyzed Negishi coupling.
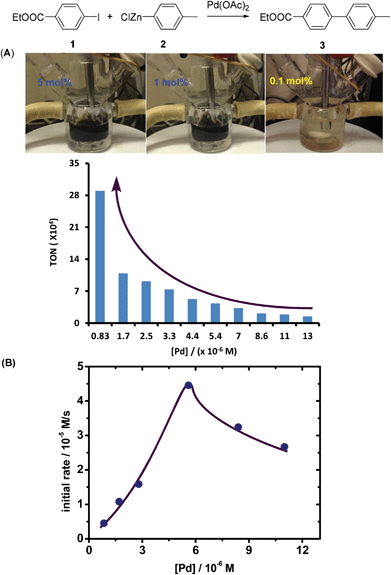
Our initial studies commenced from the curiosity about the real catalytic capability of Pd(OAc)2 in Negishi coupling. The Pd-catalyzed cross-coupling between ethyl 4-iodobenzoate (1) and p-MeC6H4ZnCl (2) using Pd(OAc)2 as the catalyst precursor was investigated. The reactions could proceed smoothly in quantitative yields with both 5 mol% and 1 mol% catalyst loadings. The TONs were 20 and 100, respectively. We also found that these reactions were very rapid (within 60 seconds) and clearly there was Pd-black formation shown in Fig. 1A when 5 mol% or 1 mol% Pd(OAc)2 was employed. However, when 0.1 mol% Pd(OAc)2 was employed, the reaction could proceed smoothly and no Pd-black was observed. It is very interesting that the TONs could reach up to 290![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 at 10 °C when the concentration of Pd was decreased (Fig. 1A). The continuous increase of TONs indicated the powerful catalytic potential.
000 at 10 °C when the concentration of Pd was decreased (Fig. 1A). The continuous increase of TONs indicated the powerful catalytic potential.
To our great surprise, when kinetic studies were performed using in situ IR, a strange [catalysts]-kinetic dependence was observed. As shown in Fig. 1B, when the reaction was performed using a lower concentration of the Pd catalyst (<8.0 × 10−6 M), the initial rates exhibited a positive dependence on the concentration of the catalyst. However, when the reaction was performed using a higher concentration of the Pd catalyst (≥8.0 × 10−6 M), a negative dependence on the concentration of the catalyst was determined. The impressive efficiency and unpredictable kinetic behavior in this Pd-catalyzed Negishi coupling inspired us to further probe the transformation in detail.
With this Pd-catalyzed cross-coupling between 1 and 2 as a model, further kinetic investigations were carried out. As mentioned above, when [Pd] ≥ 8.0 × 10−6 M, increasing the concentration of Pd would result in lower reaction rates, revealing that some Pd species might be out of the catalytic cycle and decompose under these conditions. Thus, the reaction performed at high concentration of Pd could not be employed for kinetic studies. Subsequently, reactions with different concentrations of Pd at 10 °C ([Pd] < 8.0 × 10−6 M) were carried out. As shown in Fig. 2, when plotting the initial rates vs. [Pd], a linear relationship could be determined, suggesting that the reaction exhibits first-order kinetic behavior on the concentration of Pd under those conditions. In this regard, the following kinetic experiments were performed under the obtained Pd concentration range ([Pd] ≤ 7.0 × 10−6 M).
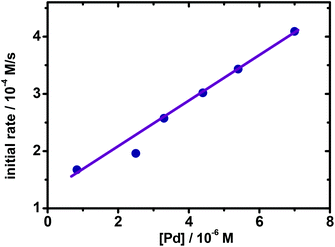 | ||
| Fig. 2 Plotting initial rates vs. [Pd]. Reaction conditions: 1 (0.25 M in THF), 2 (0.34 M in THF, 4.0 mL), Pd(OAc)2 (8.2 × 10−7–7.2 × 10−6 M in THF), 10 °C. | ||
The subsequent reactions with different concentrations of ArI were performed and monitored by in situ IR. As shown in Fig. 3, the reactions show similar reaction rates, suggesting that the reactions exhibited zero order dependence on [ArI]. Meanwhile, the kinetic behavior of ArZnCl was examined. It was observed that plotting initial rates vs. [ArZnCl] yielded a line, revealing that the reaction exhibited a first-order dependence on [ArZnCl] (Fig. 4).
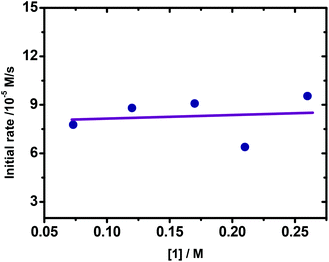 | ||
| Fig. 3 Plotting initial rates vs. [ArI]. Reaction conditions: 1 (0.073–0.26 M in THF), 2 (0.33 M in THF, 4.0 mL), Pd(OAc)2 (5.2 × 10−6 M in THF), −10 °C. | ||
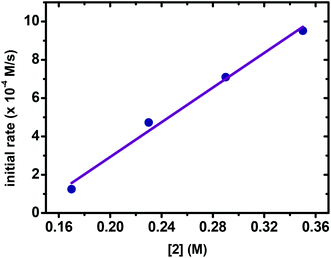 | ||
| Fig. 4 Plotting initial rates vs. [ArZnCl]. Reaction conditions: 1 (0.082 M in THF), 2 (0.17–0.35 M in THF, 6.0 mL), Pd(OAc)2 (6.6 × 10−6 M in THF), −10 °C. | ||
On the basis of the kinetic results above, the rate law of this Pd-catalyzed cross-coupling between ethyl 4-iodobenzoate and p-Me C6H4ZnCl could be derived as: rate = k × [Pd] × [ArZnCl]. Zero-order kinetic dependence on [1] and first-order dependence on [2] suggest that transmetalation might be the rate-limiting step.
Subsequently, the rates of this Negishi coupling using different para-substituted (R = MeO, Me, H, Cl) arylzinc reagents were measured. Arylzinc reagents with electron-donating substituents reacted faster than electron-deficient arylzinc reagents. As shown in Fig. 5, a linear free energy relationship was observed with a calculated ρ-value of −1.07. The negative ρ-value signified that in the rate limiting step, arylzinc should be involved in the transition state and a positive charge buildup exists in the phenyl ring of arylzinc, further supporting that the transmetalation is the rate-limiting step.
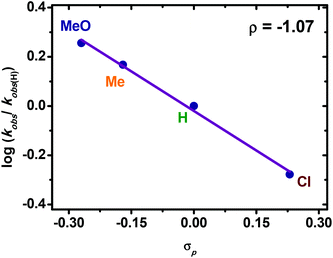 | ||
| Fig. 5 Hammett plot of Pd-catalyzed Negishi coupling between 1 and ArZnX. Reaction conditions: 1 (0.12 M in THF), ArZnX (0.31 M in THF, 4.0 mL), Pd(OAc)2 (5.2 × 10−6 M in THF), −10 °C. | ||
Having confirmed that transmetalation was the rate-limiting step, we were allowed to gain further understanding of the transmetalation of arylzinc. Then the activation parameters were determined through Eyring analysis by measuring the reaction rates from −20 to 30 °C. As shown in Fig. 6, the activation enthalpy ΔH≠ was measured to be 11.3 kcal mol−1.
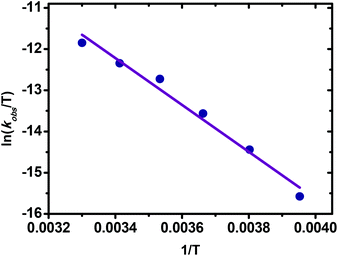 | ||
| Fig. 6 Eyring plots of Pd-catalyzed Negishi coupling between 1 and 2. Reaction conditions: 1 (0.12 M in THF), 2 (0.25 M in THF, 4.0 mL), Pd(OAc)2 (5.2 × 10−6 M in THF), −20–30 °C. | ||
Conclusions
In summary, using a Pd-catalyzed Negishi coupling between ethyl 4-iodobenzoate and p-MeC6H4ZnCl as the model, transmetalation has been identified as the rate-limiting step through kinetic investigations. Quantitative measurements have been obtained with the activation energy at a value of 11.3 kcal mol−1. This rate-limiting transmetalation could rationalize the high efficiency of this transformation, which will provide an implication for further development in increasing TONs and TOFs.Acknowledgements
This work was supported by the 973 Program (2012CB725302), the National Natural Science Foundation of China (21025206 and 21272180), and the Program for Changjiang Scholars and Innovative Research Team in University (IRT1030) and the Research Fund for the Doctoral Program of Higher Education of China (20120141130002).Notes and references
- Organozinc Reagents: A Practical Approach, ed. P. Knochel and P. Joned, 1999 Search PubMed.
- E.-i. Negishi and A. d. Meijere, Handbook of organopalladium chemistry for organic synthesis, Wiley-Interscience, New York, 2002 Search PubMed.
- X.-F. Wu, P. Anbarasan, H. Neumann and M. Beller, Angew. Chem., Int. Ed., 2010, 49, 9047–9050 CrossRef CAS PubMed.
- P. Knochel and R. D. Singer, Chem. Rev., 1993, 93, 2117–2188 CrossRef CAS.
- R. Jana, T. P. Pathak and M. S. Sigman, Chem. Rev., 2011, 111, 1417–1492 CrossRef CAS PubMed.
- V. B. Phapale and D. J. Cardenas, Chem. Soc. Rev., 2009, 38, 1598–1607 RSC.
- J. A. Casares, P. Espinet, B. Fuentes and G. Salas, J. Am. Chem. Soc., 2007, 129, 3508–3509 CrossRef CAS PubMed.
- A. d. Meijere and F. Diederich, Metal-catalyzed cross-coupling reactions, Wiley-VCH, Weinheim, 2nd, completely rev. and enlarged edn, 2004 Search PubMed.
- O. F. Wendt, Curr. Org. Chem., 2007, 11, 1417–1433 CrossRef CAS.
- Q. Liu, Y. Lan, J. Liu, G. Li, Y.-D. Wu and A. Lei, J. Am. Chem. Soc., 2009, 131, 10201–10210 CrossRef CAS PubMed.
- P. Ribagnac, M. Blug, J. Villa-Uribe, G. X.-F. Le, C. Gosmini and N. Mezailles, Chem.–Eur. J., 2011, 17, 14389–14393 CrossRef CAS PubMed.
- L. Jin and A. Lei, Org. Biomol. Chem., 2012, 10, 6817–6825 CAS.
- A. B. Gonzalez-Perez, R. Alvarez, O. N. Faza, L. A. R. de and J. M. Aurrecoechea, Organometallics, 2012, 31, 2053–2058 CrossRef CAS.
- P. Espinet and A. M. Echavarren, Angew. Chem., Int. Ed., 2004, 43, 4704–4734 CAS.
- A. L. Casado and P. Espinet, J. Am. Chem. Soc., 1998, 120, 8978–8985 CrossRef CAS.
- A. L. Casado, P. Espinet and A. M. Gallego, J. Am. Chem. Soc., 2000, 122, 11771–11782 CrossRef CAS.
- A. Ricci, F. Angelucci, M. Bassetti and S. C. Lo, J. Am. Chem. Soc., 2002, 124, 1060–1071 CrossRef CAS PubMed.
- D. Canseco-Gonzalez, V. Gomez-Benitez, S. Hernandez-Ortega, R. A. Toscano and D. Morales-Morales, J. Organomet. Chem., 2003, 679, 101–109 CrossRef CAS.
- J. Louie and J. F. Hartwig, J. Am. Chem. Soc., 1995, 117, 11598–11599 CrossRef CAS.
- A. Nova, G. Ujaque, F. Maseras, A. Lledos and P. Espinet, J. Am. Chem. Soc., 2006, 128, 14571–14578 CrossRef CAS PubMed.
- M. H. Perez-Temprano, A. Nova, J. A. Casares and P. Espinet, J. Am. Chem. Soc., 2008, 130, 10518–10520 CrossRef CAS PubMed.
- A. Ariafard, Z. Y. Lin and I. J. S. Fairlamb, Organometallics, 2006, 25, 5788–5794 CrossRef CAS.
- Y. Nishihara, H. Onodera and K. Osakada, Chem. Commun., 2004, 192–193 RSC.
- B. Crociani, S. Antonaroli, A. Marini, U. Matteoli and A. Scrivanti, Dalton Trans., 2006, 2698–2705 RSC.
- C. Adamo, C. Amatore, I. Ciofini, A. Jutand and H. Lakmini, J. Am. Chem. Soc., 2006, 128, 6829–6836 CrossRef CAS PubMed.
- B. P. Carrow and J. F. Hartwig, J. Am. Chem. Soc., 2011, 133, 2116–2119 CrossRef CAS PubMed.
- N. Miyaura, J. Organomet. Chem., 2002, 653, 54–57 CrossRef CAS.
- K. Osakada, H. Onodera and Y. Nishihara, Organometallics, 2005, 24, 190–192 CrossRef CAS.
- C. Amatore, A. Jutand and D. G. Le, Chem.–Eur. J., 2011, 17, 2492–2503 CrossRef CAS PubMed.
- X. He, S. Zhang, Y. Guo, H. Wang and G. Lin, Organometallics, 2012, 31, 2945–2948 CrossRef CAS.
- B. Fuentes, M. Garcia-Melchor, A. Lledos, F. Maseras, J. A. Casares, G. Ujaque and P. Espinet, Chem.–Eur. J., 2010, 16, 8596–8599 CrossRef CAS PubMed.
- M. Garcia-Melchor, B. Fuentes, A. Lledos, J. A. Casares, G. Ujaque and P. Espinet, J. Am. Chem. Soc., 2011, 133, 13519–13526 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures and kinetic parameters detected by a Mettler Toledo ReactIR™ 15 spectrometer. See DOI: 10.1039/c3qo00021d |
| This journal is © the Partner Organisations 2014 |