Aerobic oxidative C–B bond cleavage of arylboronic acids mediated by methylhydrazines†
Wei
Ding
,
Jia-Rong
Chen
*,
You-Quan
Zou
,
Shu-Wen
Duan
,
Liang-Qiu
Lu
and
Wen-Jing
Xiao
*
CCNU–UOttawa Joint Research Centre, Key Laboratory of Pesticide & Chemical Biology, Ministry of Education, College of Chemistry, Central China Normal University, 152 Luoyu Road, Wuhan, Hubei 430079, China. E-mail: chenjiarong@mail.ccnu.edu.cn; wxibao@mail.ccnu.edu.cn; Fax: +86 27 67862041; Tel: +86 27 67862041
First published on 27th January 2014
Abstract
The aerobic oxidative C–B bond cleavage and ipso-hydroxylation of arylboronic acids mediated by methylhydrazine have been developed under metal-free conditions. This transformation affords structurally diverse phenols in good to excellent yields (26 examples, 71–99%).
Boronic acids are valuable synthetic precursors and have attracted considerable research interest from chemical society.1 Among the various transformations of boronic acids, the oxidative C–B bond cleavage and the ipso-hydroxylation of arylboronic acid have recently been extensively investigated to prepare synthetically useful and biologically important phenols.2 In this context, great efforts have been devoted to the search for new and more efficient terminal oxidants for this transformation. Representative examples include aqueous hydrogen peroxide,3 sodium perborate,4 oxone,5 hydroxylamine,6N-oxide,7m-chloroperoxybenzoic acid,8 and tert-butyl hydroperoxide.9
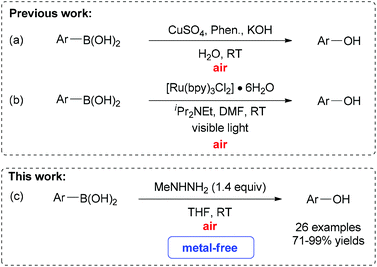
Compared to these oxidants, molecular oxygen and air have been well established as highly environmentally friendly and inexpensive oxidants for oxidation reactions.10 Notably, oxygen and air have been successfully employed as oxidants for the oxidation reaction of C–B bonds.11 For instance, Wang, Hu and co-workers have documented an elegant example of the CuSO4-catalyzed aerobic oxidative hydroxylation of arylboronic acids in the presence of KOH (Scheme 1a).11g Thereafter, other metal catalysts, such as a variety of copper or palladium complexes, have been utilized in this kind of transformation under air or oxygen atmospheres.11c–f Alternatively, our group has recently developed a visible light induced aerobic oxidative cleavage of arylboronic acids to phenols with [Ru(bpy)3Cl2]·6H2O as a photoredox catalyst (Scheme 1b).11a In this reaction, the key intermediate superoxide radical anion, generated from oxygen by photoredox catalysis, was proposed to act as the highly active terminal oxidant. Although impressive advances have been achieved, these procedures usually need to use transition-metals as the catalysts. Therefore, the search for more efficient and milder methods for the conversion of structurally diverse arylboronic acids into their corresponding phenols with oxygen or air as the oxidant remain highly desirable.11b As part of our continuing research program on the chemistry of arylboronic acids11a and hydrazones,12 we recently developed a novel protocol for the oxidative cleavage of aryl C–B bonds mediated by methylhydrazine in an open flask at room temperature under metal-free conditions. Herein, we describe the preliminary results in this communication (Scheme 1c).13
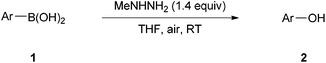
Initially, 4-methoxyphenylboronic acid 1a was chosen as the model substrate for examining the feasibility of the aerobic oxidation reaction and optimizing the reaction conditions (Table 1). To our delight, the desired product 4-methoxyphenol 2a could be obtained in a 90% yield under an oxygen atmosphere in the presence of MeNHNH2 (40% aq., 1.4 equiv.)14,15 (Table 1, entry 1). Notably, the reaction could also proceed smoothly in an open flask (under an air atmosphere) with a comparative yield within 7 h (Table 1, entry 2). Encouraged by these results, we then examined other reaction parameters to further improve the chemical yield. It was found that this transformation could proceed well in many commonly used organic solvents (Table 1, entries 3–11). Complete conversion was achieved in THF after 7 h to give the product 2a in a 94% isolated yield (Table 1, entry 8). Control experiments showed that both MeNHNH2 and air were essential for this process; otherwise, no desired product was observed when either of them was omitted from the reaction system (Table 1, entries 12–13). Screening of other reaction parameters led to no further improvement.16 Accordingly, the optimal reaction conditions were identified as follows: 1a (0.5 mmol) and MeNHNH2 (40% aq; 1.4 equiv.) in THF (0.017 M) at room temperature under an air atmosphere (Table 1, entry 8).
| Entry | Solvent | Time (h) | Yieldb (%) |
|---|---|---|---|
| a Unless otherwise noted, the reaction of 1a (0.5 mmol) with MeNHNH2 (40% aq., 1.4 equiv.) was carried out in the solvent (30 mL) at room temperature under an air atmosphere. b Isolated yield. c The reaction was carried out under an O2 atmosphere. d Performed without MeNHNH2. e Performed under an N2 atmosphere. n.r. = no reaction. | |||
| 1c | Dioxane | 7 | 90 |
| 2 | Dioxane | 7 | 89 |
| 3 | CH2Cl2 | 24 | 65 |
| 4 | MeOH | 24 | 59 |
| 5 | EtOH | 24 | 55 |
| 6 | DMF | 7 | 67 |
| 7 | Toluene | 24 | 72 |
| 8 | THF | 7 | 94 |
| 9 | Et2O | 24 | 81 |
| 10 | TBME | 24 | 91 |
| 11 | iPr2O | 24 | 81 |
| 12d | THF | 12 | n.r. |
| 13e | THF | 12 | Trace |
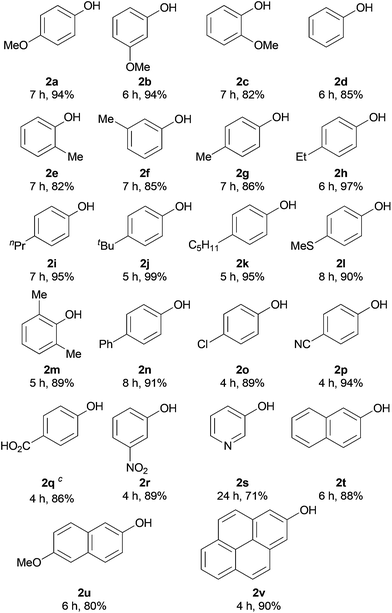
With the optimal reaction conditions in hand, we then examined the substrate scope of this transformation. As shown in Table 2, the reaction appears to be very general with respect to a variety of arylboronic acids and gives their corresponding phenols in good to excellent yields. Variation of the electronic properties and the position of the substituents on the aromatic ring of arylboronic acids has little effect on this transformation, affording the corresponding products 2a–r in 82–99% yields (Table 2). Compared to the traditional procedures for phenol preparation via the nucleophilic aromatic substitution of activated aryl halides,17 the present metal-free method showed a higher efficiency in the cases of electron-rich phenols 2a–m. In addition, the heteroaryl boronic acid 1s and polycyclic substrates 1t–v were also well tolerated to afford their corresponding phenols 2s–v in 71–90% isolated yields.
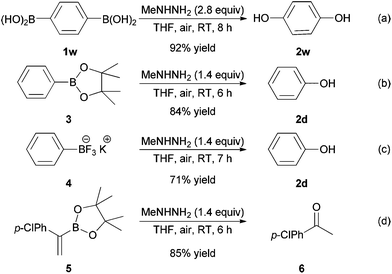
Notably, 1,4-phenylenediboronic acid 1w proved to be suitable for this process, generating hydroquinone 2w in a 92% yield (Scheme 2a). Both phenylboronic pinacol ester 3 and potassium phenyltrifluoroborate 4 could also undergo the desired oxidative cleavage easily to provide the product 2d in 84% and 71% yields, respectively (Scheme 2b and c). Interestingly, under the standard conditions, the vinylboronic ester 5 was readily transformed into the ketone 6 through an oxidative cleavage/ipso-hydroxylation/isomerization sequence in an 85% yield (Scheme 2d).
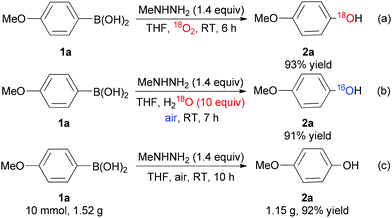
To gain more insight into the possible mechanism, we also carried out two 18O-labelling experiments by using 18O2 and H218O (Scheme 3a and b). These results show that the oxygen atom of the hydroxyl oxygen atom in the product originated from molecular oxygen rather than water. Moreover, the reaction could be carried out on a gram scale without any loss of efficiency (Scheme 3c).
In conclusion, we have developed a methylhydrazine-mediated aerobic oxidative cleavage of arylboronic acids to their corresponding phenols.18 This mild and metal-free strategy shows a wide substrate scope and high functional group tolerance with respect to arylboronic acids. Further detailed mechanistic investigations into this reaction as well as applications of this system to other processes are currently underway in our laboratory.
Acknowledgements
We are grateful to the National Natural Science Foundation of China (no. 21272087, 21202053 and 21232003) and the National Basic Research Program of China (2011CB808603) for support of this research.Notes and references
- (a) A. Suzuki, Angew. Chem., Int. Ed., 2011, 50, 6722 CrossRef CAS PubMed; (b) N. Fujita, S. Shinkai and T. D. James, Chem.–Asian J., 2008, 3, 1076 CrossRef CAS PubMed; (c) D. G. Hall, Boronic Acids: Preparation and Applications in Organic Synthesis and Medicine, Wiley-VCH, Weinheim, 2007 Search PubMed; (d) A. Suzuki, J. Organomet. Chem., 1999, 576, 147 CrossRef CAS; (e) F. Diederich and P. J. Stang, Metal-Catalyzed Cross-Coupling Reactions, Wiley-VCH, Weinheim, 1998 Search PubMed.
- (a) A. Tlili, N. Xia, F. Monnier and M. Taillefer, Angew. Chem., Int. Ed., 2009, 48, 8725 CrossRef CAS PubMed; (b) R. Bal, M. Tada, T. Sasaki and Y. Iwasawa, Angew. Chem., Int. Ed., 2006, 45, 448 CrossRef CAS PubMed; (c) J. H. P. Tyman, Synthetic and Natural Phenols, Elsevier, New York, 1996 Search PubMed; (d) Z. Rappoport, The Chemistry of Phenols, Wiley-VCH, Weinheim, 2003 CrossRef.
- (a) N. Mulakayala, Ismail, K. M. Kumar, R. K. Rapolu, B. Kandagatla, P. Rao, S. Oruganti and M. Pal, Tetrahedron Lett., 2012, 53, 6004 CrossRef CAS PubMed; (b) A. Gogoi and U. Bora, Synlett, 2012, 1079 CAS; (c) G. K. S. Prakash, S. Chacko, C. Panja, T. E. Thomas, L. Gurung, G. Rasul, T. Mathew and G. A. Olah, Adv. Synth. Catal., 2009, 351, 1567 CrossRef CAS; (d) J. Simon, S. Salzbrunn, G. K. S. Prakash, N. A. Petasis and G. A. Olah, J. Org. Chem., 2001, 66, 633 CrossRef CAS; (e) H. G. Kuivila and A. G. Armour, J. Am. Chem. Soc., 1957, 79, 5659 CrossRef CAS; (f) M. F. Hawthorne, J. Org. Chem., 1957, 22, 1001 CrossRef CAS; (g) H. G. Kuivila, J. Am. Chem. Soc., 1955, 77, 4014 CrossRef CAS; (h) H. G. Kuivila, J. Am. Chem. Soc., 1954, 76, 870 CrossRef CAS; (i) A. D. Ainley and F. Challenger, J. Chem. Soc., 1930, 2171 RSC.
- (a) P. Fontani, B. Carboni, M. Vaultier and G. Maas, Synthesis, 1991, 605 CrossRef CAS; (b) E. J. Nanni and R. Ray, J. Am. Chem. Soc., 1980, 102, 7591 CrossRef CAS.
- (a) R. E. Maleczka, Jr., F. Shi, D. Holmes and M. R. Smith, III, J. Am. Chem. Soc., 2003, 125, 7792 CrossRef PubMed; (b) B. R. Travis, B. P. Ciaramitaro and B. Borhan, Eur. J. Org. Chem., 2002, 3429 CrossRef CAS; (c) K. S. Webb and D. Levy, Tetrahedron Lett., 1995, 36, 5117 CAS.
- E. Kianmehr, M. Yahyaee and K. Tabatabai, Tetrahedron Lett., 2007, 48, 2713 CrossRef CAS PubMed.
- C. Zhu, R. Wang and J. R. Falck, Org. Lett., 2012, 14, 3494 CrossRef CAS PubMed.
- D.-S. Chen and J.-M. Huang, Synlett, 2013, 499 CAS.
- S.-M. Guo, L. Lu and H. Cai, Synlett, 2013, 1712 CrossRef CAS PubMed.
- For selected reviews, see: (a) J. Piera and J. E. Bäckvall, Angew. Chem., Int. Ed., 2008, 47, 3506 CrossRef CAS PubMed; (b) T. Matsumoto, M. Ueno, N.-W. Wang and S. Kobayashi, Chem.–Asian J., 2008, 3, 196 CrossRef CAS PubMed; (c) M. J. Schultz and M. S. Sigman, Tetrahedron, 2006, 62, 8227 CrossRef CAS PubMed; (d) T. Punniyamurthy, S. Velusamy and J. Iqbal, Chem. Rev., 2005, 105, 2329 CrossRef CAS PubMed; (e) S. S. Stahl, Angew. Chem., Int. Ed., 2004, 43, 3400 CrossRef CAS PubMed; (f) T. Mallat and A. Baiker, Chem. Rev., 2004, 104, 3037 CrossRef CAS PubMed; (g) B.-Z. Zhan and A. Thompson, Tetrahedron, 2004, 60, 2917 CrossRef CAS PubMed; (h) M. S. Sigman and M. J. Schultz, Org. Biomol. Chem., 2004, 2, 2551 RSC; (i) R. A. Sheldon, I. W. C. E. Arends, G. J. T. Brink and A. Dijksman, Acc. Chem. Res., 2002, 35, 774 CrossRef CAS PubMed.
- (a) Y.-Q. Zou, J.-R. Chen, X.-P. Liu, L.-Q. Lu, R. L. Davis, K. A. Jørgensen and W.-J. Xiao, Angew. Chem., Int. Ed., 2012, 51, 784 CrossRef CAS PubMed; (b) P. Kaewmati, E. Somsook, R. N. Dhital and H. Sakurai, Tetrahedron Lett., 2012, 53, 6104 CrossRef CAS PubMed; (c) K. Inamoto, K. Nozawa, M. Yonemoto and Y. Kondo, Chem. Commun., 2011, 47, 11775 RSC; (d) B. Kaboudin, Y. Abedi and T. Yokomatsu, Eur. J. Org. Chem., 2011, 6656 CrossRef CAS; (e) H.-J. Yang, Y. Li, M. Jiang, J.-M. Wang and H. Fu, Chem.–Eur. J., 2011, 17, 5652 CrossRef CAS PubMed; (f) A. D. Chowdhury, S. M. Mobin, S. Mukherjee, S. Bhaduri and G. K. Lahiri, Eur. J. Inorg. Chem., 2011, 3232 CrossRef CAS; (g) J.-M. Xu, X.-Y. Wang, C.-W. Shao, D.-Y. Su, G.-L. Cheng and Y.-F. Hu, Org. Lett., 2010, 12, 1964 CrossRef CAS PubMed . Electron-mediated hydroxylation of arylboronic acids: ; (h) H.-L. Qi, D.-S. Chen, J.-S. Ye and J.-M. Huang, J. Org. Chem., 2013, 78, 7482 CrossRef CAS PubMed; (i) H. Jiang, L. Lykke, S. U. Pedersen, W.-J. Xiao and K. A. Jørgensen, Chem. Commun., 2012, 48, 7203 RSC; (j) K. Hosoi, Y. Kuriyama, S. Inagi and T. Fuchigami, Chem. Commun., 2010, 46, 1284 RSC.
- X.-Q. Hu, J.-R. Chen, S. Gao, B. Feng, L.-Q. Lu and W.-J. Xiao, Chem. Commun., 2013, 49, 7905 RSC.
- During the preparation of this manuscript, Jiao developed an elegant chemoselective coupling and oxygenation of olefins, affording the corresponding alcohols, ketones, and diketones in generally good yields, see: Y. Su, X. Sun, G. Wu and N. Jiao, Angew. Chem., Int. Ed., 2013, 52, 9808 CrossRef CAS PubMed.
- H. Kawai, S. Okusu, Z. Yuan, E. Tokunaga, A. Yamano, M. Shiro and N. Shibata, Angew. Chem., Int. Ed., 2013, 52, 2221 CrossRef CAS PubMed.
- (a) T. Taniguchi, Y. Sugiura, H. Zaimoku and H. Ishibashi, Angew. Chem., Int. Ed., 2010, 49, 10154 CrossRef CAS PubMed; (b) E. G. Samsel and J. K. Kochi, Inorg. Chem., 1986, 25, 2450 CrossRef CAS; (c) E.-M. Kosower, Acc. Chem. Res., 1971, 4, 193 CrossRef CAS; (d) H. Aebi, B. Dewald and H. Suter, Helv. Chim. Acta, 1965, 48, 656 CrossRef CAS.
- Please see the ESI† for more details.
- (a) C. Hoarau and T. R. R. Pettus, Synlett, 2003, 127 CAS; (b) P. Hanson, J. R. Jones, A. B. Taylor, P. H. Walton and A. W. Timms, J. Chem. Soc., Perkin Trans. 2, 2002, 1135 RSC; (c) T. George, R. Mabon, G. Sweeney, J. B. Sweeney and A. Tavassoli, J. Chem. Soc., Perkin Trans. 1, 2000, 2529 RSC.
- Methylhydrazine-mediated aerobic oxidative cleavage of arylboronic acids. General procedure: a 50 mL round-bottom flask was charged with aryl boronic acid 1 (0.5 mmol), MeNHNH2 (40% aq. 1.4 equiv., 80 μL) (potentially dangerous, please be careful!!!) and THF (0.017 M, 30 mL). Then, the reaction mixture was stirred at room temperature in an open flask. After the reaction was completed (monitored by TLC analysis), the mixture was concentrated under reduced pressure and purified by flash chromatography on silica gel (eluted with ethyl acetate–petroleum ether = 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) to give the desired product 2. Analytical data of 4-methoxyphenol (2a): colorless solid; 1H NMR (600 MHz, CDCl3) δ (ppm) 6.80–6.76 (4 H, m), 4.65 (1 H, s), 3.77 (3 H, s). 13C NMR (100 MHz, CDCl3) δ (ppm) 153.39, 149.45, 116.07, 114.88, 55.83. MS: m/z = 124.1 (M+).
5) to give the desired product 2. Analytical data of 4-methoxyphenol (2a): colorless solid; 1H NMR (600 MHz, CDCl3) δ (ppm) 6.80–6.76 (4 H, m), 4.65 (1 H, s), 3.77 (3 H, s). 13C NMR (100 MHz, CDCl3) δ (ppm) 153.39, 149.45, 116.07, 114.88, 55.83. MS: m/z = 124.1 (M+).
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and compound characterisation data. See DOI: 10.1039/c3qo00026e |
| This journal is © the Partner Organisations 2014 |