Highly enantioselective oxidative tandem cyclization reaction: a chiral ligand and an anion cooperatively control stereoselectivity†
Yu-Ping
He
,
Hua
Wu
,
Lue
Xu
,
Yong-Liang
Su
and
Liu-Zhu
Gong
*
Hefei National Laboratory for Physical Sciences at the Microscale and Department of Chemistry, University of Science and Technology of China, Hefei, 230026, China
First published on 25th March 2014
Abstract
The unprecedented combination of a palladium(II) complex with a chiral Bu-QUOX ligand and a chiral phosphoric acid enables the highly efficient asymmetric oxidative tandem cyclization of N-(2,2-disubstituted hex-5-en-1-yl)acrylamides, providing a straightforward method to access chiral 6,5-bicyclic aza-heterocycles in moderate to good yields and with excellent enantioselectivities.
The palladium(II)-catalyzed alkene functionalization via nucleopalladation of the carbon–carbon double bond has enabled numerous transformations, providing efficient entries to diverse families of either oxygenous or N-containing molecules, in particular, heterocycles.1 However, asymmetric Pd(II)-catalyzed functionalization of alkenes2 has met with much less success than chiral palladium(0) complex-catalyzed enantioselective reactions.3 The limited number of chiral ligands compatible with oxidizing conditions,4,5 which are mostly required in Pd(II)-catalysis, has been considered one of the key factors to suppress the reality of a highly enantioselective Pd(II)-catalyzed functionalization of alkenes.
The palladium(II)-catalyzed enantioselective tandem Wacker-type oxidation/cyclization reactions are capable of directly generating complex polycyclic products, which have been commonly incorporated into natural alkaloids as core structural elements.6 Sasai established the first highly enantioselective Pd(II)-catalyzed oxidative tandem cyclization reactions of alkenyl alcohols affording bicyclic products with excellent levels of enantioselectivity (up to 95% ee).6e Yang and coworkers accomplished a Pd(II)-catalyzed enantioselective oxidative tandem reaction of nitrogen atom-based nucleophiles using readily available (–)-sparteine as a chiral ligand, generating dihydro-1H-pyrrolo[1,2-a]indol-3(2H)-one derivatives in good yields and high enantioselectivities.6f Thereafter, the same group further improved the reaction using Bu-Quox as a chiral ligand (Scheme 1, eqn (1)).6g Recently, Sasai presented a Pd(II)-SPRIX-catalyzed enantioselective cascade intramolecular C–N/C–C bond formation reaction of N-(2,2-disubstituted pent-4-en-1-yl)acrylamides for the synthesis of tetrahydro-1H-pyrrolizin-3(2H)-ones in good yields, but with moderate enantioselectivities (Scheme 1, eqn (2)).6i In contrast to these well-established methods available to access fused 5,5-bicyclic N-containing skeletons, the Pd(II)-catalyzed enantioselective oxidative tandem cyclization reaction has been much less developed for generation of fused 6,5-bicyclic aza-heterocycles.
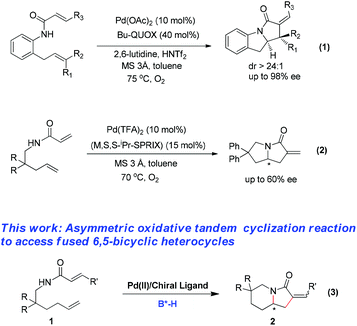 | ||
| Scheme 1 Palladium(II) catalyzed enantioselective oxidative tandem cyclization to access fused 5,5-bicyclic nitrogenous skeletons. | ||
Recently, the combination of metal catalysis with organocatalysis has been a robust strategy for the creation of enantioselective transformations.7 In particular, chiral Brønsted acids have been found to be highly compatible with palladium catalysts.8,9 Notably, the use of a chiral phosphoric acid alone was able to control the stereoselectivity in some typical Pd(II) catalyzed reactions.8 Thus, we envisioned that the use of a chiral phosphoric acid as a co-catalyst would be able to modulate the enantioselectivity of the palladium(II)-catalyzed tandem Wacker-type oxidation/cyclization reactions. Herein, we describe a highly enantioselective Wacker-type oxidative transformation catalyzed by a chiral palladium(II) complex and a chiral phosphoric acid, providing a straightforward entry to chiral 6,5-bicyclic heterocycles (eqn (3)) (Scheme 1).
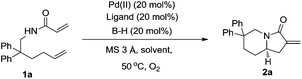
Our initial study began with a reaction of N-(2,2-diphenylhex-5-en-1-yl)acrylamide 1a in the presence of 20 mol% Pd(OAc)2, 20 mol% iPr-quinolineoxazoline 3a and 20 mol% of a phosphoric acid 4a along with 3 Å MS and molecular oxygen (balloon) as the sole oxidant in toluene at 50 °C for 20 h (Table 1, entry 1). To our delight, the desired product was isolated in 21% yield with a promising enantiomeric ratio of 77.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22.5. It should be noted that a small amount of side product was formed and 65% of starting material 1a was recovered. A further improvement in both yield (25%) and enantioselectivity (79.5
22.5. It should be noted that a small amount of side product was formed and 65% of starting material 1a was recovered. A further improvement in both yield (25%) and enantioselectivity (79.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
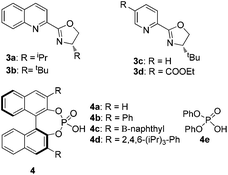
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20.5) could be achieved upon exploiting 3b as a ligand (Table 1, entries 2–4). Next, various chiral phosphoric acids 4b–4d derived from 3,3′-disubstituted BINOLs (Fig. 1). were evaluated (entries 5–7). Among them, (S)-trip-PA 4d turned out to be the preeminent catalyst and was able to provide 2a with 43% yield and 97.5
20.5) could be achieved upon exploiting 3b as a ligand (Table 1, entries 2–4). Next, various chiral phosphoric acids 4b–4d derived from 3,3′-disubstituted BINOLs (Fig. 1). were evaluated (entries 5–7). Among them, (S)-trip-PA 4d turned out to be the preeminent catalyst and was able to provide 2a with 43% yield and 97.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 er (entry 7). Notably, the use of (R)-trip-PA as a co-catalyst gave a lower yield and enantioselectivity than its enantiomer 4d, suggesting that (S)-trip-PA acts as a matched cocatalyst (entry 8 vs. 7). Although the acidity of achiral Brønsted acids, to some degree, affected the reaction performance, none of them was able to provide better results than chiral PA 4d (entries 9–11 vs. 7). The palladium(II) source also exerted a great impact on the stereochemical outcomes (entries 7 and 12–13). When Pd(CF3COO)2 was used, the cyclic product 2a was generated with 50% yield and 98
2.5 er (entry 7). Notably, the use of (R)-trip-PA as a co-catalyst gave a lower yield and enantioselectivity than its enantiomer 4d, suggesting that (S)-trip-PA acts as a matched cocatalyst (entry 8 vs. 7). Although the acidity of achiral Brønsted acids, to some degree, affected the reaction performance, none of them was able to provide better results than chiral PA 4d (entries 9–11 vs. 7). The palladium(II) source also exerted a great impact on the stereochemical outcomes (entries 7 and 12–13). When Pd(CF3COO)2 was used, the cyclic product 2a was generated with 50% yield and 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 er (entry 13). Conducting the reaction in other solvents was unable to enhance either the yield or the enantioselectivity (entries 14 and 15). Significantly, both yield (24%) and enantioselectivity (92
2 er (entry 13). Conducting the reaction in other solvents was unable to enhance either the yield or the enantioselectivity (entries 14 and 15). Significantly, both yield (24%) and enantioselectivity (92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) were diminished when the chiral ligand 3b was used alone (entry 16), suggesting that a synergistic effect exists between the chiral ligand and phosphate.
8) were diminished when the chiral ligand 3b was used alone (entry 16), suggesting that a synergistic effect exists between the chiral ligand and phosphate.
| Entry | Ligand | B-H | Yieldb (%) | erc |
|---|---|---|---|---|
| a Unless indicated otherwise, the reaction of 1a (0.1 mmol) was carried out in toluene (1 mL) at 50 °C in the presence of Pd(OAc)2 (20 mol%), ligand ( 20 mol%), Brønsted acid (20 mol%) and 3 Å (50 mg) under O2 (balloon) for 20 h. b Isolated yield. c Determined by HPLC. d Pd(MeCN)4(BF4)2 was used. e Pd(CF3COO)2 was used. f The solvent was 1,4-dixone. g The solvent was chlorobenzene. | ||||
| 1 | 3a | (S)-4a | 21 | 77.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22.5 22.5 |
| 2 | 3b | (S)-4a | 25 | 79.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20.5 20.5 |
| 3 | 3c | (S)-4a | 27 | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
| 4 | 3d | (S)-4a | 29 | 72.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27.5 27.5 |
| 5 | 3b | (S)-4b | 42 | 93.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.5 6.5 |
| 6 | 3b | (S)-4c | 38 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
| 7 | 3b | (S)-4d | 43 | 97.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 2.5 |
| 8 | 3b | (R)-4d | 37 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
| 9 | 3b | 4e | 42 | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
| 10 | 3b | PhCOOH | 10 | 66.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33.5 33.5 |
| 11 | 3b | p-TSA | 45 | 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
| 12 | 3b | (S)-4d | 35 | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15d 15d |
| 13 | 3b | (S)-4d | 50 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2e 2e |
| 14 | 3b | (S)-4d | 40 | 86.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13.5e,f 13.5e,f |
| 15 | 3b | (S)-4d | 38 | 96.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5e,g 3.5e,g |
| 16 | 3b | — | 24 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8e 8e |
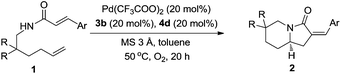
With the optimal conditions in hand, the generality and the substrate scope of the enantioselective oxidative tandem cyclization reaction were explored. When N-(2,2-diphenylhex-5-en-1-yl)cinnamamide 1b was employed under the optimized conditions, the target product 2b was obtained in 52% yield with 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 er. Substrates 1c–1j with different substituents at the phenyl group of cinnamamide were able to undergo the oxidative tandem cyclization reaction to give the corresponding 2c–2j in moderate to good yields with high levels of enantioselectivities (entries 2–9). Basically, the electronic feature of substituents exerts an obvious effect on the reaction performance (entries 4–8) while the substitution pattern of the cinnamamide moiety has little influence on the stereoselectivity (entry 2 vs. 7 and 3 vs. 6). A fairly good yield (65%) and high enantioselectivity (93
4 er. Substrates 1c–1j with different substituents at the phenyl group of cinnamamide were able to undergo the oxidative tandem cyclization reaction to give the corresponding 2c–2j in moderate to good yields with high levels of enantioselectivities (entries 2–9). Basically, the electronic feature of substituents exerts an obvious effect on the reaction performance (entries 4–8) while the substitution pattern of the cinnamamide moiety has little influence on the stereoselectivity (entry 2 vs. 7 and 3 vs. 6). A fairly good yield (65%) and high enantioselectivity (93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 er) were obtained for the highly electronically rich 2j substituted with methoxy at 3-, 4-, 5-positions, respectively (entry 9). The replacement of sterically bulky γ-diphenyl substituents of 1b with γ-dimethyl groups, as shown in 1k, was also allowed to produce the corresponding product 2k in a good yield (57%) and a high enantioselectivity (92.5
7 er) were obtained for the highly electronically rich 2j substituted with methoxy at 3-, 4-, 5-positions, respectively (entry 9). The replacement of sterically bulky γ-diphenyl substituents of 1b with γ-dimethyl groups, as shown in 1k, was also allowed to produce the corresponding product 2k in a good yield (57%) and a high enantioselectivity (92.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.5 er, entry 10). Both cyclopentyl and cyclohexyl substituted substrates (1l and 1m) underwent the reaction smoothly to yield the corresponding products 2l and 2m in good yields and high enantioselectivities, respectively (entries 11 and 12). Moreover, N-(2,2-diphenylpent-4-en-1-yl)acrylamide could also be tolerated and afforded 2n in 51% yield and with 92.5
7.5 er, entry 10). Both cyclopentyl and cyclohexyl substituted substrates (1l and 1m) underwent the reaction smoothly to yield the corresponding products 2l and 2m in good yields and high enantioselectivities, respectively (entries 11 and 12). Moreover, N-(2,2-diphenylpent-4-en-1-yl)acrylamide could also be tolerated and afforded 2n in 51% yield and with 92.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.5 er using Pd(OAc)2 as a precatalyst at 25 °C (entry 13) (Table 2) .
7.5 er using Pd(OAc)2 as a precatalyst at 25 °C (entry 13) (Table 2) .
| Entry | 2 | R | Ar | Yieldb (%) | erc |
|---|---|---|---|---|---|
| a Unless indicated otherwise, the reaction of 1 (0.1 mmol) was carried out in toluene (1 mL) at 50 °C in the presence of Pd(CF3COO)2 (20 mol%), 3b (20 mol%), 4d (20 mol%) and 3 Å (50 mg) under O2 (balloon) for 20 h. b Isolated yield. c Determined by HPLC. d The reaction time was 36 h. e The substrate was N-(2,2-diphenylpent-4-en-1-yl)acrylamide and Pd(OAc)2 was used at 25 °C. | |||||
| 1 | 2b | Ph | Ph | 52 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
| 2 | 2c | Ph | 2-ClC6H4 | 48 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| 3 | 2d | Ph | 3-FC6H4 | 55 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
| 4 | 2e | Ph | 4-MeC6H4 | 39 | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
| 5 | 2f | Ph | 4-OMeC6H4 | 56 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
| 6 | 2g | Ph | 4-FC6H4 | 55 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
| 7 | 2h | Ph | 4-ClC6H4 | 51 | 94.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.5 5.5 |
| 8 | 2i | Ph | 4-NO2C6H4 | 48 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5d 5d |
| 9 | 2j | Ph | 3,4,5-OMeC6H2 | 65 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
| 10 | 2k | Me | Ph | 57 | 92.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.5 7.5 |
| 11 | 2l | –C4H8- | Ph | 60 | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
| 12 | 2mm | –C5H10- | Ph | 54 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
| 13 | 2n | — | —e | 51 | 92.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.5 7.5 |
To gain insight into the reaction process, we investigated the kinetics of the cascade reaction. As shown in Fig. 2, the chiral palladium(II) complex of ligand 3b was able to catalyze the oxidative tandem cyclization reaction alone, but in the presence of the chiral phosphoric acid 4d, the reaction proceeded even faster. This observation, together with the difference in the enantioselectivity (entry 13 vs. 16, Table 1), strongly suggested that the synergistic effect of the chiral ligand and Brønsted acid played an important role in the enantioselective catalysis.
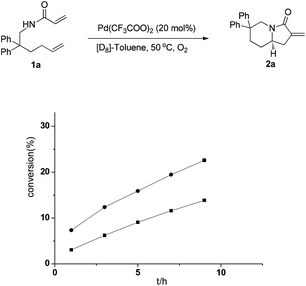 | ||
| Fig. 2 Kinetic studies on the oxidative tandem cyclization reaction catalyzed by 20 mol% ligand 3b and 20 mol% 4d (●) and by 20 mol% ligand 3b (◆). | ||
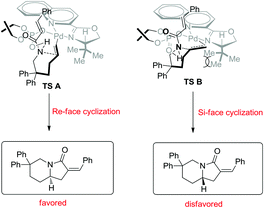
To find out the palladium species in the catalysis, a high-resolution mass spectrometry (HRMS) analysis of a reaction mixture of the palladium complex with 3b and 4d was conducted.10 The result showed that an anion exchange occurred between one molecule of the chiral phosphoric acid 4d and Pd(CF3COO)2, which coordinated to one molecule of a chiral ligand 3b, leading to the formation of the catalytically active palladium(II) complex possessing both a chiral ligand and a chiral phosphate.8a,b,9 On the basis of this fact and the experimental observations, a transition-state model was proposed. As shown in Scheme 2, the alkene coordinates to the Pd(II) and cinnamamide was activated by hydrogen bonding interaction with phosphoric acid.11 The formation of TS-A is favored and leads to a Re-face cyclization because the orientation of the vinyl group of the substrate is sterically matched with the chiral environment of 3b. In contrast, the Si-face cyclization via TS-B is disfavored due to the steric repulsion between the vinyl moiety of the substrate and the tert-butyl group of 3b.
In summary, we have demonstrated that the combined use of a palladium(II) complex of a chiral Bu-QUOX ligand and a chiral phosphoric acid enabled a highly enantioselective oxidative cascade cyclization reaction of N-(2,2-disubstituted hex-5-en-1-yl)acrylamides, providing a straightforward method to access chiral 6,5-bicyclic aza-heterocycles in moderate yields and with excellent enantioselectivities. A synergistic effect between the ligand and counterion was found in the catalysis. The chiral ligand and anion cooperatively control the stereoselectivity. The findings suggest that the combined catalysts of the chiral Pd(II) complex and the chiral Brønsted acid might be amenable to the development of other asymmetric catalytic oxidative reactions.
Acknowledgements
We are grateful for financial support from MOST (973 project 2010CB833300), NSFC (21232007 and 21072181) and the Ministry of Education.Notes and references
- For reviews see: (a) T. Nishimura and S. Uemura, Synlett, 2004, 201 CAS; (b) B. M. Stoltz, Chem. Lett., 2004, 33, 362 CrossRef CAS; (c) S. S. Stahl, Angew. Chem., Int. Ed., 2004, 43, 3347 CrossRef; (d) M. S. Sigman and M. J. Schultz, Org. Biomol. Chem., 2004, 2, 2551 RSC; (e) T. Punniyamurthy, S. Velusamy and J. Iqbal, Chem. Rev., 2005, 105, 2329 CrossRef CAS PubMed; (f) S. S. Stahl, Science, 2005, 309, 1824 CrossRef CAS PubMed; (g) J. Muzart, Chem. – Asian J., 2006, 1, 508 CrossRef CAS PubMed; (h) K. M. Gligorich and M. S. Sigman, Angew. Chem., Int. Ed., 2006, 45, 6612 CrossRef CAS PubMed; (i) E. M. Beccalli, G. Broggini, M. Martinelli and S. Sottocornola, Chem. Rev., 2007, 107, 5318 CrossRef CAS PubMed; (j) T. W. Lyons and M. S. Sanford, Chem. Rev., 2010, 110, 1147 CrossRef CAS PubMed; (k) D. M. Schultz and J. P. Wolfe, Synthesis-Stuttgart, 2012, 44, 351 CrossRef CAS PubMed; (l) K. C. Majumdar, S. Samanta and B. Sinaha, Synthesis-Stuttgart, 2012, 44, 814 Search PubMed.
- (a) L. F. Tietze, H. Ila and H. P. Bell, Chem. Rev., 2004, 104, 3453 CrossRef CAS PubMed; (b) R. M. Trend, Y. K. Ramtohul and B. M. Stoltz, J. Am. Chem. Soc., 2005, 127, 17778 CrossRef CAS PubMed; (c) G. S. Liu and S. S. Stahl, J. Am. Chem. Soc., 2007, 129, 6328 CrossRef CAS PubMed; (d) R. I. Mcdonald, G. S. Liu and S. S. Stahl, Chem. Rev., 2011, 111, 2981 CrossRef CAS PubMed.
- (a) M. Shibasaki, C. D. J. Boden and A. Kojima, Tetrahedron, 1997, 53, 7371 CrossRef CAS; (b) A. B. Dounay and L. E. Overman, Chem. Rev., 2003, 103, 2945 CrossRef CAS PubMed; (c) M. Shibasaki, E. M. Vogl and T. Ohshima, Adv. Synth. Catal., 2004, 346, 1533 CrossRef CAS.
- For reviews, see: (a) R. Giri, B. F. Shi, K. M. Engle, N. Maugel and J. Q. Yu, Chem. Soc. Rev., 2009, 38, 3242 RSC; (b) Ref. 2d .
- For selected examples, see: (a) T. Hosokawa, S. Miyagi, S. I. Murahashi and A. Sonoda, J. Chem. Soc., Chem. Commun., 1978, 687 RSC; (b) Y. Uozumi, K. Kato and T. Hayashi, J. Am. Chem. Soc., 1997, 119, 5063 CrossRef CAS; (c) A. E. Qisairi, H. A. Qaseer and P. M. Henry, J. Organomet. Chem., 2002, 656, 168 CrossRef; (d) R. M. Trend, Y. K. Ramtohul, E. M. Ferrira and B. M. Stoltz, Angew. Chem., Int. Ed., 2003, 42, 2892 CrossRef CAS PubMed; (e) L. F. Tietze, K. M. Sommer, J. Zinngrebe and F. Stecker, Angew. Chem., Int. Ed., 2004, 44, 257 CrossRef PubMed; (f) G. S. Liu and S. S. Stahl, J. Am. Chem. Soc., 2006, 128, 7179 CrossRef CAS PubMed; (g) F. J. Wang, Y. J. Zhang, H. Wei, J. M. Zhang and W. B. Zhang, Tetrahedron Lett., 2007, 48, 4083 CrossRef CAS; (h) Y. Zhang and M. S. Sigman, J. Am. Chem. Soc., 2007, 129, 3076 CrossRef CAS PubMed; (i) B. F. Shi, N. Maugel, Y. H. Zhang and J. Q. Yu, Angew. Chem., Int. Ed., 2008, 47, 4882 CrossRef CAS PubMed; (j) T. Tsujihara, K. Takenaka, K. Onitsuka, M. Hatanaka and H. Sasai, J. Am. Chem. Soc., 2009, 131, 3452 CrossRef CAS PubMed; (k) C. C. Scarborough, A. Bergant, G. T. Sazamaa, I. A. Guzeia, L. C. Spencera and S. S. Stahl, Tetrahedron, 2009, 65, 5084 CrossRef CAS PubMed; (l) B. F. Shi, Y. H. Zhang, J. K. Lam, D. H. Wang and J. Q. Yu, J. Am. Chem. Soc., 2010, 132, 460 CrossRef CAS PubMed; (m) M. Wasa, K. M. Engle, D. W. Lin, E. J. Yoo and J. Q. Yu, J. Am. Chem. Soc., 2011, 133, 19598 CrossRef CAS PubMed; (n) A. B. Weinstein and S. S. Stahl, Angew. Chem., Int. Ed., 2012, 51, 11505 CrossRef CAS PubMed; (o) X. F. Chen, Y. Li, Y. M. Su, F. Yin, J. Y. Wang, J. Sheng, H. U. Vora, X. S. Wang and J. Q. Yu, J. Am. Chem. Soc., 2013, 135, 1236 CrossRef PubMed; (p) G. Q. Yang and W. B. Zhang, Angew. Chem., Int. Ed., 2013, 52, 7540 CrossRef CAS PubMed; (q) L. Chu, X. C. Wang, C. E. Moore, A. L. Rheingold and J. Q. Yu, J. Am. Chem. Soc., 2013, 135, 16344 CrossRef CAS PubMed.
- For reviews of palladium-catalyzed cascade cyclization reactions, see: (a) E. I. Negishi, C. Copéret, S. Ma, S. Y. Liou and F. Liu, Chem. Rev., 1996, 96, 365 CrossRef CAS; (b) G. Poli, G. Giambastiani and A. Heumann, Tetrahedron, 2000, 56, 5959 CrossRef CAS; (c) G. Battistuzzi, S. Cacchi and G. Fabrizi, Eur. J. Org. Chem., 2002, 2671 CrossRef CAS; (d) J. T. Link, in Organic Reactions, ed. L. E. Overman, Wiley, New York, 2002, vol. 60, p. 157 Search PubMed For excellent examples of the palladium(II)-catalyzed enantioselective Wacker-type oxidative tandem reaction, see: (e) M. A. Arai, K. Minori, A. Takayoshi and H. Sasai, J. Am. Chem. Soc., 2001, 123, 2907 CrossRef CAS PubMed; (f) K. T. Yip, M. Yang, K. L. Law, N. Y. Zhu and D. Yang, J. Am. Chem. Soc., 2006, 128, 3130 CrossRef CAS PubMed; (g) W. He, K. T. Yip, N. Y. Zhang and D. Yang, Org. Lett., 2009, 11, 5626 CrossRef CAS PubMed; (h) K. T. Yip and D. Yang, Chem. – Asian. J., 2011, 6, 2166 CrossRef CAS PubMed; (i) C. Ramalingan, K. Takenaka and H. Sasai, Tetrahedron, 2011, 67, 2889 CrossRef CAS.
- For reviews on combining organocatalysis with metal catalysis, see: (a) Z. Shao and H. Zhang, Chem. Soc. Rev., 2009, 38, 2745 RSC; (b) P. de Armas, D. Tejedor and F. García-Tellado, Angew. Chem., Int. Ed., 2010, 49, 1013 CrossRef CAS PubMed; (c) M. Rueping, R. M. Koenigs and I. Atodiresei, Chem. – Eur. J., 2010, 16, 9350 CrossRef CAS PubMed; (d) C. Zhong and X. Shi, Eur. J. Org. Chem., 2010, 2999 CrossRef CAS; (e) J. Zhou, Chem. – Asian. J., 2010, 5, 422 CrossRef CAS PubMed; (f) Z. Y. Han, C. Wang and L. Z. Gong, in Science of Synthesis, Asymmetric Organocatalysis, ed. B. List and K. Maruoka, Georg Thieme Verlag, Stuttgart, 2012, vol. 2, p. 697 Search PubMed; (g) A. E. Allen and D. W. C. MacMillan, Chem. Sci., 2012, 3, 633 RSC; (h) L. Stegbauer, F. Sladojevich and D. J. Dixone, Chem. Sci., 2012, 3, 942 RSC; (i) N. T. Patil, V. S. Shinde and B. Gajula, Org. Biomol. Chem., 2012, 10, 211 RSC; (j) C. C. J. Loh and D. Enders, Chem. – Eur. J., 2012, 18, 10212 CrossRef CAS PubMed; (k) X. Wu, M. L. Li and L. Z. Gong, Acta Chim. Sin., 2013, 71, 1091 CrossRef CAS.
- (a) H. Alper and N. Hamel, J. Am. Chem. Soc., 1990, 112, 2803 CrossRef CAS; (b) Z. Cai and T. J. Rainey, J. Am. Chem. Soc., 2012, 134, 3615 CrossRef PubMed; (c) S. Y. Yu, H. Zhang, Y. Gao, L. Mo, S. Z. Wang and Z. J. Yao, J. Am. Chem. Soc., 2013, 135, 11402 CrossRef CAS PubMed; (d) G. X. Jiang, R. Halder, Y. Fang and B. List, Angew. Chem., Int. Ed., 2011, 50, 9752 CrossRef CAS PubMed.
- (a) S. Mukherjee and B. List, J. Am. Chem. Soc., 2007, 129, 11336 CrossRef CAS PubMed; (b) G. X. Jiang and B. List, Angew. Chem., Int. Ed., 2011, 50, 9471 CrossRef CAS PubMed; (c) Z. L. Tao, W. Q. Zhang, D. F. Chen, A. Adele and L. Z. Gong, J. Am. Chem. Soc., 2013, 135, 9255 CrossRef CAS PubMed.
- The details of HRMS data are described in the ESI.†.
- (a) M. Terada, K. Machioka and K. Sorimachi, Angew. Chem., Int. Ed., 2006, 45, 2254 CrossRef CAS PubMed; (b) M. Terada and K. Sorimachi, J. Am. Chem. Soc., 2007, 129, 292 CrossRef CAS PubMed; (c) Y. X. Jia, J. Zhong, S. F. Zhu and Q. L. Zhou, Angew. Chem., Int. Ed., 2007, 46, 5565 CrossRef CAS PubMed; (d) M. Terada, K. Soga and N. Momiyama, Angew. Chem., Int. Ed., 2008, 47, 4122 CrossRef CAS PubMed; (e) M. Terada, K. Machioka and K. Sorimachi, Angew. Chem., Int. Ed., 2009, 48, 2533 CrossRef PubMed; (f) Q. X. Guo, Y. G. Peng, J. W. Zhang, L. Song, Z. Feng and L. Z. Gong, Org. Lett., 2009, 11, 4620 CrossRef CAS PubMed; (g) C. Guo, J. Song, J. Z. Huang, P. H. Chen, S. W. Luo and L. Z. Gong, Angew. Chem., Int. Ed., 2012, 51, 1046 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 972713. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4qo00042k |
| This journal is © the Partner Organisations 2014 |