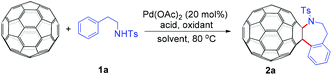Palladium-catalyzed heteroannulation of [60]fullerene with N-(2-arylethyl) sulfonamides via C–H bond activation†
Yi-Tan
Su
a,
You-Liang
Wang
a and
Guan-Wu
Wang
*ab
aHefei National Laboratory for Physical Sciences at Microscale, CAS Key Laboratory of Soft Matter Chemistry, and Department of Chemistry, University of Science and Technology of China, Hefei, Anhui 230026, P. R. China. E-mail: gwang@ustc.edu.cn
bState Key Laboratory of Applied Organic Chemistry, Lanzhou University, Lanzhou, Gansu 730000, P. R. China
First published on 28th May 2014
Abstract
The palladium-catalyzed heteroannulation of [60]fullerene with various N-(2-arylethyl) sulfonamides afforded a variety of [60]fullerene-fused tetrahydrobenzazepines. These reactions were initiated by C–H bond activation and followed by cyclization. In addition, further transformation and electrochemistry of the obtained [60]fullerene-fused tetrahydrobenzazepines were investigated.
Due to their immense potential applications in materials science and biological science, fullerenes and their derivatives have attracted significant attention.1 A vast number of chemical reactions have been discovered to functionalize fullerenes over the past two decades.2 Among them, transition-metal-mediated or -catalyzed reactions of [60]fullerene (C60) have attracted increasing attention.3 Recently, our group has investigated reactions of C60 mediated by transition metal salts such as Mn(OAc)3,3a,c Fe(ClO4)3,4 Cu(OAc)2,5 Pb(OAc)4
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 and Ag2CO3
6 and Ag2CO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 to obtain a variety of novel fullerene products. Functionalization of C60 through a Pd-catalyzed C–H bond activation strategy has also been disclosed. However, this protocol is relatively underdeveloped and limited to a few Pd-catalyzed heteroannulations of C60 with anilides,8a benzamides,8b arylsulfonic acids,8cN-benzyl sulfonamides,8d phenylethyl/benzyl alcohols8e and N-sulfonyl-2-aminobiaryls.9 Therefore, there is still a demand to prepare more fullerene compounds with different appended moieties through Pd-catalyzed C–H bond activation.
7 to obtain a variety of novel fullerene products. Functionalization of C60 through a Pd-catalyzed C–H bond activation strategy has also been disclosed. However, this protocol is relatively underdeveloped and limited to a few Pd-catalyzed heteroannulations of C60 with anilides,8a benzamides,8b arylsulfonic acids,8cN-benzyl sulfonamides,8d phenylethyl/benzyl alcohols8e and N-sulfonyl-2-aminobiaryls.9 Therefore, there is still a demand to prepare more fullerene compounds with different appended moieties through Pd-catalyzed C–H bond activation.
On the other hand, Pd-catalyzed ligand-directed C–H bond activation has emerged as one of the most powerful tools to construct C–C and C–X (X = heteroatom) bonds in organic synthesis.10 2-Arylethylamines have been applied to construct heterocycles via C–H activation reactions.11 Orito et al. reported the Pd-catalyzed direct aromatic carbonylation of 2-arylethylamines.11a The Yu group developed Pd-catalyzed intramolecular C–H aminations to prepare indolines from 2-arylethyl triflamides and 2-arylethyl 2-pyridylsulfonamides.11b–d However, using 2-arylethylmine derivatives to construct seven-membered tetrahydrobenzazepine via a C–H activation strategy has been unknown until now.
As the formation of C60-fused seven-membered-ring compounds through Pd-catalyzed C–H bond activation presumably proceeds through a relatively scarce eight-membered palladacycle intermediate, the synthesis of C60-fused seven-membered-ring compounds remains a great challenge.8e,9 In continuation of our Pd-catalyzed reactions of C60,8 herein we report a novel heteroannulation of C60 with various N-(2-arylethyl) sulfonamides to give the rare C60-fused tetrahydrobenzazepines through a Pd-catalyzed C–H activation protocol. Furthermore, these C60-fused tetrahydrobenzazepines were found to undergo TfOH-promoted rearrangements.
We chose the reaction of C60 with N-phenethyl-p-toluenesulfonamide (1a) as the model reaction to screen the optimal conditions. At the onset, C60 (36 mg, 0.05 mmol) was allowed to react with 3 equiv. of 1a in the presence of 3 equiv. of K2S2O8, 1 equiv. of mesitylenesulfonic acid dihydrate (MesSA) and 20 mol% of Pd(OAc)2 in 6 mL of o-dichlorobenzene (ODCB) at 80 °C for 10 h. To our satisfaction, the desired fullerotetrahydrobenzazepine 2a was obtained in 6% yield (Table 1, entry 1). Replacing MesSA with p-toluenesulfonic acid (PTSA) led to 2a in 14% yield (Table 1, entry 2). When the reaction was performed in a mixture of PTSA (1 equiv.) and trifluoroacetic acid (TFA, 0.3 mL),9 the product yield could be further improved to 18% yield (Table 1, entry 3). However, TFA itself also provided a comparative yield with much more recovered C60 (Table 1, entry 4 vs. entry 3). The use of acetic acid (AcOH) or pivalic acid (PivOH) to replace TFA was unsuccessful (Table 1, entries 5 and 6). All these results suggested that TFA was more suitable for this reaction. The critical role played by TFA was believed to be to participate in the in situ formation of a more reactive Pd(TFA)2 species from the Pd(OAc)2 precatalyst,12 thus promoting the current heteroannulation of C60. After the acid was selected, several oxidants were screened. Compared to K2S2O8, oxone, AgOAc and p-benzoquinone (BQ), Cu(OAc)2 afforded the best result with a yield of 30% (Table 1, entry 10 vs. entries 4 and 7–9). Notably, the fruitless attempt to replace TFA with PTSA further confirmed that TFA was the best acid for this reaction (Table 1, entry 11). In addition, the desired reaction did not take place in the presence of DMSO, CH3CN or DMF (Table 1, entries 12–14). Reducing the Pd(OAc)2 loading decreased the product yield dramatically (Table 1, entry 15 vs. entry 10). Gratifyingly, decreasing the amount of TFA from 0.3 mL to 0.2 mL could give a slightly better yield (32%) (Table 1, entry 16 vs. entry 10). Therefore, 0.05 mmol of C60, 20 mol% of Pd(OAc)2, 3 equiv. of 1a, 3 equiv. of Cu(OAc)2 with ODCB–TFA (6.2 mL, v/v = 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the solvent at 80 °C were selected as the optimal reaction conditions.
1) as the solvent at 80 °C were selected as the optimal reaction conditions.
| Entry | Acid | Oxidant | Solvent (mL) | Yield of 2ab (%) |
|---|---|---|---|---|
| a Unless otherwise specified, all reactions were performed with 0.05 mmol of C60, 0.15 mmol of 1a, 20 mol% of Pd(OAc)2, 0.15 mmol of oxidant and 0.3 mL of acid in 6 mL of ODCB at 80 °C for 10 h. b Isolated yield, values in parentheses were based on consumed C60. c 1 equiv. of the solid acid was used. d 0.3 mL of AcOH was used. e 193 mg of PivOH was used. f 10 mol% of Pd(OAc)2 was used, and the reaction time was 18 h. g 0.2 mL of TFA was used. Ts = p-toluenesulfonyl, DMSO = dimethyl sulfoxide, DMF = N,N-dimethylformamide. | ||||
| 1c | MesSA | K2S2O8 | ODCB (6) | 6 (30) |
| 2c | PTSA | K2S2O8 | ODCB (6) | 14 (50) |
| 3c | PTSA/TFA | K2S2O8 | ODCB (6) | 18 (36) |
| 4 | TFA | K2S2O8 | ODCB (6) | 18 (60) |
| 5d | AcOH | K2S2O8 | ODCB (6) | Trace |
| 6e | PivOH | K2S2O8 | ODCB (6) | Trace |
| 7 | TFA | Oxone | ODCB (6) | 8 (36) |
| 8 | TFA | AgOAc | ODCB (6) | < 3% |
| 9 | TFA | BQ | ODCB (6) | 7 (58) |
| 10 | TFA | Cu(OAc)2 | ODCB (6) | 30 (55) |
| 11c | PTSA | Cu(OAc)2 | ODCB (6) | Trace |
| 12 | TFA | Cu(OAc)2 | ODCB–DMSO (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0 |
| 13 | TFA | Cu(OAc)2 | ODCB–CH3CN (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0 |
| 14 | TFA | Cu(OAc)2 | ODCB–DMF (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0 |
| 15f | TFA | Cu(OAc)2 | ODCB (6) | 17 (49) |
| 16g | TFA | Cu(OAc)2 | ODCB (6) | 32 (59) |
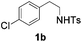
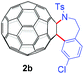
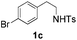
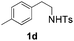
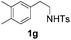
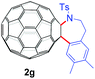
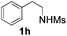
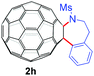
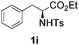
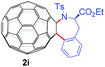
With the optimal conditions in hand, the scope of this annulation was explored using a variety of substrates as illustrated in Table 2. We were pleased to find that all of the examined N-(2-arylethyl) sulfonamides 1a–1i could be applied to furnish the desired C60-fused tetrahydrobenzazepines 2a–2i in synthetically valuable yields. Substrates bearing either an electron-withdrawing group or an electron-donating group on the 2-arylethylamine ring worked well and gave the desired products 2b–2g in 12–47% yields (Table 2, entries 2–7). The substrate with an electron-withdrawing chloro or bromo group at the para position of the phenyl ring (1b, 1c) gave a lower yield based on consumed C60 because it tended to generate some fullerene byproducts, which were most probably formed from the reaction of C60 with bulk ODCB (Table 2, entries 2 and 3). The efforts to increase the product yields and inhibit the formation of byproducts by adjusting the reaction temperature and reaction time proved fruitless. The substrate 1d with a methyl group at the para position of the phenyl ring provided 2d in 40% yield (Table 2, entry 4), while the substrate 1f with a strong electron-donating methoxy group at the para position of the phenyl ring resulted in a decreased yield (Table 2, entry 6). When substrates were substituted at the meta position (1e, 1g), products resulting from the reactions at the less sterically hindered positions were regioselectively obtained in 37% and 47% yields, respectively (Table 2, entries 5 and 7). In addition, 2-phenethylamine with the methanesulfonyl (Ms) group attached to the nitrogen atom (1h) gave a comparable product yield to that of 1a (Table 2, entry 8 vs. entry 1). It is of interest to note that the tosylamide of L-phenylalanine (1i) was also reactive under our optimal conditions, and a novel C60-fused amino acid derivative was isolated in 24% yield (Table 2, entry 9).
| Entry | Substrate 1 | Product 2 | Yieldb (%) | Recovered C60 (%) |
|---|---|---|---|---|
| a Unless otherwise specified, all reactions were performed with 0.05 mmol of C60, 0.15 mmol of 1a–1i, 0.01 mmol of Pd(OAc)2, 0.15 mmol of Cu(OAc)2 and 0.2 mL of TFA in 6 mL of ODCB at 80 °C for 10 h. b Isolated yield, values in parentheses were based on consumed C60. c The reaction was performed for 6 h. | ||||
| 1 |
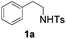
|
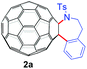
|
32 (59) | 46 |
| 2 |
|
|
24 (39) | 38 |
| 3 |
|
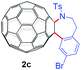
|
12 (34) | 65 |
| 4 |
|
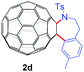
|
40 (65) | 38 |
| 5 |
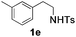
|
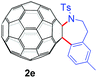
|
37 (61) | 39 |
| 6 |
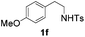
|
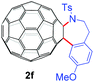
|
20 (77) | 74 |
| 7c |
|
|
47 (63) | 25 |
| 8 |
|
|
37 (56) | 34 |
| 9 |
|
|
24 (69) | 65 |
All products 2a–2i were unambiguously characterized using HRMS, 1H NMR, 13C NMR, FT-IR and UV-vis spectra. The ESI mass spectra of 2a–2i gave the correct molecular ion peaks. In their 13C NMR spectra, the observation of at least 51 lines in the range of 133–156 ppm for the sp2-carbons of the C60 skeleton, and two peaks at 78–80 ppm and 70–72 ppm for the two sp3-carbons of the fullerene cage, is consistent with the C1 symmetry of their molecular structures. The IR spectra of 2a–2i showed two strong absorptions at 1330–1350 cm−1 and 1140–1160 cm−1 due to the sulfonamide group. Furthermore, their UV-vis spectra displayed a characteristic peak at 433–437 nm, which is the diagnostic absorption for the 1,2-adduct of C60.7,9
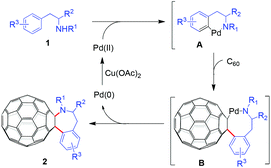
Based on our experimental results and previously suggested mechanisms in the literature,8,9 a plausible mechanism for the formation of the C60-fused tetrahydrobenzazepines is shown in Scheme 1. Initially, N-(2-arylethyl) sulfonamide 1 is coordinated to the Pd(II) species, followed by ortho C–H activation to produce the intermediate A. Insertion of C60 into the arylpalladium bond in A yields the intermediate B. Subsequent reductive elimination of the intermediate B generates C60-fused tetrahydrobenzazepine 2 and Pd(0). The latter is reoxidized to a Pd(II) species by Cu(OAc)2 to complete the catalytic cycle.
The alternative possible five-membered-ring [60]fulleroindanes resulting from annulation at the benzylic position and the ortho position of N-(2-arylethyl) sulfonamides were not observed, indicating the preference of the ligand-directed C–H activation pathway. It should be noted that the formation of [60]fulleroazepines via Pd-catalyzed C–H bond activation has been reported.9 However, only rigid N-sulfonyl-2-aminobiaryls were used as the substrates, and a hybrid acid system (PTSA/TFA) was crucial for the efficient formation of the seven-membered-ring products.9 In our present work, flexible N-(2-arylethyl) sulfonamides incorporating an alkyl chain, even the chiral amino acid moiety, were employed as the substrates, and only TFA was required as the acid additive (Table 1, entry 3 vs. entry 4). In addition, Pd(OAc)2/Cu(OAc)2 in ODCB–TFA (7 mL, v/v = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), which was similar to our system, was totally inert in Chuang's work.9 A related study on C60-fused tetrahydroazepinones and -azepinonimines formed from the Cu(OAc)2-promoted heteroannulations of C60 with N-sulfonylated o-amino-aromatic methyl ketones or O-alkyl oximes was recently disclosed.13 Besides the sulfonamide group, another functional group such as ketone or oxime group was required to facilitate the radical pathway.
1), which was similar to our system, was totally inert in Chuang's work.9 A related study on C60-fused tetrahydroazepinones and -azepinonimines formed from the Cu(OAc)2-promoted heteroannulations of C60 with N-sulfonylated o-amino-aromatic methyl ketones or O-alkyl oximes was recently disclosed.13 Besides the sulfonamide group, another functional group such as ketone or oxime group was required to facilitate the radical pathway.
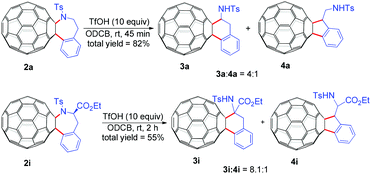
Further transformations of representative C60-fused tetrahydrobenzazepines 2a and 2i were also investigated (Scheme 2). Intriguingly, treatment of C60-fused tetrahydrobenzazepine 2a with 10 equiv. of trifluoromethanesulfonic acid (TfOH) at ambient temperature for 45 min afforded a mixture of fullerotetrahydronaphthalene 3a and fulleroindane 4a (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in a total yield of 82%. The attempt to obtain 3a and 4a directly from the Pd-catalyzed reaction of C60 with 1a (Table 1, entry 16) by adding 10 equiv. of TfOH led to a yield of only 5% along with 79% of recovered C60, showing extremely low efficiency of the direct transformation. Similarly, treatment of C60-fused tetrahydrobenzazepine 2i with 10 equiv. of TfOH for 2 h afforded an 8.1
1) in a total yield of 82%. The attempt to obtain 3a and 4a directly from the Pd-catalyzed reaction of C60 with 1a (Table 1, entry 16) by adding 10 equiv. of TfOH led to a yield of only 5% along with 79% of recovered C60, showing extremely low efficiency of the direct transformation. Similarly, treatment of C60-fused tetrahydrobenzazepine 2i with 10 equiv. of TfOH for 2 h afforded an 8.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 3i and 4i in 55% yield. Both 3i and 4i bear a phenylalanine moiety which is a biologically active motif, hinting potential application of 3i and 4i in biomedical sciences. In addition, the preparation of C60-fused tetrahydronaphthalene14 and indane8d,14,15 derivatives is still limited; this method provides a new route to synthesize these two types of compounds.
1 mixture of 3i and 4i in 55% yield. Both 3i and 4i bear a phenylalanine moiety which is a biologically active motif, hinting potential application of 3i and 4i in biomedical sciences. In addition, the preparation of C60-fused tetrahydronaphthalene14 and indane8d,14,15 derivatives is still limited; this method provides a new route to synthesize these two types of compounds.
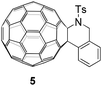
The half-wave reduction potentials of 2a–2i along with 5 (Fig. 1) and C60 were measured by cyclic voltammetry and are summarized in Table 3. We noted that the reduction potentials of C60-fused tetrahydrobenzazepines were dependent on the substituents on the 2-arylethylamine moiety. Products bearing an electron-withdrawing group are reduced more easily, whereas products with electron-donating groups give more negative first redox reduction potentials (2b–2cvs.2d–2g). Compound 2h bearing the mesyl group has a more positive first redox reduction potential than that substituted by the tosyl group (2hvs.2a). Compared with the seven-membered-ring compound 2a, the first reduction potential of its six-membered-ring analogue 5 shows a positive shift (–1.120 V for 5vs. −1.133 V for 2a), exhibiting an effect of the C60-fused ring size. In addition, the first reduction potentials of 2a–2i and 5 are 40–73 mV negatively shifted compared to that of C60. In consideration of their high solubility in common solvents such as CS2, CHCl3, chlorobenzene and o-dichlorobenzene, C60-fused tetrahydrobenzazepines may have potential application in organic photovoltaic devices when combined with suitable polymer donors.16,17 A close examination of the cyclic voltammograms indicated that the first two redoxes of 2a–2i were reversible, while their third redoxes were irreversible. Therefore, this unique electrochemical property may be exploited to generate ring-opened trianions of 2a–2i by controlled potential electrolysis (CPE), and then undergo nucleophilic reactions with various electrophiles to give other diversified fullerene derivatives.18
| Compound | E 1 | E 2 | E 3 |
|---|---|---|---|
| a Potential values versus Fc/Fc+ reference electrode. Conditions: ca. 1 mM of the title compound and 0.1 mM of n-Bu4NClO4 in anhydrous ODCB; reference electrode: SCE; working electrode: Pt; auxiliary electrode: Pt wire; scanning rate: 20 mV s−1. b Saturated solution of 2i. | |||
| 2a | −1.133 | −1.533 | −2.004 |
| 2b | −1.128 | −1.532 | −2.041 |
| 2c | −1.124 | −1.528 | −2.013 |
| 2d | −1.135 | −1.522 | −2.008 |
| 2e | −1.132 | −1.517 | −1.997 |
| 2g | −1.140 | −1.535 | −2.043 |
| 2g | −1.149 | −1.545 | −2.048 |
| 2h | −1.118 | −1.509 | −1.992 |
| 2i | −1.122 | −1.515 | −1.964 |
| 5 | −1.120 | −1.514 | −2.000 |
| C60 | −1.076 | −1.460 | −1.925 |
In summary, we have successfully synthesized the C60-fused tetrahydrobenzazepines by the Pd-catalyzed heteroannulation of [60]fullerene with various N-(2-arylethyl) sulfonamides via a C–H bond activation strategy. The rare seven-membered products are supposed to be generated via a hard-to-form eight-membered palladacycle intermediate. In the presence of TfOH, the C60-fused tetrahydrobenzazepines could be converted to the C60-fused tetrahydronaphthalene and indane derivatives.
Acknowledgements
We are grateful for financial support from the National Natural Science Foundation of China (no. 21132007) and the Specialized Research Fund for the Doctoral Program of Higher Education (no. 20123402130011).Notes and references
- For reviews, see: (a) M. Prato, Top. Curr. Chem., 1999, 199, 173 CrossRef CAS; (b) F. Diederich and M. Gomez-Lopez, Chem. Soc. Rev., 1999, 28, 263 RSC; (c) E. Nakamura and H. Isobe, Acc. Chem. Res., 2003, 36, 807 CrossRef CAS PubMed; (d) D. M. Guldi, F. Zerbetto, V. Georgakilas and M. Prato, Acc. Chem. Res., 2005, 38, 38 CrossRef CAS PubMed.
- For selected reviews, see: (a) A. Hirsch, Synthesis, 1995, 895 CrossRef CAS PubMed; (b) C. Thilgen and F. Diederich, Chem. Rev., 2006, 106, 5049 CrossRef CAS PubMed; (c) F. Giacalone and N. Martín, Chem. Rev., 2006, 106, 5136 CrossRef CAS PubMed; (d) M. Murata, Y. Murata and K. Komatsu, Chem. Commun., 2008, 6083 RSC.
- For reviews, see: (a) G.-W. Wang and F.-B. Li, J. Nanosci. Nanotechnol., 2007, 7, 1162 CrossRef CAS PubMed; (b) Y. Matsuo and E. Nakamura, Chem. Rev., 2008, 108, 3016 CrossRef CAS PubMed; (c) G.-W. Wang and F.-B. Li, Curr. Org. Chem., 2012, 16, 1109 CrossRef CAS; (d) M. D. Tzirakis and M. Orfanopoulos, Chem. Rev., 2013, 113, 5262 CrossRef CAS PubMed.
- For a review, see: F.-B. Li and G.-W. Wang, Sci. China Chem., 2012, 55, 2009 CrossRef CAS.
- G.-W. Wang and F.-B. Li, Org. Biomol. Chem., 2005, 3, 794 CAS.
- F.-B. Li, T.-X. Liu, Y.-S. Huang and G.-W. Wang, J. Org. Chem., 2009, 74, 7743 CrossRef CAS PubMed.
- C.-L. He, R. Liu, D.-D. Li, S.-E. Zhu and G.-W. Wang, Org. Lett., 2013, 15, 1532 CrossRef CAS PubMed.
- (a) B. Zhu and G.-W. Wang, Org. Lett., 2009, 11, 4334 CrossRef CAS PubMed; (b) S.-C. Chuang, V. Rajeshkumar, C.-A. Cheng, J.-C. Deng and G.-W. Wang, J. Org. Chem., 2011, 76, 1599 CrossRef CAS PubMed; (c) F. Li, T.-X. Liu and G.-W. Wang, Org. Lett., 2012, 14, 2176 CrossRef CAS PubMed; (d) Y.-T. Su, Y.-L. Wang and G.-W. Wang, Chem. Commun., 2012, 48, 8132 RSC; (e) W.-Q. Zhai, R.-F. Peng, B. Jin and G.-W. Wang, Org. Lett., 2014, 16, 1638 CrossRef CAS PubMed.
- V. Rajeshkumar, F.-W. Chan and S.-C. Chuang, Adv. Synth. Catal., 2012, 354, 2473 CAS.
- For selected recent reviews on C–H activations, see: (a) T. W. Lyons and M. S. Sanford, Chem. Rev., 2010, 110, 1147 CrossRef CAS PubMed; (b) C. S. Yeung and V. M. Dong, Chem. Rev., 2011, 111, 1215 CrossRef CAS PubMed; (c) K. M. Engle, T.-S. Mei, M. Wasa and J.-Q. Yu, Acc. Chem. Res., 2012, 45, 788 CrossRef CAS PubMed; (d) B.-J. Li and Z.-J. Shi, Chem. Soc. Rev., 2012, 41, 5588 RSC.
- (a) K. Orito, A. Horibata, T. Nakamura, H. Ushito, H. Nagasaki, M. Yuguchi, S. Yamashita and M. Tokuda, J. Am. Chem. Soc., 2004, 126, 14342 CrossRef CAS PubMed; (b) J.-J. Li, T.-S. Mei and J.-Q. Yu, Angew. Chem., Int. Ed., 2008, 47, 6452 CrossRef CAS PubMed; (c) T.-S. Mei, X. Wang and J.-Q. Yu, J. Am. Chem. Soc., 2009, 131, 10806 CrossRef CAS PubMed; (d) T.-S. Mei, D. Leow, H. Xiao, B. N. Laforteza and J.-Q. Yu, Org. Lett., 2013, 15, 3058 CrossRef CAS PubMed.
- (a) S. White, B. L. Bennett and D. M. Roddick, Organometallics, 1999, 18, 2356 CrossRef; (b) C. S. Yeung, N. Borduas, X. Zhao and V. M. Dong, Chem. Sci., 2010, 1, 331 RSC.
- T.-X. Liu, Z. Zhang, Q. Liu, P. Zhang, P. Jia, Z. Zhang and G. Zhang, Org. Lett., 2014, 16, 1020 CrossRef CAS PubMed.
- T.-X. Liu, F.-B. Li and G.-W. Wang, Org. Lett., 2011, 13, 6130 CrossRef CAS PubMed.
- (a) M. Saunders, H. A. Jiménez-Vázquez, R. J. Cross, E. Billups, C. Gesenberg and D. J. McCord, Tetrahedron Lett., 1994, 35, 3869 CrossRef CAS; (b) K. Kokubo, S. Tochika, M. Kato, Y. Sol and T. Oshima, Org. Lett., 2008, 10, 3335 CrossRef CAS PubMed; (c) Y.-T. Su and G.-W. Wang, Org. Lett., 2013, 15, 3408 CrossRef CAS PubMed.
- For a recent review, see: C.-Z. Li, H.-L. Yip and A. K.-Y. Jen, J. Mater. Chem., 2012, 22, 4161 RSC.
- M. Hashiguchi, N. Obata, M. Maruyama, K. S. Yeo, T. Ueno, T. Ikebe, I. Takahashi and Y. Matsuo, Org. Lett., 2012, 14, 3276 CrossRef CAS PubMed.
- (a) R. Liu, F. Li, Y. Xiao, D.-D. Li, C.-L. He, W.-W. Yang, X. Gao and G.-W. Wang, J. Org. Chem., 2013, 78, 7093 CrossRef CAS PubMed; (b) Y. Xiao, S.-E. Zhu, D.-J. Liu, M. Suzuki, X. Lu and G.-W. Wang, Angew. Chem., Int. Ed., 2014, 53, 3006 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and characterization data. See DOI: 10.1039/c4qo00106k |
| This journal is © the Partner Organisations 2014 |