Ferrocene as a functional group enhances the inhibitive effect of dihydropyrimidine on radical-induced oxidation of DNA
Rui
Wang
and
Zai-Qun
Liu
*
Department of Organic Chemistry, College of Chemistry, Jilin University, Changchun 130021, China. E-mail: zaiqun-liu@jlu.edu.cn
First published on 10th July 2014
Abstract
Phenyl and ferrocenyl groups are involved in 3,4-dihydropyrimidin-2(1H)-one (-thione) to form eleven dihydropyrimidines (DHPMs) in this work, aiming to explore the effects of the ferrocene moiety, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bond, and the phenolic hydroxyl group on the abilities of DHPMs to protect DNA against 2,2′-azobis(2-amidinopropane hydrochloride) (AAPH)-induced oxidation. It is found that the antioxidant abilities of DHPMs containing C
S bond, and the phenolic hydroxyl group on the abilities of DHPMs to protect DNA against 2,2′-azobis(2-amidinopropane hydrochloride) (AAPH)-induced oxidation. It is found that the antioxidant abilities of DHPMs containing C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S are higher than those of DHPMs containing C
S are higher than those of DHPMs containing C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. In addition, the phenolic hydroxyl group and the C
O. In addition, the phenolic hydroxyl group and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bond show similar effects on the antioxidant capacities of DHPMs. On the other hand, the ferrocenyl group remarkably increases the antioxidant capacities of DHPMs, while the antioxidant effects of ferrocenyl-appended DHPMs are further improved by the C
S bond show similar effects on the antioxidant capacities of DHPMs. On the other hand, the ferrocenyl group remarkably increases the antioxidant capacities of DHPMs, while the antioxidant effects of ferrocenyl-appended DHPMs are further improved by the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bond. Therefore, the influence of the functional group on the antioxidant abilities of DHPMs follows the order: ferrocenyl group > C
S bond. Therefore, the influence of the functional group on the antioxidant abilities of DHPMs follows the order: ferrocenyl group > C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bond > phenolic hydroxyl group.
S bond > phenolic hydroxyl group.
1. Introduction
Dihydropyrimidine dehydrogenase plays a key role in the metabolism,1 and the lack of this enzyme may cause some neurological symptoms.2 The measurement of the enzyme is of importance for evaluating the treatment results for some diseases,3 and the supplementation of dihydropyrimidine- and pyrimidine-related drugs may increase the level of the corresponding enzymes.4–6 Thus, the synthesis and the investigation of the biologically active pyrimidines and dihydropyrimidines have become attractive topics in organic and medicinal chemistry.7 Many efforts have been made to design novel strategies for preparing pyrimidines and dihydropyrimidines8 and to evaluate their pharmacological effects.9In the synthesis of DHPMs (dihydropyrimidines), the yield depends on the activity of the catalyst, and many studies focus on screening the activities of catalysts.10 Recently, we found that benzoyl and ferrocenoyl 3,4-dihydropyrimidin-2(1H)-one (-thione) (DHPM) can be synthesized under catalyst-free and solvent-free conditions. The radical-scavenging properties of the eleven obtained DHPMs (the structures and nomenclatures are listed in Table 1) have been screened by reacting with some radicals, and it is found that the phenolic hydroxyl group, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bond, and the ferrocene group exhibit different activities in trapping radicals.11 As a following research study, we herein determine the activities of these DHPMs in protecting DNA against radical-induced oxidation of DNA, aiming to reveal the functions of the phenolic hydroxyl group, the C
S bond, and the ferrocene group exhibit different activities in trapping radicals.11 As a following research study, we herein determine the activities of these DHPMs in protecting DNA against radical-induced oxidation of DNA, aiming to reveal the functions of the phenolic hydroxyl group, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bond, and the ferrocene group in inhibiting radical-induced oxidation of DNA.
S bond, and the ferrocene group in inhibiting radical-induced oxidation of DNA.
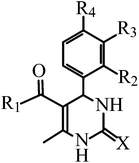
| Compound | X | R1 | R2 | R3 | R4 | |
|---|---|---|---|---|---|---|
| Abbreviation | Nomenclature | |||||
| a Fc = ferrocenyl group. | ||||||
| PDO | 5-Benzoyl-6-methyl-4-phenyl-3,4-dihydropyrimidin-2(1H)-one | O | Ph | H | H | H |
| PDS | (6-Methyl-4-phenyl-2-thioxo-1,2,3,4-tetrahydropyrimidin-5-yl)phenylmethanone | S | Ph | H | H | H |
| PDO-OH | 5-Benzoyl-4-(4′-hydroxyphenyl)-6-methyl-3,4-dihydropyrimidin-2(1H)-one | O | Ph | H | H | OH |
| PDS-OH | (4-(4′-Hydroxyphenyl)-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidin-5-yl)phenylmethanone | S | Ph | H | H | OH |
| PDO-2OH | 5-Benzoyl-4-(3′,4′-dihydroxyphenyl)-6-methyl-3,4-dihydropyrimidin-2(1H)-one | O | Ph | H | OH | OH |
| PDS-2OH | (4-(3′,4′-Dihydroxyphenyl)-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidin-5-yl)phenylmethanone | S | Ph | H | OH | OH |
| FDO | 5-Ferrocenoyl-6-methyl-4-phenyl-3,4-dihydropyrimidin-2(1H)-one | O | Fca | H | H | H |
| FDS | 5-Ferrocenoyl-6-methyl-4-phenyl-3,4-dihydropyrimidin-2(1H)-thione | S | Fc | H | H | H |
| FDO-OH | 5-Ferrocenoyl-6-methyl-4-(4′-hydroxyphenyl)-3,4-dihydropyrimidin-2(1H)-one | O | Fc | H | H | OH |
| FDS-OH | 5-Ferrocenoyl-6-methyl-4-(4′-hydroxyphenyl)-3,4-dihydropyrimidin-2(1H)-thione | S | Fc | H | H | OH |
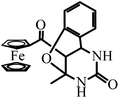
| FTC | 13-Ferrocenoyl-9-methyl-11-oxo-8-oxa-10,12-diazatricyclo[7.3.1.02,7]trideca-2,4,6-triene |
|
||||
The radical-induced oxidation of DNA is regarded as a carcinogenesis,12 and some experimental systems and measurement methods are applied to mimic DNA undergoing oxidation and to follow the oxidation process.13 2,2′-Azobis(2-amidinopropane hydrochloride) (AAPH, R–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–R, R = –CMe2C(
N–R, R = –CMe2C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH)NH2) as a radical resource can provide peroxyl radical (ROO˙) with a stable rate (Rg = (1.4 ± 0.2) × 10−6 [AAPH] s−1).14 The ROO˙ can abstract a hydrogen atom from the C-4′ position in the DNA strand, leading to the damage of DNA and producing carbonyl species eventually.15 The formed carbonyl species can be detected after reacting with thiobarbituric acid (TBA). Thus, it is convenient to follow the oxidative process of DNA by measuring the thiobarbituric acid reactive substance (TBARS) at 535 nm.16 Presented herein is a study on the antioxidant effects of eleven DHPMs (see Table 1) on AAPH-induced oxidation of DNA, and the ferrocene group is found to enhance the antioxidant capacity of DHPMs markedly, followed by the C
NH)NH2) as a radical resource can provide peroxyl radical (ROO˙) with a stable rate (Rg = (1.4 ± 0.2) × 10−6 [AAPH] s−1).14 The ROO˙ can abstract a hydrogen atom from the C-4′ position in the DNA strand, leading to the damage of DNA and producing carbonyl species eventually.15 The formed carbonyl species can be detected after reacting with thiobarbituric acid (TBA). Thus, it is convenient to follow the oxidative process of DNA by measuring the thiobarbituric acid reactive substance (TBARS) at 535 nm.16 Presented herein is a study on the antioxidant effects of eleven DHPMs (see Table 1) on AAPH-induced oxidation of DNA, and the ferrocene group is found to enhance the antioxidant capacity of DHPMs markedly, followed by the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bond and the phenolic hydroxyl group.
S bond and the phenolic hydroxyl group.
2. Experimental procedure
2.1. Materials and instrumentation
AAPH and the naked DNA sodium salt were purchased from ACROS ORGANICS, Geel, Belgium. Other agents were of analytical grade and used without treatment. DHPMs were synthesized and identified according to the description in our previous report.112.2. AAPH-induced oxidation of DNA and the test of the antioxidant abilities of DHPMs
The oxidation of DNA induced by AAPH was performed following our previous report.17 DNA and AAPH were dissolved in phosphate-buffered solution (PBS: 8.1 mM Na2HPO4, 1.9 mM NaH2PO4, 10.0 μM EDTA), and the final concentrations of DNA and AAPH were 2.0 mg mL−1 and 40 mM, respectively. Various concentrations of DHPMs were dissolved in DMSO and added to the aforementioned mixture. Then, the mixture was dispensed into test tubes, and each contained 2.0 mL. All the tubes were incubated in a water bath (37 °C) to initiate the reaction. Three tubes were taken out at appropriate intervals and cooled immediately, to which 1.0 mL of thiobarbituric acid (TBA) solution (1.00 g TBA and 0.40 g NaOH dissolved in 100 mL PBS) and 1.0 mL of 3.0% trichloroacetic acid aqueous solution were added. The tubes were heated in a boiling water bath for 15 min. After cooling, 1.5 mL of n-butanol was added and shaken vigorously to extract the thiobarbituric acid reactive substance (TBARS, λmax = 535 nm). The absorbance of the n-butanol phase was measured and plotted against incubation time.2.3. Statistical analysis
All the data were the average value from at least three independent measurements with the experimental error within 10%. The relationships between the concentrations of DHPMs and the reaction time were fitted in Origin 7.5 professional software, and p < 0.001 indicate a significant difference.3. Results
The DHPMs used herein can be cataloged as phenyl and ferrocenyl groups according to the kind of R1 group (see Table 1). Fig. 1 and 2 outline the variation of the absorbance of TBARS in the presence of different concentrations of phenyl and ferrocenyl DHPMs, respectively.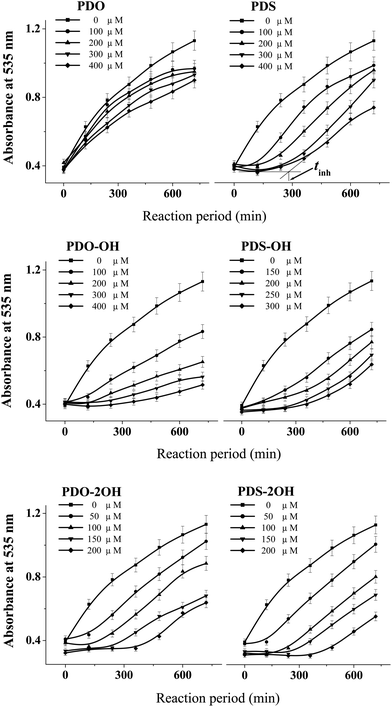 | ||
| Fig. 1 The variation of the absorbance of TBARS in the mixture of DNA (2.0 mg mL−1) and 40 mM AAPH at 37 °C in the presence of various concentrations of phenyl DHPMs. | ||
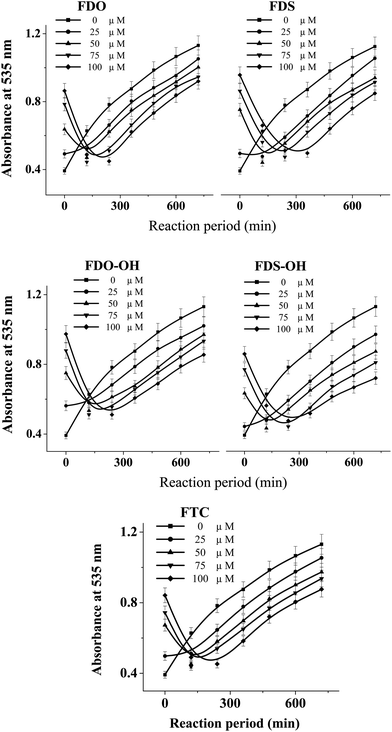 | ||
| Fig. 2 The variation of the absorbance of TBARS in the mixture of DNA (2.0 mg mL−1) and 40 mM AAPH at 37 °C in the presence of various concentrations of ferrocenyl DHPMs. | ||
The absorbance lines in the figure indicate that the amounts of TBARS increase with the reaction time, revealing that much more carbonyl species are produced in AAPH-induced oxidation of DNA. As can be seen from the panel of PDS in Fig. 1, the addition of PDS hinders the increase of the absorbance line in the first period, indicating that PDS can protect DNA against AAPH-induced oxidation of DNA and generate an inhibition period (tinh) eventually. The tinh can be measured from the cross point of the tangents for the inhibition and the increase period of the absorbance line. The relationships between the tinh and the concentration of phenyl DHPMs are outlined in Fig. 3. As can be seen in Fig. 3, tinh increases with the concentration of these phenyl DHPMs added into the oxidation system of DNA. However, Fig. 2 exhibits a different picture when ferrocenyl DHPMs are applied to protect DNA against AAPH-induced oxidation. The first point of the absorbance is higher than the second one in the case when high concentration of ferrocenyl DHPMs is used, because ferrocenyl DHPMs as colorful compounds have the absorbance at 535 nm. The absorbance at 535 nm decreases with the increase of the reaction period. After ferrocenyl DHPMs are exhausted completely, the absorbance at 535 nm increases owing to the formation of TBARS. The cross point of the tangent from these two periods can be assigned as the tinh. This phenomenon has been found in our previous report on the antioxidant effectiveness of carminic acid in the same experimental system.18 On the other hand, the introduction of a ferrocenyl group into organic scaffolds generates a colorful compound with the absorbance at 535 nm as well. We have synthesized ferrocenyl-appended ailanthoidol and have found that the introduction of a ferrocenyl group into ailanthoidol generates an absorbance at 535 nm, and this absorbance does not affect the measurement of tinh.19 Thus, the relationships between the tinh and the concentration of ferrocenyl DHPMs are illustrated in Fig. 3 as well. The ferrocenyl DHPMs can also increase tinh in a concentration-dependent manner. Therefore, both phenyl and ferrocenyl DHPMs are concentration-dependent antioxidants in protecting DNA against AAPH-induced oxidation. The linear correlations of the tinh with the concentration of DHPMs are quantitatively expressed by equations as shown in Table 2.
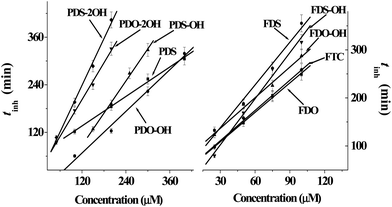 | ||
| Fig. 3 The linear relationship between the concentrations of phenyl and ferrocenyl DHPMs and inhibition period (tinh) in protecting DNA against AAPH-induced oxidation. | ||
| Compound | t inh (min) = (n/Ri) [DHPM (μM)] | n |
|---|---|---|
| a The value of n is the product of the coefficient of tinh ∼ [DHPM] and Ri = 1.4 × 10−6 × 40 mM s−1 = 3.36 μM min−1 when 40 mM AAPH is employed. | ||
| PDO | — | — |
| PDS | t inh = 0.62 (±0.03) [PDS] + 61.9 (±3.1) | 2.1 (±0.1) |
| PDO-OH | t inh = 0.87 (±0.04) [PDO-OH] − 37.2 (±1.9) | 2.9 (±0.1) |
| PDS-OH | t inh = 1.35 (±0.07) [PDS-OH] − 75.2 (±3.8) | 4.5 (±0.2) |
| PDO-2OH | t inh = 1.55 (±0.08) [PDO-2OH] + 17.0 (±0.8) | 5.2 (±0.3) |
| PDS-2OH | t inh = 1.96 (±0.10) [PDS-2OH] + 3.3 (±0.3) | 6.6 (±0.3) |
| FDO | t inh = 2.04 (±0.10) [FDO] + 47.2 (±2.4) | 6.8 (±0.3) |
| FDS | t inh = 2.97 (±0.15) [FDS] + 49.1 (±2.4) | 10.0 (±0.5) |
| FDO-OH | t inh = 2.13 (±0.11) [FDO-OH] + 72.7 (±3.6) | 7.2 (±0.3) |
| FDS-OH | t inh = 3.20 (±0.16) [FDS-OH] + 6.2 (±0.31) | 10.7 (±0.5) |
| FTC | t inh = 2.10 (±0.10) [FTC] + 47.8 (±2.39) | 7.1 (±0.3) |
Chemical kinetics reveals that the tinh is proportionally related to the concentration of the antioxidant as shown by eqn (1).20
| tinh = (n/Ri) [antioxidant] | (1) |
In eqn (1), n, the stoichiometric factor, denotes the number of the radical-propagation terminated by one molecule of the antioxidant. Ri is the initiation rate of the radical-induced reaction. We have used this equation to treat the data obtained from homoisoflavonoids in the same experimental system.21 Because both sodium salts of DNA and AAPH are water-soluble compounds and radicals generated from AAPH attack DNA in the same phase, Ri is assumed equal to the radical-generation rate, Rg = (1.4 ± 0.2) × 10−6 [AAPH] s−1.20 Thus, the n values of DHPMs are the products of coefficients in the corresponding equation multiplying Ri = Rg = 1.4 × 10−6 × 40 mM s−1 = 3.36 μM min−1 and are listed in Table 2. As can be seen in Table 2, the n values of ferrocenyl DHPMs are higher than those of phenyl DHPMs, indicating that ferrocenyl DHPMs possess higher antioxidant capacities than phenyl DHPMs. In addition, PDO does not have n value because the addition of PDO cannot generate tinh. But the panel of PDO in Fig. 1 shows that the absorbance lines in the presence of PDO are lower than those of the blank experiment, revealing that the addition of PDO also inhibits the formation of TBARS, and PDO can protect DNA against AAPH-induced oxidation although it cannot generate tinh.
4. Discussion
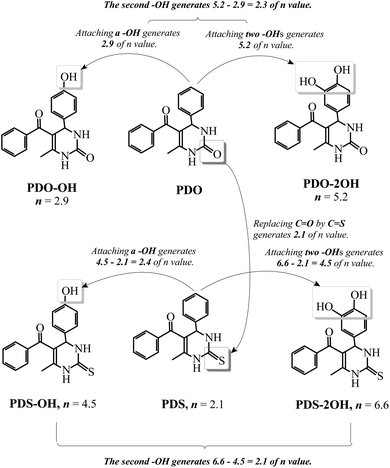
We have investigated the antioxidant abilities of Schiff bases to protect DNA against AAPH-induced oxidation by comparing n values.22 This comparison clarifies the contribution of every structural feature to the antioxidant capacity of the whole molecule. As can be seen in Scheme 1, by comparing the n of PDO with that of PDS, it can be found that replacing C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in DHPM by C
O in DHPM by C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S generates an n value of 2.1. This result reveals that C
S generates an n value of 2.1. This result reveals that C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S is able to improve the antioxidant effectiveness of DHPM. The n of PDO-OH is 2.9, indicating that one hydroxyl group can generate an n value of 2.9. The n of PDO-2OH is 5.2, demonstrating that introducing the second hydroxyl group into PDO just increases the n value by 5.2–2.9 = 2.3. Moreover, comparing the n value of PDS-OH with that of PDS, one can find that introducing one hydroxyl group into PDS leads to an n value of 4.5–2.1 = 2.4. The difference between the n values of PDS-2OH and PDS-OH is 6.6–4.5 = 2.1, indicating that the second hydroxyl group results in an n value of 2.1. This is very close to the result from comparing the n value of PDO-2OH with that of PDO-OH. The difference of n values of PDO-2OH and PDO-OH is 2.3. Based on the aforementioned comparison, it can be concluded that replacing C
S is able to improve the antioxidant effectiveness of DHPM. The n of PDO-OH is 2.9, indicating that one hydroxyl group can generate an n value of 2.9. The n of PDO-2OH is 5.2, demonstrating that introducing the second hydroxyl group into PDO just increases the n value by 5.2–2.9 = 2.3. Moreover, comparing the n value of PDS-OH with that of PDS, one can find that introducing one hydroxyl group into PDS leads to an n value of 4.5–2.1 = 2.4. The difference between the n values of PDS-2OH and PDS-OH is 6.6–4.5 = 2.1, indicating that the second hydroxyl group results in an n value of 2.1. This is very close to the result from comparing the n value of PDO-2OH with that of PDO-OH. The difference of n values of PDO-2OH and PDO-OH is 2.3. Based on the aforementioned comparison, it can be concluded that replacing C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O by C
O by C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S and introducing one hydroxyl group into DHPM result in an n value of 2–3, and thus, the influence of one phenolic hydroxyl group on the antioxidant effect of phenyl DHPM is similar to that of C
S and introducing one hydroxyl group into DHPM result in an n value of 2–3, and thus, the influence of one phenolic hydroxyl group on the antioxidant effect of phenyl DHPM is similar to that of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S.
S.
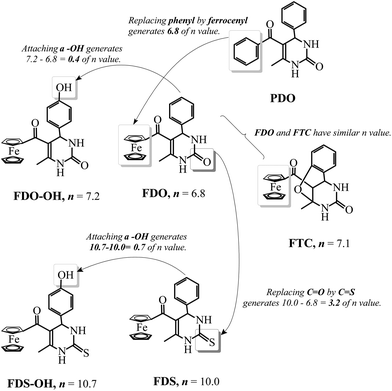
Recently, organometallic moieties are widely used in drug design for improving the pharmacological effect.23 The ferrocene moiety plays an important role in organometallic drugs because of the special affinity of the ferrocene group toward amino acids, proteins, DNA, and carbohydrates.24 Introducing the ferrocene group into organic scaffolds may change the redox property, potential cytotoxicity, and the lipophilicity of the drugs.25 We have synthesized some ferrocene-appended compounds including ferrocenyl-substituted ailanthoidol,19 Schiff bases,26 curcumin,27 and chalcones.28 It is found that the ferrocene group can enhance the abilities of the aforementioned compounds to inhibit the oxidation of DNA29 and to scavenge radicals.30 This research background motivates us to evaluate the influence of the ferrocene group on the antioxidant capacity of DHPM in protecting DNA against AAPH-induced oxidation. Scheme 2 outlines the variation of the n value when the ferrocene group, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S, and the phenolic hydroxyl group are introduced into DHPM scaffolds. By comparing the n values of FDO and PDO, it can be found that replacing the phenyl group in DHPM by the ferrocenyl group generates an n value of 6.8, demonstrating that the function of the ferrocenyl group is more important than the phenyl group for DHPM in protecting DNA against AAPH-induced oxidation. FTC is a product resulting from ortho-hydroxylbenzaldehyde as the reagent. The hydroxyl group adds to the C
S, and the phenolic hydroxyl group are introduced into DHPM scaffolds. By comparing the n values of FDO and PDO, it can be found that replacing the phenyl group in DHPM by the ferrocenyl group generates an n value of 6.8, demonstrating that the function of the ferrocenyl group is more important than the phenyl group for DHPM in protecting DNA against AAPH-induced oxidation. FTC is a product resulting from ortho-hydroxylbenzaldehyde as the reagent. The hydroxyl group adds to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C in DHPM via Michael addition, producing a tricyclic compound. The ferrocenyl group in FTC as the only active group protects DNA and generates an n value of 7.1, which is similar to that of FDO (6.8). Moreover, it can be found that the n value increases from 6.8 for FDO to 10.0 for FDS, indicating that replacing C
C in DHPM via Michael addition, producing a tricyclic compound. The ferrocenyl group in FTC as the only active group protects DNA and generates an n value of 7.1, which is similar to that of FDO (6.8). Moreover, it can be found that the n value increases from 6.8 for FDO to 10.0 for FDS, indicating that replacing C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O by C
O by C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S results in an n value of 10.0–6.8 = 3.2. Thus, the C
S results in an n value of 10.0–6.8 = 3.2. Thus, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S enhances the antioxidant ability more markedly in the presence of a ferrocenyl group than a phenyl group (see Scheme 1). On the other hand, comparing the n values of FDO-OH and FDO, it can be found that introducing a hydroxyl group into ferrocenyl DHPM just increases the n value by 0.4. In addition, the n value just increases by 0.7 when a hydroxyl group attaches to FDS to form FDS-OH, revealing that the phenolic hydroxyl group does not play a key role in the antioxidant capacity of ferrocenyl DHPM.
S enhances the antioxidant ability more markedly in the presence of a ferrocenyl group than a phenyl group (see Scheme 1). On the other hand, comparing the n values of FDO-OH and FDO, it can be found that introducing a hydroxyl group into ferrocenyl DHPM just increases the n value by 0.4. In addition, the n value just increases by 0.7 when a hydroxyl group attaches to FDS to form FDS-OH, revealing that the phenolic hydroxyl group does not play a key role in the antioxidant capacity of ferrocenyl DHPM.
5. Conclusion
As a kind of important antioxidant, DHPM is able to inhibit radical-induced oxidation of DNA for a period. The inhibition period is proportionally related to the concentration of DHPM employed. A chemical kinetic treatment results in the stoichiometric factor (n), and the n value makes it possible to quantitatively compare the antioxidant capacity of DHPM. In phenyl DHPM, the phenolic hydroxyl group and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S exhibit similar abilities to increase the antioxidant effectiveness, and more phenolic hydroxyl groups are beneficial for the antioxidant effect of phenyl DHPM. However, replacing the phenyl group by the ferrocenyl group remarkably enhances the antioxidant capacity of ferrocenyl DHPM, and the C
S exhibit similar abilities to increase the antioxidant effectiveness, and more phenolic hydroxyl groups are beneficial for the antioxidant effect of phenyl DHPM. However, replacing the phenyl group by the ferrocenyl group remarkably enhances the antioxidant capacity of ferrocenyl DHPM, and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S can markedly increase the antioxidant effect of ferrocenyl DHPM. Thus, the influence of the functional group on the antioxidant effectiveness of DHPM follows the order: ferrocenyl group > C
S can markedly increase the antioxidant effect of ferrocenyl DHPM. Thus, the influence of the functional group on the antioxidant effectiveness of DHPM follows the order: ferrocenyl group > C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bond > phenolic hydroxyl group. This information may be helpful for designing DHPM-related drugs.
S bond > phenolic hydroxyl group. This information may be helpful for designing DHPM-related drugs.
Acknowledgements
Financial support from Jilin Provincial Science and Technology Department, China, is gratefully acknowledged (20130206075GX).References
- K.-I. Sakata, M. Someya, Y. Matsumoto, H. Tauchi, M. Kai, M. Toyota, M. Takagi, M. Hareyama and M. Fukushima, Cancer Sci., 2011, 102, 1712–1716 CrossRef CAS PubMed.
- A. B. P. van Kuilenburg, J. Meijer, A. N. P. M. Mul, R. C. M. Hennekam, J. M. N. Hoovers, C. E. M. de Die-Smulders, P. Weber, A. C. Mori, J. Bierau, B. Fowler, K. Macke, J. O. Sass, R. Meinsma, J. B. Hennermann, P. Miny, L. Zoetekouw, R. Vijzelaar, J. Nicolai, B. Ylstra and M. E. Rubio-Gozalbo, Hum. Genet., 2009, 125, 581–590 CrossRef CAS PubMed.
- J. Brabender, R. Metzger, D. Vallböhmer, F. Ling, S. Neiss, E. Bollschweiler, P. M. Schneider, A. H. Hölscher and P. P. Grimminger, Surgery, 2012, 151, 306–312 CrossRef PubMed.
- J. Matsubara, T. Nishina, Y. Yamada, T. Moriwaki, T. Shimoda, T. Kajiwara, T. E. Nakajima, K. Kato, T. Hamaguchi, Y. Shimada, Y. Okayama, T. Oka and K. Shirao, Br. J. Cancer, 2008, 98, 832–839 CrossRef CAS PubMed.
- M. T. Ong, G. C. S. Chow and R. E. Morton, Pediatr. Neurol., 2012, 46, 178–181 CrossRef PubMed.
- W. B. Parker, Chem. Rev., 2009, 109, 2880–2893 CrossRef CAS PubMed.
- M. Y. Cha, K.-O. Lee, S.-J. Kang, Y. H. Jung, J. Y. Song, K. J. Choi, J. Y. Byun, H.-J. Lee, G. S. Lee, S. B. Park and M. S. Kim, J. Med. Chem., 2012, 55, 2846–2857 CrossRef CAS PubMed.
- A. Adhikari, B. Kalluraya, K. V. Sujith, Gouthamchandra and R. Mahmood, Saudi Pharm. J., 2012, 20, 75–79 CrossRef PubMed.
- J. E. Biggs-Houck, A. Younai and J. T. Shaw, Curr. Opin. Chem. Biol., 2010, 14, 371–382 CrossRef CAS PubMed.
- J. Yu, F. Shi and L.-Z. Gong, Acc. Chem. Res., 2011, 44, 1156–1171 CrossRef CAS PubMed.
- R. Wang and Z.-Q. Liu, J. Org. Chem., 2012, 77, 3952–3958 CrossRef CAS PubMed.
- T. B. Kryston, A. B. Georgiev, P. Pissis and A. G. Georgakilas, Mutat. Res., 2011, 711, 193–201 CrossRef CAS PubMed.
- M. S. Cooke, M. D. Evans, M. Dizdaroglu and J. Lunec, FASEB J., 2003, 17, 1195–1214 CrossRef CAS PubMed.
- J. Shao, N. E. Geacintov and V. Shafirovich, J. Phys. Chem. B, 2010, 114, 6685–6692 CrossRef CAS PubMed.
- P. Zhang and S. T. Omaye, Food Chem. Toxicol., 2001, 39, 239–246 CrossRef CAS.
- P. Møller and S. Loft, Environ. Health Perspect., 2010, 118, 1126–1136 CrossRef PubMed.
- C. Zhao and Z.-Q. Liu, Biochimie, 2011, 93, 1755–1760 CrossRef CAS PubMed.
- G.-X. Li, Z.-Q. Liu and D. Wu, J. Phys. Org. Chem., 2009, 22, 883–887 CrossRef CAS.
- C. Zhao and Z.-Q. Liu, Biochimie, 2012, 94, 1805–1811 CrossRef CAS PubMed.
- V. W. Bowry and R. Stocker, J. Am. Chem. Soc., 1993, 115, 6029–6044 CrossRef CAS.
- Y.-F. Li, Z.-Q. Liu and X.-Y. Luo, J. Agric. Food Chem., 2010, 58, 4126–4131 CrossRef CAS PubMed.
- F. Zhao and Z.-Q. Liu, J. Phys. Org. Chem., 2009, 22, 791–798 CrossRef CAS.
- C. S. Allardyce, A. Dorcier, C. Scolaro and P. J. Dyson, Appl. Organomet. Chem., 2005, 19, 1–10 CrossRef CAS.
- D. R. van Staveren and N. Metzler-Nolte, Chem. Rev., 2004, 104, 5931–5985 CrossRef CAS PubMed.
- U. Schatzschneider and N. Metzler-Nolte, Angew. Chem., Int. Ed., 2006, 45, 1504–1507 CrossRef CAS PubMed.
- Y.-F. Li and Z.-Q. Liu, Eur. J. Pharm. Sci., 2011, 44, 158–163 CrossRef CAS PubMed.
- P.-Z. Li and Z.-Q. Liu, Eur. J. Med. Chem., 2011, 46, 1821–1826 CrossRef CAS PubMed.
- G. Nabi and Z.-Q. Liu, Bioorg. Med. Chem. Lett., 2011, 21, 944–646 CrossRef CAS PubMed.
- F. Zhao, C. Zhao and Z.-Q. Liu, J. Biol. Inorg. Chem., 2011, 16, 1169–1176 CrossRef CAS PubMed.
- G. Nabi and Z.-Q. Liu, Med. Chem. Res., 2012, 21, 3015–3020 CrossRef CAS.
| This journal is © the Partner Organisations 2014 |