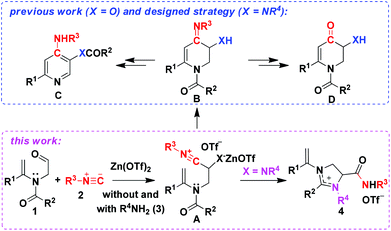
Functionalized imidazoliniums from the three-component domino reaction of N-formylmethylcarboxamides with amines and isocyanides†
Chuan-Hu
Lei
ab,
Liang
Zhao
a,
De-Xian
Wang
b,
Jieping
Zhu
c and
Mei-Xiang
Wang
*a
aKey Laboratory of Bioorganic Phosphorus Chemistry and Chemical Biology (Ministry of Education), Department of Chemistry, Tsinghua University, Beijing 100084, China. E-mail: wangmx@mail.tsinghua.edu.cn
bBeijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Molecular Recognition and Function, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China
cLaboratory of Synthesis and Natural Products, Institute of Chemical Sciences and Engineering, Ecole Polytechnique Fédérale de Lausanne, EPFL-SB-ISIC-LSPN, BCH 5304, 1015 Lausanne, Switzerland
First published on 18th August 2014
Abstract
In the presence of Zn(OTf)2, the three-component domino reaction of N-formylmethyl-substituted tertiary amides and enamides with amines and isocyanides in acetonitrile at room temperature produced functionalized imidazoliniums in good to excellent yields.
2-Substituted imidazolinium salts constitute an interesting and useful class of heterocyclic compounds. Because of resistance to bases, for example, ionic liquid 2-alkyl and 2-aryl imidazolinium salts are used as green media under basic reaction conditions.1 Functionalized 2-aryl imidazolinium salts have also been found to be able to catalyze Diels–Alder and aza-Diels–Alder reactions.2 Furthermore, it has been shown that 2-aryl imidazolinium salts derived from chiral diamines act as chiral shift reagents to discriminate a racemic sample of potassium Mosher's carboxylate.2a,3 Moreover, imidazoliniums provide a simple model of tetrahydrofolate (THF) coenzymes, facilitating the study of the group transfer reaction in biosynthesis and metabolism.4 Among the syntheses of 2-substituted imidazolinium salts reported in the literature, N-alkylation of 2-substituted imidazolines is the most frequently used method.3,5 Oxidation of imidazolidines,1b,2a,b and palladium-catalyzed coupling of imines, CO and acid chlorides6 have also provided synthetic routes to 2-substituted imidazolinium salts. However, these methods suffer from limitations and drawbacks such as (1) multi-step synthesis of precursors, (2) low selectivity or (3) difficulty in functionalization. Development of efficient and practical methods for the construction of functionalized 2-substituted imidazolinium salts7 is challenging and highly desirable.
Multicomponent reactions8 and domino reactions9 have become very popular strategies in organic synthesis because they provide opportunities for constructing molecules of complexity and diversity from single and generally easy operations. Successful and fruitful multicomponent and domino syntheses rely on, however, the versatility of reactants and the designed reaction pathways. Based on their unique and versatile reactivities,10 for instance, isocyanides have been extensively employed in multicomponent and domino reactions, yielding a wide variety of compounds, including natural product-like and biologically active ones, that are not readily accessible by other methods.11
For years, we12 have been exploring the reactions and synthetic applications of tertiary enamides. Being variants of conventional enamines, tertiary enamides exhibit diminished nucleophilicity due to the effect of the N-electron-withdrawing group that alleviates the delocalization of nitrogen lone-pair electrons into enaminic carbon.13 By means of regulating the cross-conjugation system of the enamide segment, we have demonstrated that tertiary enamides are valuable starting materials in synthesis.12 Very recently, we discovered that N-formylmethyl-substituted tertiary enamides 1 undergo Zn(OTf)2-promoted [5 + 1] cycloaddition reaction with isocyanides 2 to form six-membered heterocyclic intermediate B through A. Termination of the reaction cascade by oxidative aromatization and hydrolysis furnished poly-substituted pyridines C and 2,3-dihydropyridin-4(1H)-ones D, respectively (see previous work in Scheme 1).14 As a logical extension, we then became interested in the three-component reaction of tertiary enamides, amines and isocyanides, aiming at the synthesis of amino-substituted pyridine and 2,3-dihydropyridin-4(1H)-one derivatives (see the designed strategy in Scheme 1) that possess interesting biological activities such as blocking the voltage-dependent potassium channel, promoting the release of acetylcholine in the nerve endings, and thus can be used for the treatment of Lambert–Eaton myasthenic syndrome (LEMS), reducing the symptoms of downbeat nystagmus (DBN) and improving the cognitive functions during aging.15 Surprisingly, no targeted products were produced at all from the designed three-component reaction. Instead, the reaction afforded unexpectedly imidazolinium salts as the sole products (see this work in Scheme 1). We report herein a novel and efficient synthesis of functionalized imidazoliniums by a one-pot three-component domino reaction of N-alkenyl- and N-alkyl-bearing N-formylmethylcarboxamides with isocyanides and aliphatic amines under very mild conditions. The 18O-labeling experiment reveals an intriguing reaction pathway which comprises the formation and fragmentation of a bridged heterocyclic intermediate.
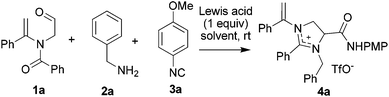
We initiated our study with the examination of the reaction of N-formylmethyl-substituted enamide 1a with benzylamine 2a and para-methoxyphenyl (PMP) isocyanide 3a (Table 1). Initially, the reaction was conducted by preparation of imine from the reaction of 1a and 2a followed by the addition of 3a in the presence of one equivalent of Zn(OTf)2 under the standard conditions14 for the reaction of N-formylmethyl-substituted enamide 1a with para-methoxyphenyl (PMP) isocyanide 3a. Outside of our expectation, the reaction did not form the designed six-membered product. Instead, a five-membered heterocyclic compound, viz. imidazolinium triflate 4a, was obtained in 70% yield as the sole product (entry 1, Table 1). The structure of 4a was elucidated on the basis of spectroscopic data and determined unambiguously by single crystal X-ray diffraction analysis (Fig. 1). In order to alter the reaction pathways, other Lewis acids and Brønsted acids were surveyed. As indicated by the results summarized in Table 1, however, formation of products other than 4a was not observed. The use of Sc(OTf)3, Sm(OTf)3 and In(OTf)3, for example, afforded 4a in yield ranging from 42% to 50% (entries 2–4, Table 1). The reaction mediated by Cu(OTf)2 gave rise to a very low chemical yield of 4a (entry 5, Table 1) whereas no three-component reaction took place when [Cu(CH3CN)4]PF6 was applied. In the latter case, the reaction resulted in nearly quantitative formation of tetra(p-methoxyphenyl isocyanide)copper(I) hexafluorophosphate whose structure was determined by X-ray crystallography (entry 6, Table 1).16 It was interesting to note that trifluoromethanesulfonic acid (TfOH) was also able to effect the three-component reaction rapidly albeit the yield of product 4a was only moderate (entry 7, Table 1). The reaction promoted by ZnCl2 proceeded analogously, affording imidazolinium chloride in 52% yield (entry 8, Table 1). An almost equally efficient reaction was observed when acetonitrile was replaced by chloroform (entry 9, Table 1). An elongated reaction time was required for the reaction in toluene, THF and, particularly, in methanol, with product 4a being isolated in slightly diminished yields (entries 10–12, Table 1).
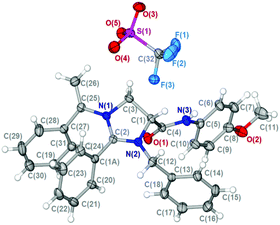 | ||
| Fig. 1 X-ray molecular structure of 4a (CCDC 1015230). | ||
| Entry | Lewis acid | Solvent | Time | 4a (%) |
|---|---|---|---|---|
a The ratio between 1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2a 2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3a was 1 3a was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2. Imine was firstly prepared from the reaction of 1a and 2a, and was then treated with 3a.
b Isolated yield.
c [Cu(PMPNC)4]PF6 complex was obtained in 97% yield.
d The chloride salt complexed with ZnCl2 was obtained as a product.
e One-pot reaction of 1a with 2a and 3a. 1.2. Imine was firstly prepared from the reaction of 1a and 2a, and was then treated with 3a.
b Isolated yield.
c [Cu(PMPNC)4]PF6 complex was obtained in 97% yield.
d The chloride salt complexed with ZnCl2 was obtained as a product.
e One-pot reaction of 1a with 2a and 3a.
|
||||
| 1 | Zn(OTf)2 | CH3CN | 2 h | 70 |
| 2 | Sc(OTf)3 | CH3CN | 1.5 h | 42 |
| 3 | Sm(OTf)3 | CH3CN | 1.5 h | 50 |
| 4 | In(OTf)3 | CH3CN | 2 h | 48 |
| 5 | Cu(OTf)2 | CH3CN | 2 h | 21 |
| 6 | [Cu(CH3CN)4]PF6 | CH3CN | 7 h | —c |
| 7 | TfOH | CH3CN | 10 min | 41 |
| 8 | ZnCl2 | CH3CN | 2 h | 52d |
| 9 | Zn(OTf)2 | CHCl3 | 1 h | 64 |
| 10 | Zn(OTf)2 | Toluene | 6 h | 55 |
| 11 | Zn(OTf)2 | THF | 6 h | 67 |
| 12 | Zn(OTf)2 | CH3OH | 48 h | 50 |
| 13 | Zn(OTf)2 | CH3CN | 2 h | 85 |
To simplify the operation, a one-pot reaction was executed simply by mixing N-formylmethyl-substituted enamide 1a with para-methoxyphenyl (PMP) isocyanide 2a and benzylamine 3a in the presence of one equivalent of Zn(OTf)2 in acetonitrile. Pleasingly, the reaction was found to proceed smoothly at ambient temperature to afford product 4a in an improved yield (85%) in 2 h (entry 13, Table 1).
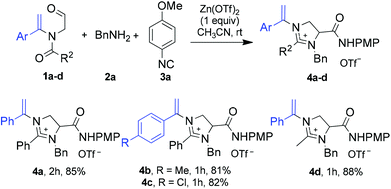
Under optimized one-pot reaction conditions, the scope and limitations of this unprecedented three-component reaction were investigated using other tertiary enamides (Scheme 2). Irrespective of the electronic feature of the substituent on the benzene ring, N-aroyl-containing tertiary enamides 1b (R = 4-MeC6H4) and 1c (4-ClC6H4) underwent a three-component reaction as efficiently as 1a to produce 2-aryl-substituted imidazolinium salts 4b and 4c in 81% and 82% yield, respectively. In the case of N-acetyl-substituted enamide 1d (Ar = Ph, R2 = Me), the reaction led to the formation of 2-methylated imidazolinium salt 4d in 88% yield. The higher chemical yield obtained for 4d is probably due to the lower enaminic reactivity of N-acetyl-bearing enamide reactant 1d than that of N-aroyl-substituted analogues 1a–1c. It appeared that higher enaminic reactivity would cause competitive side reactions such as intramolecular cyclization and ring opening reaction (vide infra), corroding the yield of imidazolinium product.
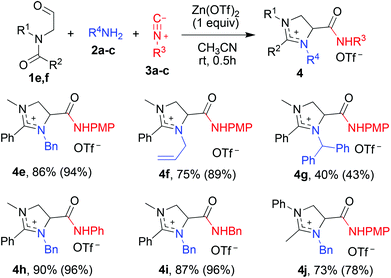
Since the carbon–carbon double bond of tertiary enamides did not participate in the cyclization, we then extended substrates from tertiary enamides 1a–d into N-formylmethyl-N-methylbenzamide 1e (R1 = Me, R2 = Ph) and N-formylmethyl-N-phenylacetamide 1f (R1 = Ph, R2 = Me, Scheme 3). In addition to benzylamine 2a and para-methoxyphenyl isocyanide 3a, aliphatic amines such as allylamine 2b and (1,1-diphenylmethyl)amine 2c, and isocyanides such as phenyl isocyanide 3b and benzyl isocyanide 3c were employed in the study. To our delight, as depicted in Scheme 3, all substrates tested underwent the same reaction effectively, demonstrating the versatility of diversity-orientated three-component domino reaction. When N-formylmethyl-N-methylbenzamide 1e was applied as the input, for example, the domino reaction with benzylamine 2a and para-methoxyphenyl isocyanide 3a gave imidazolinium salt 4e in 86% yield. A good yield (75%) of N-allylated product 4f was obtained from the reaction with allylamine 2b. (Diphenylmethyl)amine 2c, a steric bulky amine, was also accepted as a substrate, albeit the corresponding product 4g was isolated in a diminished yield. Remarkably, both aromatic isocyanides like 2a and 2b and aliphatic one 2c underwent highly efficient reaction to generate products 4e, 4h and 4i, respectively, in excellent yields. N-Formylmethyl-N-phenylacetamide 1f acted similarly as benzamide analog 1e, and its reaction with 2a and 3a yielded 3-benzyl-2-methyl-1-phenylimidazolinium 4j in 73% yield (Scheme 3). It should be noted that all three-component reactions employing N-formylmethylcarboxamides gave improved chemical yields of 2-substituted imidazolinium salts when imines were pre-formed from the interaction of aldehydes and amines (Scheme 3, chemical yields in parentheses). The slightly lower chemical yield obtained from the three-component reaction in a one-pot manner than in a step-wise fashion was most likely due to the side reactions between N-formylmethylcarboxamides and isocyanides.
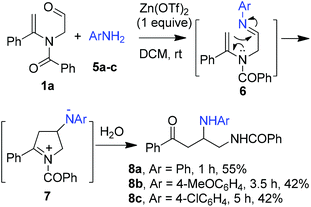
It should be pointed out that the same three-component domino reaction did not proceed when anilines 5 were utilized instead of aliphatic amines. Under the identical conditions, the reaction of N-formylmethyl-substituted enamide 1a, for example, gave diamine derivatives 8 as the major products in which isocyanides were not involved. It is probably the higher reactivity of aromatic imines 6 generated in situ from the interaction of aldehyde 1 and anilines 5 that is in favor of intramolecular cyclization with the enamide moiety to form intermediate 7. Further hydrolysis of iminium ion 7 led to the ring opening products 8 (Scheme 4).
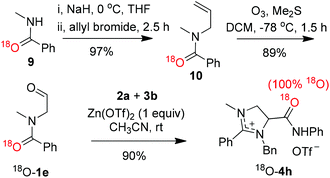
The formation of imidazolinium salts 4 from the reaction of N-formylmethyl-substituted amides 1 with aliphatic amines 2 and isocyanides 3 implied an intriguing and complex reaction pathway. To shed light on the reaction mechanism, especially the origin of the oxygen of amide group in molecule 4, an 18O-labeling experiment was carried out. Scheme 5 shows the synthesis of 18O-labeled amide 18O-1e from N-allylation and subsequent ozonization of 18O-labeled N-methylbenzamide 9.17 The same three-component reaction involving 18O-1e, benzylamine 2a and phenyl isocyanide 3b afforded almost quantitatively 18O-labeled imidazolinium salt 18O-4h. The formation of 18O-4h as the sole product, as evidenced by mass spectral data, indicated clearly the complete conversion of 18O-1e into 18O-4h. It also excluded the possibility of transformation of the aldehyde moiety of 1 into the amide functionality of the product and of the involvement of moisture in the reaction.
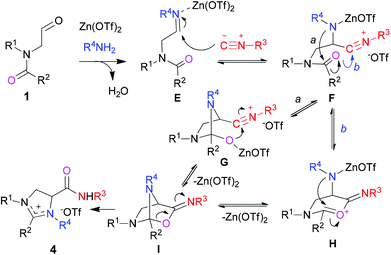
On the basis of the aforementioned results, a plausible mechanism for the three-component domino reaction was proposed. As depicted in Scheme 6, condensation reaction between aliphatic amines 2 and aldehydes 1 gives imine intermediates E with the release of water. Activated by Zn(OTf)2, imines E are attacked by isocyanides 3 to afford nitrilium intermediates F which undergo intramolecular nucleophilic addition of the nitrogen anion to the amide group (pathway a). Cyclization of the resulting heterocyclic intermediates G between alkoxide and nitrilium moieties leads to the formation of 3-imino-2-oxa-6,7-diazabicyclo[2.2.1]heptane intermediates I. Alternatively, the nitrilium F was first trapped by the amido carbonyl oxygen to give six-membered oxonium intermediate H which was further captured by the nitrogen anion to form intermediate I (pathway b). Finally, ring opening reaction via the cleavage of the C–O bond assisted by lone-pair electrons of nitrogen furnishes the production of imidazolinium triflates 4. It is worth addressing that since the nucleophilicity of the nitrogen anion is stronger than the enaminic carbon of tertiary enamide (R1 = CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(Ar)–), the resulting intermediate F tends to undergo addition reaction between the nitrogen anion and carbonyl rather than between enamide and nitrilium. In the case of reactions using aromatic amines, on the other hand, intramolecular addition reaction between enamide (R1 = CH2
C(Ar)–), the resulting intermediate F tends to undergo addition reaction between the nitrogen anion and carbonyl rather than between enamide and nitrilium. In the case of reactions using aromatic amines, on the other hand, intramolecular addition reaction between enamide (R1 = CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(Ar)–) and aromatic imine takes place preferentially in comparison to intermolecular addition of isocyanide to aromatic imine within intermediate E, thus diverting the reaction pathway to the formation of diamine product 8 (see Scheme 4).
C(Ar)–) and aromatic imine takes place preferentially in comparison to intermolecular addition of isocyanide to aromatic imine within intermediate E, thus diverting the reaction pathway to the formation of diamine product 8 (see Scheme 4).
Conclusions
In conclusion, we have developed an unprecedented three-component domino reaction of various N-formylmethyl-substituted amides with isocyanides and aliphatic amines. The Zn(OTf)2-mediated reaction provides an expedient synthetic route to functionalized imidazoliniums under very mild conditions. An 18O-labeling experiment reveals an intriguing mechanism involving most likely the bridged heterocyclic intermediate.Acknowledgements
We thank the National Natural Science Foundation of China (21320102002) and Tsinghua University for financial support.Notes and references
- (a) J.-C. Hsu, Y.-H. Yen and Y.-H. Chu, Tetrahedron Lett., 2004, 45, 4673 CrossRef CAS PubMed; (b) V. Jurčík and R. Wilhelm, Green Chem., 2005, 7, 844 RSC.
- (a) V. Jurčík and R. Wilhelm, Tetrahedron: Asymmetry, 2006, 17, 801 CrossRef PubMed; (b) V. Jurčík and R. Wilhelm, Org. Biomol. Chem., 2005, 3, 239 RSC; (c) O. Sereda, N. Clemens, T. Heckel and R. Wilhelm, Beilstein J. Org. Chem., 2012, 8, 1798 CrossRef CAS PubMed.
- A. Winkel and R. Wilhelm, Tetrahedron: Asymmetry, 2009, 20, 2344 CrossRef CAS PubMed.
- (a) Y. Zhang, D. Li, C. Xia and W. Guo, Heterocycles, 2005, 65, 2893 CrossRef CAS; (b) C. Xia, J. Hao, Y. Tang, Y. Ni and P. Zhou, Synth. Commun., 2002, 32, 1457 CrossRef CAS PubMed; (c) C. Xia, J. Chen, B. J. Zhao, H. Wang, C. Kang, Y. Ni and P. Zhou, Synth. Commun., 2002, 32, 1129 CrossRef CAS PubMed; (d) C. Xia, J. Chen, H. Wang, C. Kang, B. Zhao, Y. Ni and P. Zhou, Synth. Commun., 2002, 32, 2979 CrossRef CAS PubMed.
- C. Xia, H. Wang, B. Zhao, J. Chen, C. Kang, Y. Ni and P. Zhou, Synth. Commun., 2002, 32, 1447 CrossRef CAS PubMed.
- (a) K. Worrall, B. Xu, S. Bontemps and B. A. Arndtsen, J. Org. Chem., 2011, 76, 170 CrossRef CAS PubMed; (b) B. Xu, K. Worrall and B. A. Arndtsen, Molecules, 2012, 17, 13759 CrossRef CAS PubMed.
- Some long chain substituted imidazolinium salts exhibit the good properties of surfactants: (a) D. Bajpai and V. K. Tyagi, Eur. J. Lipid Sci. Technol., 2008, 110, 935 CrossRef CAS PubMed; (b) D. Bajpai and V. K. Tyagi, J. Surfactants Deterg., 2008, 11, 79 CrossRef CAS.
- (a) J. Zhu and H. Bienaymé, in Multicomponent Reactions, Wiley-VCH, Weinheim, 2005 Search PubMed; (b) R. C. Cioc, E. Ruijter and R. V. A. Orru, Green Chem., 2014, 16, 2958 RSC.
- For reviews, see: (a) L. F. Tietze, Chem. Rev., 1996, 96, 115 CrossRef CAS PubMed; (b) D. Enders, C. Grondal and M. R. M. Hüttl, Angew. Chem., Int. Ed., 2007, 46, 1570 CrossRef CAS PubMed.
- (a) V. G. Nenajdenko, in Isocyanide Chemistry: Applications in Synthesis and Material Science, Wile-VCH, Weinheim, 2012 Search PubMed; (b) I. Ugi, in Isonitrile Chemistry, Academic Press, New York, 1971 Search PubMed.
- (a) J. Zhu, Eur. J. Org. Chem., 2003, 1133 CrossRef CAS PubMed; (b) A. Dömling, Chem. Rev., 2006, 106, 17 CrossRef PubMed; (c) V. Nair, C. Rajesh, A. U. Vinod, S. Bindu, A. R. Sreekanth, J. S. Mathen and L. Balagopal, Acc. Chem. Res., 2003, 36, 899 CrossRef CAS PubMed.
- (a) S. Tong, D.-X. Wang, L. Zhao, J. Zhu and M.-X. Wang, Angew. Chem., Int. Ed., 2012, 51, 4417 CrossRef CAS PubMed; (b) S. Tong, X. Yang, D.-X. Wang, L. Zhao, J. Zhu and M.-X. Wang, Tetrahedron, 2012, 68, 6492 CrossRef CAS PubMed; (c) L. Yang, C.-H. Lei, D.-X. Wang, Z.-T. Huang and M.-X. Wang, Org. Lett., 2010, 12, 3918 CrossRef CAS PubMed; (d) L. Yang, D.-X. Wang, Z.-T. Huang and M.-X. Wang, J. Am. Chem. Soc., 2009, 131, 10390 CrossRef CAS PubMed; (e) L. Yang, Q.-Y. Zheng, D.-X. Wang, Z.-T. Huang and M.-X. Wang, Org. Lett., 2008, 10, 2461 CrossRef CAS PubMed; (f) L. Yang, G. Deng, D.-X. Wang, Z.-T. Huang, J.-P. Zhu and M.-X. Wang, Org. Lett., 2007, 9, 1387 CrossRef CAS PubMed.
- (a) M. Ōki, in Applications of Dynamic NMR Spectroscopy to Organic Chemistry, Wiley-VCH, Weinheim, 1985, pp. 43–46 Search PubMed; (b) M. T. Rogers and J. C. Woodbrey, J. Phys. Chem., 1962, 66, 540 CrossRef CAS.
- (a) C.-H. Lei, D.-X. Wang, L. Zhao, J. Zhu and M.-X. Wang, J. Am. Chem. Soc., 2013, 135, 4708 CrossRef CAS PubMed; (b) C.-H. Lei, D.-X. Wang, L. Zhao, J. Zhu and M.-X. Wang, Chem. – Eur. J., 2013, 19, 16981 CrossRef CAS PubMed.
- (a) D. M. Green, A. C. Jones and K. R. Brain, J. Clin. Pharm. Ther., 2012, 37, 53 CrossRef CAS PubMed; (b) T. Tsunemi, K. Ishikawa, K. Tsukui, T. Sumi, K. Kitamura and H. Mizusawa, J. Neurol. Sci., 2010, 292, 81 CrossRef CAS PubMed; (c) M. R. Garovoy, P. E. Haroldsen and D. G. Musson, WO003708 A1, 2013 Search PubMed; (d) S. Sedehizadeh, P. Maddison and M. Keogh, Orphan Drugs: Res. Rev., 2014, 4, 11 CrossRef; (e) F. Guyou, D. Pradeau, M. D. L. Hoang and J.-J. Houri, US0106651 A1, 2004 Search PubMed.
- Tetra(p-methoxyphenyl isocyanide)copper(I) hexafluorophosphate complex has been reported previously: C. L. Perrine, M. Zeller, J. Woolcock, T. M. Styranec and A. D. Hunter, J. Chem. Crystallogr., 2009, 40, 289 CrossRef.
- PhC18O2H was synthesized from PhCCl3 and H218O by slight modification of a previously reported procedure: M. Kobayashi and R. Kiritani, Bull. Chem. Soc. Jpn., 1966, 39, 1782 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1015230. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4qo00203b |
| This journal is © the Partner Organisations 2014 |