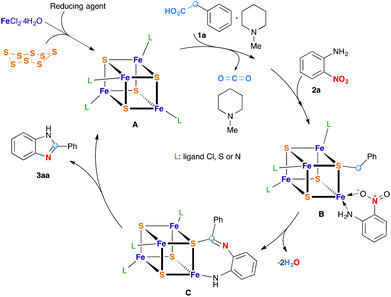
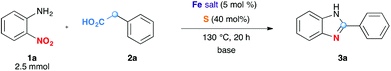Fe/S-catalyzed decarboxylative redox condensation of arylacetic acids with nitroarenes†
Thanh Binh
Nguyen
*,
Ludmila
Ermolenko
,
Mathilde
Corbin
and
Ali
Al-Mourabit
*
Centre de Recherche de Gif-sur-Yvette, Institut de Chimie des Substances Naturelles, CNRS, 91198 Gif-sur-Yvette Cedex, France. E-mail: nguyen@icsn.cnrs-gif.fr; ali.almourabit@cnrs.fr
First published on 22nd September 2014
Abstract
Fe/S clusters generated in situ from simple iron salts and sulfur S8 were found to be highly efficient to catalyze the decarboxylative redox condensation of arylacetic acids with nitroarenes in the presence of N-methylpiperidine as a basic additive. A wide range of aza-heterocycles was obtained in an atom-, step-, and redox-economical manner with water and carbon dioxide as the only by-products.
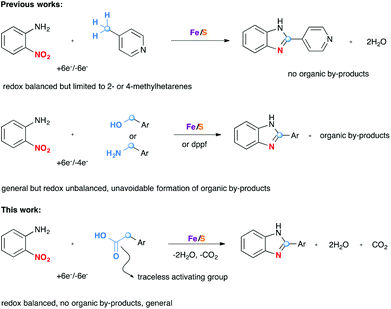
Enzymatic transformations have been an inexhaustible source of inspiration for progress in organic synthesis and catalysis. Decarboxylative coupling reaction of carboxylic acids is a versatile method to create carbon–carbon and carbon–heteroatom bonds.1 Although the process is thermodynamically favorable due to the formation of stable carbon dioxide by-products, the non-enzymatic reaction requires in general high temperatures and/or strong oxidizing agents in the presence of metal catalysts. Intriguingly, such transformations which are common and fundamentally important in biology can be conducted efficiently under physiological conditions in the presence of some decarboxylases.2 The active sites of some of these enzymes were found to contain iron–sulfur clusters.3 The oxidation states of the iron atoms in these clusters change between +2 and +3, the effect being the charge and discharge process of multiple electrons of these biological capacitors. This way the enzyme acts as electron transfer agents in biological redox reactions. Organic synthesis can take inspiration from nature to design versatile assemblage for biomimetic transformations and catalysts.
Recently, several groups4–6 have begun to explore catalytic redox coupling reactions starting directly from aromatic nitro compounds as a convenient and direct method to aza heterocycles without prior reduction of the nitro group. In connection with the widespread interest in the development of a more sustainable redox chemistry, we are particularly interested in exploring the versatility of biomimetic catalysts based on iron/sulfur clusters generated from iron or its simple salts and elemental sulfur.4,6 This idea is linked to the redox active iron–sulfur clusters due to their capacity of reversibly taking up electrons from a reducing partner and transferring them to an oxidant partner. Different reducing partners such as methylhetarenes, benzyl alcohols, and amines have been found to be successful substrates in these reactions (Scheme 1). Although methylhetarene substrates are efficient in terms of the number of electrons transferred, the methyl group must be located at the 2- or 4-position of the aza-heterocycles for an efficient stabilization by resonance.4a The high inertness of the methyl group of other hetarenes or arenes (for example 3-picoline or toluene) required the use of other prefunctionalized compounds such as ArCH2OH5c or RCH2NH2.6a Indeed, reaction of benzyl alcohols or amines with o-nitroanilines led to benzimidazoles. However, excess of alcohols/amines must be used to compensate the difference in electron numbers between the nitro group and the –CH2OH/–CH2NH2 group during the redox process. This unbalanced exchange led consequently to the formation of organic by-products in the oxidation of CH2OH/CH2NH2 groups of the reducing partners.
We reasoned that the use of an acetic acid function (HO2CCH2–), with a traceless activating carboxylic acid group, as a synthetic equivalent of a methyl group, would greatly expand the scope while maintaining a full electronic balance of the redox process. Moreover, this idea is supported by the fact that carboxylic acids are in general readily available starting materials in great structural diversity. As far as we are aware, no example of such a decarboxylative redox condensation has been reported prior to this study.
To test this idea, we chose o-nitroaniline and phenylacetic acid as prototypical substrates. Based on our previous experience on using Fe/S as the redox condensation catalyst, FeCl3·6H2O (5 mol%) and S (40 mol%) were used as precursors for the catalyst (Table 1).7
| Entry | 2a equiv. | Base (equiv.) | Iron salt | Conversionb (%) | |
|---|---|---|---|---|---|
| a Conditions: 1a (2.5 mmol), 2a (1.2–2 mmol), iron salt (5 mol%), sulfur (40 mol%, 1 mmol, 32 mg), base (0.4–2 equiv.). b Determined by 1H NMR of the crude mixture. c Yield of isolated 3aa. | |||||
| 1 | 2 |
|
2 | FeCl3·6H2O | 16 |
| 2 | 2 |
|
2 | FeCl3·6H2O | 16 |
| 3 | 2 |
|
2 | FeCl3·6H2O | 35 |
| 4 | 2 |
|
2 | FeCl3·6H2O | 87 |
| 5 | 2 |
|
2 | FeCl3·6H2O | 83 |
| 6 | 2 |
|
2 | FeCl3·6H2O | 74 |
| 7 | 2 |
|
1.5 | FeCl3·6H2O | 89(53)c |
| 8 | 1.2 |
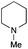
|
1.5 | FeCl3·6H2O | 91 |
| 9 | 1.2 |
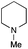
|
0.4 | FeCl3·6H2O | 93 |
| 10 | 1.2 |
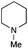
|
0.4 | FeCl2·4H2O | >95(82) |
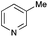
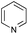
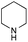
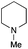
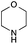
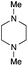
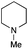
The reaction in 3-picoline at 130 °C resulted in the formation of 2-phenylbenzimidazole in low conversion (entry 1). The same result was observed with pyridine as an additive (entry 2). We hypothesized that basic conditions are necessary because the decarboxylation is initiated preferentially with a carboxylate form. To improve the conversion, the screening of different basic additives was envisioned. A range of aza bases which are stable to the oxidation of elemental sulfur has been tested (entries 3–6). In all cases investigated, we noticed that all the bases stronger than 3-picoline are more efficient to promote this condensation.8 The best result was observed with N-methylpiperidine (entry 4). Finally, strong inorganic bases such as NaOH and Na2S were also tested but lower conversions were observed in these cases (results not shown).
Encouraged by these results, we continued the optimization by lowering the quantity of the base additive N-methylpiperidine. Interestingly, the conversion was improved (entry 7) but the purification remained inefficient due to the presence of the base additive and excess phenylacetic acid. The reaction was thus conducted by lowering the quantities of both 2a and N-methylpiperidine (entries 8 and 9) and led to higher conversions. Finally, replacing FeCl3·6H2O by FeCl2·4H2O gave a slightly better conversion (entry 12) and the purification was simplified and more efficient (82%).9
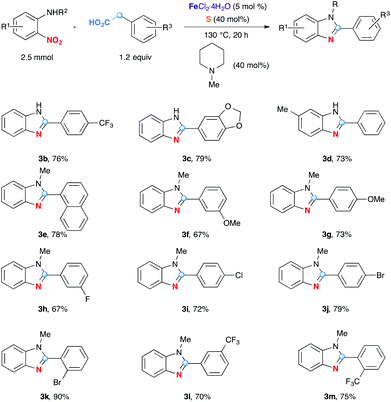
The next series of experiments evaluated the effect of substituents on the efficiency of the reaction. As shown in Scheme 2, this decarboxylative redox condensation can be applied to a variety of substrates to produce the corresponding 2-arylbenzimidazoles. In addition to NH benzimidazoles 3b–d, N-methyl analogues could also be obtained in high yields 3e–m. Functional groups such as OMe (3f–g), dioxymethylene (3c), F (3h), Cl (3i), Br (3j–k), CF3 (3b, l–m) at different positions are well tolerated.
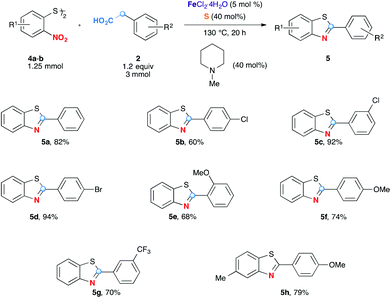
The method was further successfully extended to the formation of benzothiazole analogues (Scheme 3). Although o-nitrothiophenols are not stable and readily oxidatively dimerized into 2,2′-dinitrodiphenyl disulfides 4, the latter, commercially available can be used conveniently as oxidizing partners. Gratifyingly, while this redox process is unbalanced (each moiety of 2,2′-dinitrodiphenyl disulfide molecule 4 receives 7 electrons), excellent yields were observed in all cases tested even when arylacetic acids were used only in slight excess quantities (1.2 equiv.). Benzothiazoles are less polar and more soluble in organic solvents than benzimidazoles 3, consequently the purifications by column chromatography are easier. As for 2-arylbenzimidazoles 3, in addition to the simplest 5a, arylacetic acids bearing Cl-, Br-, MeO- and CF3-functional groups also served as good substrates to yield the benzothiazoles efficiently.
Although the mechanism of the transformation is not clear at this moment, some important observations may be discussed. First, an aryl group is necessary for the decarboxylative redox condensation of acetic acid derivatives 2, suggesting its role as a stabilizing group. Second, the presence of a base is required. A plausible mechanism for this redox condensation reaction is proposed in Scheme 4 on the basis of the experiments described above and literature examples.4–6 Since in the absence of either iron salt or elemental sulfur, the reaction does not proceed, the presence of a Fe/S cluster plays a central role in this reaction. First, iron–sulfur clusters are produced from iron salts, elemental sulfur and a reducing agent. Redox flexible, these clusters can possess a wide range of oxidation states. One of the oxidized forms of these clusters can oxidize a phenylacetate anion into a phenylacetyloxy radical which then undergoes a decarboxylation into a benzyl radical fixed to one of the sulfur atoms of the cluster. In parallel with this process, the reduction of the nitro group of o-nitroaniline was effected with one of the reduced forms of Fe/S clusters. Subsequent redox and condensation steps, catalyzed by Fe/S clusters thanks to their redox flexible and acido-basic nature, led to the final product 2-phenylbenzimidazole. Although iron and sulfur appear to function cooperatively in the redox system, the question regarding how it mediates between the decarboxylation and reduction reactions leading to benzazoles is under investigation.
Conclusions
In summary, we have developed an efficient decarboxylative redox condensation between nitroarenes and arylacetic acids. This straightforward and simple route to a wide range of valuable aza-heterocycles relies on the complex interplay between four critical components: the N-methylpiperidine base and the low-cost Fe/S catalyst generated in situ from simple iron salts and S. This approach is a good example of atom-, step- and redox economical transformation. We continue to expand the scope of the Fe/S catalyst for the synthesis of complex molecules from readily available starting materials.Notes and references
- N. Rodriguez and L. J. Goossen, Chem. Soc. Rev., 2011, 40, 5030 RSC.
- (a) T. Li, L. Huo, C. Pulley and A. Liu, Bioorg. Chem., 2012, 43, 2 CrossRef CAS PubMedM. El-Said Mohamed, B. Seyfried, A. Tschech and G. Fuchs, Arch. Microbiol., 1993, 159, 563 CrossRef.
- (a) B. M. Martins, M. Blaser, M. Feliks, G. M. Ullmann, W. Buckel and T. Selmer, J. Am. Chem. Soc., 2011, 133, 14666 CrossRef CAS PubMed; (b) M. Feliks, B. M. Martins and G. M. Ullmann, J. Am. Chem. Soc., 2013, 135, 14574 CrossRef CAS PubMed; (c) K. A. Shisler and J. B. Broderick, Arch. Biochem. Biophys., 2014, 546, 64 CrossRef CAS PubMed; (d) T. Selmer and P. I. Andrei, Eur. J. Biochem., 2001, 268, 1363 CrossRef CAS.
- 2- or 4-methylhetarenes as reducing partners: (a) T. B. Nguyen, L. Ermolenko and A. Al-Mourabit, J. Am. Chem. Soc., 2013, 135, 118 CrossRef CAS PubMed; (b) T. B. Nguyen, L. Ermolenko and A. Al-Mourabit, Org. Lett., 2013, 15, 4218 CrossRef CAS PubMed.
- Benzyl alcohols as reducing partners: (a) With o-nitrophenols: M. Wu, X. Hu, J. Liu, Y. Liao and G. Deng, Org. Lett., 2012, 14, 2722 CrossRef CAS PubMed; (b) With o-nitrobenzamides: H. Wang, X. Cao, F. Xiao, S. Liu and G. Deng, Org. Lett., 2013, 15, 4900 CrossRef CAS PubMed; with o-nitroanilines; (c) G. Li, J. Wang, B. Yuan, D. Zhang, Z. Lin, P. Li and H. Huang, Tetrahedron Lett., 2013, 54, 6934 CrossRef CAS PubMed; (d) With nitrobenzenes: F. Xiao, Y. Liu, C. Tang and G. Deng, Org. Lett., 2012, 14, 984 CrossRef CAS PubMed.
- Aliphatic amines as reducing partners: (a) T. B. Nguyen, J. Le Bescont, L. Ermolenko and A. Al-Mourabit, Org. Lett., 2013, 15, 6218 CrossRef CAS PubMed; (b) T. B. Nguyen, P. Retailleau and A. Al-Mourabit, Org. Lett., 2013, 15, 5238 CrossRef CAS PubMed.
- Without either iron salt or sulfur as a catalyst in the absence/presence of a base additive (vide infra) no conversion at all or low conversions (<5%) were observed.
- 1H NMR study of an equimolar mixture of phenylacetic acid and an organic base (N-methylpiperidine or 3-picoline) in deuterated methanol (0.2 M) showed that while N-methylpiperidine resulted in total deprotonation, 3-picoline did not lead to any change (see ESI†).
- Iron chlorides FeCl2·4H2O and FeCl3·6H2O as well as other chloride salts of the first row transition metals such as MnCl2·4H2O, CoCl2·6H2O, NiCl2·6H2O, CuCl, CuCl2·2H2O were also tested as catalysts (5 mol%) under standard conditions without sulfur and did not lead to any trace of 3a. With the concomitant presence of chloride salts (5 mol%) and sulfur (40 mol%), low conversions were obtained with MnCl2·4H2O (23%), NiCl2·6H2O (5%), CuCl (9%).
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, compounds characterization data, and copies of NMR spectra. See DOI: 10.1039/c4qo00221k |
| This journal is © the Partner Organisations 2014 |