Recyclable electrochemical allylation in aqueous ZnCl2medium: synthesis and reactivity of a wire-shaped nano zinc architecture†
Arun Kumar
Sinha
a,
Bibhas
Mondal
b,
Mousumi
Kundu
b,
Biswajit
Chakraborty
*c and
Ujjal Kanti
Roy
*b
aDepartment of Physics, Indian Institute of Technology, Kharagpur 721302, India
bDepartment of Chemistry, Deshabandhu Mahavidyalaya, Chittaranjan 713331, India
cDepartment of Chemistry, Vivekananda Mahavidyalaya, Burdwan 751013, India. E-mail: uroccu@gmail.com
First published on 13th October 2014
Abstract
Electrochemistry is used in an aqueous allylation reaction using zinc chloride as the reagent. A unit galvanic cell using a beaker containing an aqueous zinc chloride solution, zinc rod, TCO glass plate and connecting copper wire has been designed. Instead of using a connecting copper wire, an external bias can be used for applying voltage from the outside. The zinc rod served as a self-decaying anode and the TCO glass substrate as the cathode. During the process, a wire-shaped nano zinc architecture was formed from zinc chloride on the cathode. Wire-shaped nano zinc is the active reagent for the allylation of aldehydes or imines. The corresponding homoallylic alcohols or amines are prepared in good yields. Both the zinc salts and water can be recycled.
Electrochemical methods are an excellent approach to synthesis because they can eliminate (or dispose of) waste materials and recycle metal reagents.1 During the electrochemical conversion (here allylation), if a new metal nano architecture is formed it will be of huge interest from a materials chemistry point of view. The reactivity of the nano-material deposited on the electrode will also be increased due to its higher surface area. Materials chemists are trying to synthesize nano architectures of different metals of a particular shape and size because of their applications in nanotechnology. Similarly, metallic nano-wires also have a huge number of technological applications.2 So far, to the best of our knowledge, the electrochemical synthesis of a wire-like nano architecture of metallic zinc from aqueous ZnCl2 is unknown.3 Here, we wish to report a very easy green synthesis of a wire nano architecture of pure metallic zinc using aqueous ZnCl2 as the starting material during an electrochemical allylation process.
The synthesis of homoallylic alcohols/amines by allylation of carbonyls/imines is one of the most important processes in organic synthesis because of their use as building blocks in natural product synthesis. Metal-mediated allylation is a well-known example of an organic reaction in aqueous media.4 However, excessive metal is used in most cases and the corresponding metal salt is generated as waste material. Lots of research has been done on developing metal-mediated allylation reactions in aqueous media. Zinc is very cheap and has low toxicity among the successful metals to mediate this Barbier-type reaction.5 In most cases, physical or chemical activation is necessary. Among the various activation methods, the addition of an acidic co-reagent is very common. Here, we wish to report a Zn-mediated electrochemical allylation of carbonyls/imines in aqueous medium (pH 2–2.5). This is efficient and a range of functional groups are stable under these mild reaction conditions.
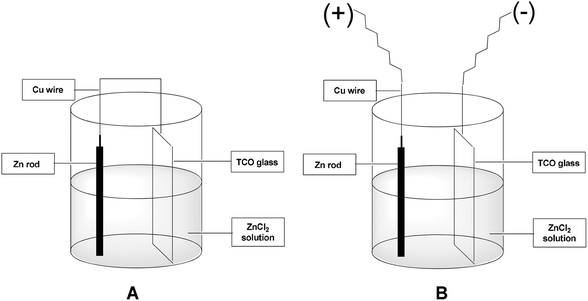
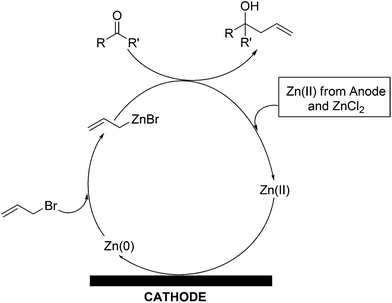
We designed a unit galvanic cell using a beaker containing an aqueous zinc chloride solution, zinc rod, TCO (e.g. ITO) glass plate and connecting copper wire (Fig. 1A). The zinc rod and TCO glass substrate (3 × 1 cm2) were short-circuited externally through a copper wire. Instead of using a connecting copper wire we can also use an external bias for applying voltage from the outside (Fig. 1B). The zinc rod served as a self-decaying anode and the TCO glass substrate as the cathode (Fig. 1). The pH of the solution was kept within 2–2.5 which was found to be optimum for the desired zinc film deposition. Regeneration of the metallic zinc species is based on using the cathode as the reducing agent for the spent zinc reagent. We chose the zinc-mediated allylation of benzaldehyde with allyl bromide as a test reaction for carbon–carbon bond formation. Our idea was to use the in situ generated metallic zinc for the allylation reaction. We found that efficient allylation can take place and in the first attempt we obtained a 32% yield of homoallyl benzyl alcohol in an undivided galvanic cell after 17 h using no external bias. We concluded that the allylation process in an undivided cell mainly takes place at the cathode, where metallic zinc is deposited.
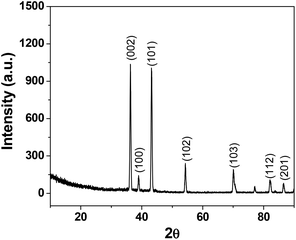
The deposition of Zn(0) from solutions of Zn(II) both in the presence and absence of organic reactants (aldehyde and allyl bromide) was studied. It was found that from the 0.02 M ZnCl2 solutions (pH 2–2.5), ∼70 mg of powder was deposited on the cathode within 10 min as a thick film (F1) when no allylation reaction was performed in an unit galvanic cell and without the presence of an external bias. Energy-dispersive X-ray (EDX) spectroscopy, EDX mapping (ESI†, Fig. S1 and S2) and XRD (Fig. 2) analysis showed that the deposits from the ZnCl2 solutions consisted of pure Zn(0) with a good crystalline structure (F1). The crystal planes for metallic zinc that were identified include (002), (100), (101), (102), (103), (112) and (201) and perfectly match with pure Zn metal. An ultra-thin oxide layer (ZnO) may have been grown on the zinc wire due to aerial oxidation, but no signature is found for the oxide in the XRD spectrum. Therefore, our synthesized Zn nano wire is phase-pure.6
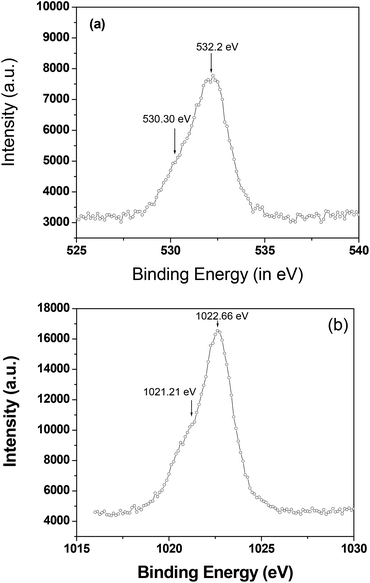
The phase purity of the as-synthesized nano-wires was further analyzed using X-ray photoelectron spectroscopy (XPS, Fig. 3). A very thin layer of ZnO or Zn(OH)2 was formed on the outer surface of the Zn nano-wire, which is not detectable by XRD analysis. Fig. 3(a) and (b) show the XPS spectra of the O 1s and Zn 2p3/2 regions for the nano-wires, respectively. The O 1s shows that there are two types of oxygen is present, a Zn(OH)2 component at 532.20 eV and a ZnO component at 530.30 eV. Zn(OH)2 is the intermediate form of ZnO, and appeared as a thin amorphous over layer on top of the ZnO. The Zn 2p3/2 feature has two components at 1022.66 eV for Zn2+ and 1021.21 eV for Zn0. Therefore, the electrodeposited thin film exists as Zn-ZnO at room temperature.
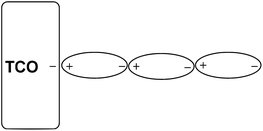
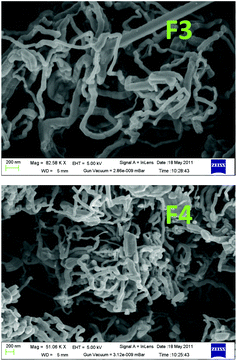
However, the SEM image (Fig. 4, F1) showed that the thick deposits were of a wire-like nano architecture. The nano structure of Zn(0) is probably responsible for its reactivity in the allylation reaction. The wire-like nano architecture may be formed through a head to tail, tail to head polar deposition of charged nano zinc particles on the charged TCO cathode plate. The as-grown Zn(0) nucleus grows in size and is eventually polarized by the charged TCO plate (with or without external bias) and longitudinally aligned on the already polarized Zn(0) present at the TCO. During the progress of the reaction, the nano wires grow in length via dipole–dipole attraction. Here, the charge on the TCO plate directs the 1D growth and slowly the whole surface is covered with the Zn nano wires.7 The growth mechanism of a 1D Zn nano wire on the negatively charged TCO plate has been shown in Scheme 1.
 | ||
| Fig. 4 Field emission scanning electron microscopy (FESEM) images of Zn nano wires on the outer surface of TCO in the absence (F1) and presence (F2) of external bias. | ||
In the presence of an external bias (2 V, 20 mA), ∼83 mg of Zn(0) powder was deposited as a thick film (F2) within 10 min in the absence of any organic reaction. We used SEM images for qualitative characterization of the Zn(0) thick films. In the absence of any allylation reaction the Zn(0) films obtained from a unit galvanic cell in the absence and presence of an external bias, FI and F2 respectively (Fig. 4), are very much similar with respect to their SEM images. From the SEM images the diameter of the Zn nano-wires is about 80–200 nm and the length is a few micro meters.
The reactivity of Zn(0) in aqueous medium is noteworthy. After the initial success, we applied the two electrode configuration using an external bias (2 V, 20 mA for 17 h) for the allylation reaction of benzaldehyde. A 0.02 M aqueous ZnCl2 solution (pH 2–2.5) was stirred at room temperature for 17 h in a one-compartment galvanic cell (Fig. 1B, TCO glass foil 3 × 1 cm2) to afford a 45% yield of the product. To increase the yield of the allylation reaction, a miscible organic solvent was added and the temperature was increased. The standardisation of the allylation reaction has been described in Table 1. The pH of the reaction mixture was measured as 2.3 (entries 1 & 2). The pH of the solution can be varied by varying the concentration of ZnCl2. By changing the pH of the reaction mixture, the yield of the homoallyl alcohol is decreased (entries 3 & 4). Therefore, the pH of the solution was optimised to be 2.3. The yield of the homoallyl alcohol increases when increasing the solubility of the organic reactants by adding a miscible organic solvent. The effect of THF addition is more prominent compared to DMF and MeOH (entry 7 vs. entries 5 & 6). Reducing the amount of the THF resulted in lower yields (entries 7 vs. 8). The effects of any acid or base additives like NaOH, HCl, HOAc are found to be negative, probably because they change the pH of the solution. The effect of other additives like NH4Cl and NH4Br were found to be negligible. By increasing the temperature of the reaction mixture up to 70 °C the yield of the reaction is increased to 74% (entries 7 vs. 9). The optimized reaction conditions are those given in entry 9.
| Sl. no. | Electrochemical conditions | Solvent | Temp. (°C) | Yield (%) |
|---|---|---|---|---|
a Aldehyde 2 mmol, allyl bromide 4 mmol, solvent 10 ml H2O–THF (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) containing 0.02 M ZnCl2. 2) containing 0.02 M ZnCl2.
|
||||
| 1 | No external bias, pH 2.3 | H2O | 40 | 32 |
| 2 | 2 V, 20 mA, pH 2.3 | H2O | 40 | 45 |
| 3 | 2 V, 2 mA, pH 1.8 | H2O | 40 | 39 |
| 4 | 2 V, 2 mA, pH 4.0 | H2O | 40 | 20 |
| 5 | 2 V, 20 mA, pH 2.3 | H2O–MeOH (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
40 | 53 |
| 6 | 2 V, 20 mA, pH 2.3 | H2O–DMF (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
40 | 50 |
| 7 | 2 V, 20 mA, pH 2.3 | H2O–THF (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
40 | 65 |
| 8 | 2 V, 20 mA, pH 2.3 | H2O–THF (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
40 | 55 |
| 9 | 2 V, 20 mA, pH 2.3 | H2O–THF (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
70 | 74 |
Recycling of the reagent (ZnCl2) can be easily achieved for this electrochemical process. After three extractions of the reaction mixture with ethyl acetate the remaining aqueous layer can be reused for the next cycle without further purification. To reuse the remaining ZnCl2 solution, some THF (20%) should be added to obtain a good yield of the homoallyl alcohol for the next cycle. The yields of the second and third cycles are very much similar (68% and 65%, respectively). Up to the 5th cycle the decrease of the yield of homoallyl alcohol is not very significant. The yields of the 4th and 5th cycles are 62% and 59%, respectively.
The deposition of zinc on the TCO plate was reproduced in the reaction mixture (in the presence of aldehyde, allyl bromide, water and THF). The Zn(0) thick films obtained after the completion of the organic reaction are very much similar in the absence (F3) and presence (F4) of an external bias (Fig. 5). By comparing F1 with F3 and F2 with F4 it can also be said that the nano structure of Zn(0) is very much similar before and after the organic reaction. During the electrochemical allylation process, we observed that the zinc deposited on the cathode was of a metallic luster, instead of the dark-gray colour of the commercial zinc powder. Scanning electron microscopy (SEM; Fig. 4 and 5) showed that the Zn had a three-dimensional (3D) wire-like architecture. The effects of aldehyde, allyl bromide and co-solvent (THF) on controlling the morphology were (as investigated by SEM) found to be negligible. It was found that in all cases the F1 to F4 nano-architectures could be obtained with similar SEM pictures. After completion of the allylation reaction (17 h), F3 and F4 were obtained as 86 and 80 mg of zinc, respectively.
 | ||
| Fig. 5 FESEM images of Zn nano-wire thick film after the completion of the organic reaction in the absence (F3) and presence (F4) of external bias. | ||
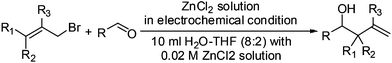
Subsequently, a variety of aldehydes and allyl bromides were used for the allylation reaction using the optimised conditions from Table 1, entry 9. Table 2 summarizes the details of the carbonyl allylation results. Both aromatic and aliphatic aldehydes can be utilized in this methodology. For the aromatic aldehydes, homoallylic alcohols can be prepared smoothly in good to excellent yields, and functionalities including nitro and chloro groups (entries 1, 4 & 5) are tolerated under these mild conditions. Aliphatic aldehydes are also allylated (entries 3 & 7–9). A regioselectivity study using crotyl bromide and 3,3-dimethylallyl bromide showed that a γ-substitution product was obtained exclusively (entries 5–7 & 9) with very poor diastereoselectivity (entry 9, syn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) anti ∼ 50
anti ∼ 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50). However, ketones did not undergo allylation under these reaction conditions, with a negligible conversion of ketone observed even after 17 h.
50). However, ketones did not undergo allylation under these reaction conditions, with a negligible conversion of ketone observed even after 17 h.
| # | Halide | Aldehyde | Time (h) | Yield (%) | Products |
|---|---|---|---|---|---|
a Aldehyde 2 mmol, allyl bromide 4 mmol, 10 ml H2O–THF (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 0.02 M ZnCl2 solution, 2 V, 20 mA, pH 2.3, 70 °C. 2) 0.02 M ZnCl2 solution, 2 V, 20 mA, pH 2.3, 70 °C.
|
|||||
| 1 | R1 = R2 = R3 = H | 4-ClPh- | 17 | 74 | 1a |
| 2 | R1 = R2 = R3 = H | Ph- | 14 | 67 | 1b |
| 3 | R1 = R2 = R3 = H | PhCH2- | 13 | 65 | 1c |
| 4 | R1 = R2 = R3 = H | 4-NO2C6H4- | 15 | 60 | 1d |
| 5 | R1 = R2 = Me, R3 = H | 4-ClPh- | 14 | 75 | 1e |
| 6 | R1 = R2 = Me, R3 = H | Ph- | 15 | 79 | 1f |
| 7 | R1 = R2 = Me, R3 = H | PhCH2- | 14 | 66 | 1g |
| 8 | R1 = R2 = H, R3 = Me | PhCH2- | 14 | 32 | 1h |
| 9 | R1 = Me, R2 = R3 = H | PhCH2- | 16 | 70 | 1i |
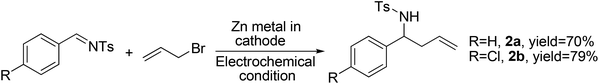
Encouraged by the above success, we applied these reaction conditions (Table 1, entry 9) to imines. The allylation of N-benzylbenzaldimine led to the isolation of the desired N-benzyl homoallylamine in a trace amount, along with 68% of the corresponding homoallyl alcohol. This is due to the hydrolysis of imines at lower pH (2–2.5). However, the electrochemical allylation of N-tosylbenzaldimine and N-tosyl-4-chlorobenzaldimine were facile, free from side reactions and were complete within 15 h and 13 h, respectively (Scheme 2). Work-up gave the desired homoallyl sulfonamides in 70% and 79% yields, respectively. This indicates that sulfonimines are suitable for the present Barbier allylation reaction due to their hydrolytic stability.
The activity of Zn towards the allylation of simple aldehydes and conjugated imines might be attributed to its special wire-like 3D nano-architecture. A preliminary stoichiometric comparative reactivity study of different types of zinc based upon surface morphology was carried out using Rieke zinc,8 commercial zinc powders, granules, foils etc. under non-electrochemical conditions (Table 3). In 3 ml water, metallic zinc (2 mmol, 131 mg), allyl bromide (2 mmol, 0.2 ml) and 4-chlorobenzaldehyde (1 mmol, 141 mg) were added and stirred at 80 °C for 17 hours. The aqueous reaction mixture was extracted twice with diethyl ether (2 × 10 ml). The combined organic layer was washed with brine (10 mL), dried over Na2SO4, filtered, and concentrated under vacuum. The crude product was purified by column chromatography on silica gel (petroleum ether–ethyl acetate 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). F1 showed the highest reactivity towards the allylation of benzaldehyde in water (yield 62%). The metallic zinc layer (F1) was removed from the TCO plate after electrochemical deposition and 131 mg used for the comparative reactivity study under the same non-electrochemical reaction conditions as stated above. Under the non-electrochemical conditions, the allylation reaction mediated by metallic zinc from preformed F1 was less effective compared to the electrochemical process (yield 74%). However, in the non-electrochemical process, the pH value during the reaction was different (pH 6.5–7). Rieke zinc was prepared according to the literature procedure in dry THF using 2 mmol ZnCl2 (please see ESI†). THF was removed by evaporation from the activated Rieke zinc solution. Water (3 ml), allyl bromide (2 mmol, 0.2 ml) and 4-chlorobenzaldehyde (1 mmol, 141 mg) were subsequently added to the residual activated zinc (2 mmol) and stirred at 80 °C for 17 h to give the corresponding homoallyl alcohol (yield 60%).
1). F1 showed the highest reactivity towards the allylation of benzaldehyde in water (yield 62%). The metallic zinc layer (F1) was removed from the TCO plate after electrochemical deposition and 131 mg used for the comparative reactivity study under the same non-electrochemical reaction conditions as stated above. Under the non-electrochemical conditions, the allylation reaction mediated by metallic zinc from preformed F1 was less effective compared to the electrochemical process (yield 74%). However, in the non-electrochemical process, the pH value during the reaction was different (pH 6.5–7). Rieke zinc was prepared according to the literature procedure in dry THF using 2 mmol ZnCl2 (please see ESI†). THF was removed by evaporation from the activated Rieke zinc solution. Water (3 ml), allyl bromide (2 mmol, 0.2 ml) and 4-chlorobenzaldehyde (1 mmol, 141 mg) were subsequently added to the residual activated zinc (2 mmol) and stirred at 80 °C for 17 h to give the corresponding homoallyl alcohol (yield 60%).
| Sl. no. | Zinc reagents | Time (h) | Yield (%) |
|---|---|---|---|
| Condition: aldehyde 1 mmol, allyl bromide 2 mmol, H2O 3 ml, time 17 h. | |||
| 1 | Rieke zinc synthesized | 17 | 60 |
| 2 | Zinc powder 325 mesh (SRL) | 17 | 51 |
| 3 | Zinc powder 200 mesh (Merck) | 17 | 45 |
| 4 | Zinc granules 30 mesh (Sigma Aldrich) | 17 | 23 |
| 5 | Zinc foil 0.38 mm (commercial) | 17 | 15 |
| 6 | F1 zinc (this work) | 17 | 62 |
The mechanism of the allylation reaction is very simple. A plausible reaction mechanism has been demonstrated schematically in Scheme 3. Allyl bromide oxidatively adds across metallic nano zinc to form reactive allyl zinc(II)bromide which reacts in situ with aldehyde to form homoallyloxyzinc(II) bromide which is hydrolysed under the reaction conditions to form the desired homoallyl alcohol and zinc(II) salts. This reaction increases the concentration of Zn2+ in solution. Zinc(II) salt(s) thus generated from the allylation of aldehyde, along with the zinc(II) from zinc chloride and the sacrificial anode, are reduced at the cathode to generate metallic zinc. In this way, the concentration of Zn2+ in solution is maintained. Metallic zinc deposits are thus generated on the cathode as nano-wires, which are more reactive compared to commercial zinc.
An efficient aqueous electro-synthesis of homoallyl alcohols/amines from simple aldehydes/imines with allylic bromides has been developed using ZnCl2 as the reagent.9 The deposited zinc(0) powder with a nano-wire architecture played an important role in the reaction. This methodology is an attractive synthetic route to homoallyl alcohols/amines as well as zinc nano-wires. Further investigation to determine the nature and properties of the nano-wires10 is under way in our laboratory. We are also trying to determine the exact mechanism of the allylation reaction and expand its scope for other organic reactions.
Acknowledgements
Financial support of this work by DST-New Delhi (SR/FT/CS-137/2011 dated 12.07.2012) to UKR is gratefully acknowledged.Notes and references
- (a) G. Hilt and K. I. Smolko, Angew. Chem., Int. Ed., 2001, 40, 3399 CrossRef CAS; (b) G. Hilt, K. I. Smolko and C. Waloch, Tetrahedron Lett., 2002, 43, 1437 CrossRef CAS; (c) M. Durandetti, C. Meignein and J. Perichon, J. Org. Chem., 2003, 68, 3121 CrossRef CAS PubMed; (d) Z. Zha, A. Hui, Y. Zhou, Q. Miao, Z. Wang and H. Zhang, Org. Lett., 2005, 7, 1903 CrossRef CAS PubMed; (e) J.-M. Huang and Y. Dong, Chem. Commun., 2009, 3943 RSC; (f) J.-M. Huang and H.-R. Ren, Chem. Commun., 2010, 2286 RSC; (g) B. A. Frontana-Uribe, R. D. Little, J. G. Ibanez, A. Palma and R. Vasquez-Medrano, Green Chem., 2010, 12, 2099 RSC; (h) J.-M. Huang, X.-X. Wang and Y. Dong, Angew. Chem., Int. Ed., 2011, 50, 924 CrossRef CAS PubMed; (i) K. Sahloul, L. Sun, A. Requet, Y. Chahine and M. Mellah, Chem. – Eur. J., 2012, 18, 11205 CrossRef CAS PubMed.
- (a) M. Li, R. B. Bhiladvala, T. J. Morrow, J. A. Sioss, K. K. Lew, J. M. Redwing, C. D. Keating and T. S. Mayer, Nat. Nanotechnol., 2008, 3, 88 CrossRef CAS PubMed; (b) A. Singh, T. P. Sai and A. Ghosh, Appl. Phys. Lett., 2008, 93, 102107 CrossRef.
- Nano wire by solution chemistry: (a) S.-S. Chang, S. O. Yoon, H. J. Park and A. Sakai, Mater. Lett., 2002, 53, 432 CrossRef CAS; (b) J.-G. Wang, M.-L. Tian, N. Kumar and T. E. Mallouk, Nano Lett., 2005, 5, 1247 CrossRef CAS PubMed; (c) Y. Wang, H. Zhao, Y. Hu, C. Ye and I. Zhang, J. Cryst. Growth, 2007, 305, 8 CrossRef CAS; (d) D. Pradhan, S. Sindhwani and K. T. Leung, J. Phys. Chem. C, 2009, 113, 15788 CrossRef CAS.
- (a) Y. Yamamoto and N. Asao, Chem. Rev., 1993, 93, 2207 CrossRef CAS; (b) J. A. Marshall, Chem. Rev., 1996, 96, 31 CrossRef CAS PubMed; (c) J. A. Marshall, Chem. Rev., 2000, 100, 3163 CrossRef CAS PubMed; (d) U. K. Roy and S. Roy, Chem. Rev., 2010, 110, 2472 CrossRef CAS PubMed.
- Zinc mediated Barbier allylation: (a) K. T. Tan, S. S. Chng, H. S. Cheng and T. P. Loh, J. Am. Chem. Soc., 2003, 125, 2958 CrossRef CAS PubMed; (b) X.-W. Sun, M.-H. Xu and G.-Q. Lin, Org. Lett., 2006, 8, 4979 CrossRef CAS PubMed; (c) X. Ma, J.-X. Wang, S. Li, K.-H. Wang and D. Huang, Tetrahedron, 2009, 65, 8683 CrossRef CAS; (d) A. Wolan, A. Joachimczak, M. Budny, A. Kozakiewicz, J. W. Lim, K. H. Kim, B. R. Park and J. N. Kim, Tetrahedron Lett., 2011, 52, 6545 CrossRef; (e) L.-M. Zhao, H.-S. Jin, L.-J. Wan and L.-M. Zhang, J. Org. Chem., 2011, 76, 1831 CrossRef CAS PubMed.
- X. Y. Kong, Y. Ding and Z. L. Wang, J. Phys. Chem. B, 2004, 108, 570 CrossRef CAS.
- A. K. Sinha, M. Basu, S. Sarkar, M. Pradhan and T. Pal, Langmuir, 2010, 26, 17419 CrossRef CAS PubMed.
- Rieke Zn-metal is also very reactive. Here the reactivity of nano-Zn is very much similar to Rieke zinc; please see: (a) R. D. Rieke, Science, 1989, 246, 1260 CAS; (b) S. Kudret, J. D. Haen, L. Lutsen, D. Vanderzande and W. Maes, Adv. Synth. Catal., 2013, 355, 569 CrossRef CAS.
- General procedure: aldehyde (2 mmol) and allyl bromide (4 mmol) were added to 10 ml H2O–THF (8
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) containing 0.02 M ZnCl2 (pH 2.3). The stirring mixture was then electrolyzed in a unit cell (two-electrode configuration) using an external bias of 2 V (±0.1 V) and 20 mA in a beaker equipped with a zinc electrode (1.5 cm2) and a TCO electrode (1 × 3 cm2) at 70 °C with slow stirring. The electrolysis was stopped when the aldehyde had been completely consumed (TLC monitoring). After the electrolysis, zinc film (F4) was collected and THF was removed by evaporation. The aqueous reaction mixture was extracted twice with diethyl ether (2 × 10 ml). The combined organic layer was washed with brine (10 mL), dried over Na2SO4, filtered, and concentrated under vacuum. The crude product was purified by column chromatography on silica gel (petroleum ether–ethyl acetate 9
2) containing 0.02 M ZnCl2 (pH 2.3). The stirring mixture was then electrolyzed in a unit cell (two-electrode configuration) using an external bias of 2 V (±0.1 V) and 20 mA in a beaker equipped with a zinc electrode (1.5 cm2) and a TCO electrode (1 × 3 cm2) at 70 °C with slow stirring. The electrolysis was stopped when the aldehyde had been completely consumed (TLC monitoring). After the electrolysis, zinc film (F4) was collected and THF was removed by evaporation. The aqueous reaction mixture was extracted twice with diethyl ether (2 × 10 ml). The combined organic layer was washed with brine (10 mL), dried over Na2SO4, filtered, and concentrated under vacuum. The crude product was purified by column chromatography on silica gel (petroleum ether–ethyl acetate 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1). - For the synthesis of nano-wire zinc film in the absence of organic reactants please see the Supporting Information.
Footnote |
| † Electronic supplementary information (ESI) available: Additional experimental details including EDX, EDX mapping, XRD analysis, synthesis of starting materials, spectral data for all compounds, NMR (1H and 13C) spectra of all compounds. See DOI: 10.1039/c4qo00235k |
| This journal is © the Partner Organisations 2014 |