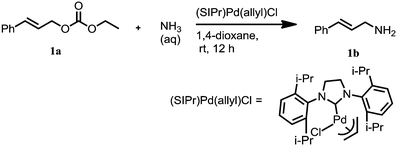
Palladium-catalyzed amination of allylic carbonates with ammonia: access to primary amines†
Peng
Yin
ab,
Mun Yee
Wong
b,
Jieying
Tham
b and
Teck-Peng
Loh
*bc
aKey Laboratory of Chemical Biology and Traditional Chinese Medicine Research (Ministry of Education), College of Chemistry and Chemical Engineering, Hunan Normal University, Changsha 410081, PR China
bDivision of Chemistry and Biological Chemistry, Nanyang Technological University, Singapore 637371, Singapore. E-mail: teckpeng@ntu.edu.sg; Fax: +65 6515 8229; Tel: +65 6513 8203
cHefei National Laboratory for Physical Science at the Microscale and Department of Chemistry, University of Science and Technology of China, Hefei, Anhui 230026, P.R. China
First published on 23rd October 2014
Abstract
Allylic amination of allylic carbonates with ammonia gas or aqueous ammonia was successfully carried out using (SIPr)Pd(allyl)Cl as the catalyst and Ph3P, which is essential for the reaction. The aqueous ammonia reactions proceeded smoothly at room temperature, using a low catalyst loading. But a higher temperature and catalyst loading were needed for the corresponding ammonia gas reactions. Moderate to good yields were achieved for both reactions. This study demonstrates the feasibility of using ammonia as an aminating reagent, and it opens the field for further development of metal-catalyzed allylic amination in the future.
Introduction
Primary amines are important building blocks and are featured prominently in a wide variety of functional compounds, such as pharmaceuticals, agrochemicals, and advanced materials.1 Therefore, the synthesis of primary amines has attracted significant attention. Among the methods reported, the transition metal-catalyzed allylic amination provides one of the most powerful methods for the synthesis of allylic amines.1d,2 However, conventional metal-catalyzed allylic amination methods to access primary amines require the use of protecting groups for the amine functionality thereby reducing the transformation efficiency.3 The direct use of low-cost and easily available ammonia as the nitrogen source is thus an attractive solution to this major synthetic challenge. Unfortunately, the direct use of ammonia in metal-catalyzed amination that leads to unprotected primary amines remains poorly explored.4 This is probably because ammonia rapidly displaces ligands on the metal center of many metal complexes to give stable non-chiral amine complexes. Furthermore, the resulting primary amine product is more reactive than ammonia, which can cause undesired reaction on the product.5 Consequently, it is not surprising that the direct use of ammonia in metal-catalyzed reactions remains a synthetic challenge to chemists.6,2b In 2009, Kobayashi made a major breakthrough in this area by describing a Pd(PPh3)4 catalyzed allylic amination reaction using aqueous ammonia as the nitrogen source, providing the primary amine products in high yields.4a Despite this breakthrough, the direct use of ammonia gas in allylic amination reactions catalyzed by palladium catalysts is still unavailable. Therefore, an intense effort is underway to discover a palladium-catalyzed allylic amination using ammonia solution or ammonia gas, and apply the methodology to a wide range of substrates.We envisage that palladium carbene complexes may be applicable to the allylic amination using ammonia gas or aqueous ammonia.5b,7 This hypothesis was made according to the following premises: it is well established that N-heterocyclic carbene (NHC) palladium complexes are easily accessible, thermally stable, tunable (both electronically and sterically), and they are inert towards air and moisture. In contrast to palladium phosphine complexes, carbene dissociation from NHC palladium complexes is not a favorable process. This special property should suppress the potential displacement of the ligands by ammonia. Accordingly, in this paper, we report the results of amination of allylic carbonates with ammonia gas or aqueous ammonia using (SIPr)Pd(allyl)Cl as a catalyst, and in the presence of Ph3P.
Results and discussion
Ethyl cinnamyl carbonate was selected as a model substrate for screening of the reaction conditions with aqueous ammonia. As shown in Table 1, when the concentration of the carbonate was kept at 0.03 M, the yield increased with the amount of PPh3 (entries 1–4, Table 1). Increasing the concentration of carbonate to 0.04 M, the yield dropped to 57% (entry 5, Table 1). Changing the ratio of aqueous NH3 to 1,4-dioxane from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to 1
2 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 led to a further drop in yield (compare entries 6 to 3, Table 1). Increasing the amount of aqueous ammonia did not increase the yield either (entry 6, Table 1); however, in this reaction the concentration of carbonate was also reduced to 0.02 M. Next, we reduced the catalyst loading from 10 mol% to 5 mol%, and the yield was not affected (entry 8, Table 1). But no reaction occurred when the catalyst loading was further decreased to 1 mol% (entry 9, Table 1). As a control reaction, no reaction took place without the addition of Ph3P (entry 10, Table 1). Furthermore, 4% of the branched primary amine was observed, and no dialkylamine or trialkylamine was obtained (entries 1–8, Table 1).
1 led to a further drop in yield (compare entries 6 to 3, Table 1). Increasing the amount of aqueous ammonia did not increase the yield either (entry 6, Table 1); however, in this reaction the concentration of carbonate was also reduced to 0.02 M. Next, we reduced the catalyst loading from 10 mol% to 5 mol%, and the yield was not affected (entry 8, Table 1). But no reaction occurred when the catalyst loading was further decreased to 1 mol% (entry 9, Table 1). As a control reaction, no reaction took place without the addition of Ph3P (entry 10, Table 1). Furthermore, 4% of the branched primary amine was observed, and no dialkylamine or trialkylamine was obtained (entries 1–8, Table 1).
| Entry | NH3/1,4-dioxane | Conc. (M) | Cat. (mol%) | PPh3 (mol%) | Yieldb (%) |
|---|---|---|---|---|---|
| a Conditions: (SIPr)Pd(allyl)Cl and PPh3 were added to 1,4-dioxane and stirred for 30 min. Next, ethyl cinnamyl carbonate (0.3 mmol) and aqueous ammonia (25%) were added to the solution successively. The solution was stirred under an atmosphere of Ar at room temperature for 12 h. b Isolated yields containing 4% of the branched primary amines. c No PPh3 was added. d No desired product was obtained. | |||||
| 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
0.03 | 10 | 10 | 43 |
| 2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
0.03 | 10 | 20 | 50 |
| 3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
0.03 | 10 | 30 | 63 |
| 4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
0.03 | 10 | 40 | 62 |
| 5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
0.04 | 10 | 30 | 57 |
| 6 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.03 | 10 | 30 | 55 |
| 7 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.02 | 10 | 30 | 43 |
| 8 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
0.03 | 5 | 15 | 62 |
| 9 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
0.03 | 1 | 3 | —d |
| 10 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
0.03 | 10 | —c | —d |
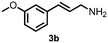
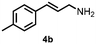
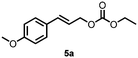
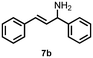
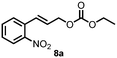
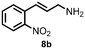
Using the optimized reaction conditions, the substrate scope was investigated. Good yields were achieved for four different substrates (entries 1–4, Table 2). No secondary amines were obtained, but judging from the 1H NMR spectra, 4% of the branched primary amines were formed. For substrates 5a and 6a, the ratio of branched primary amines increased to 15% and 45% respectively (entries 5 and 6, Table 2). In the case of substrate 7a, only 35% yield was obtained, which may be due to significant steric hindrance on the allylic carbon (entry 7, Table 2). For the less reactive substrate 8a, longer reaction time was needed for completion, and a moderate yield of 52% was obtained (entry 8, Table 2).
| Entry | Substrate | Product | Yieldb (%) |
|---|---|---|---|
| a Conditions: (SIPr)Pd(allyl)Cl (5 mol%) and PPh3 (15 mol%) were added to 1,4-dioxane (6.8 mL) and stirred for 30 min. Then substrates (0.3 mmol) and aqueous ammonia (25%, 3.4 mL) were added to the solution successively. The solution was stirred at room temperature for 12 h. b Isolated yields containing 4% of the branched primary amines. c The combined yield. d The ratio of the linear primary amine to the branched primary amine. e The reaction time was 40 h. Only a linear primary amine was obtained. | |||
| 1 |
|
|
62 |
| 2 |
|
|
68 |
| 3 |
|
|
61 |
| 4 |
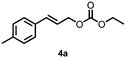
|
|
60 |
| 5 |
|
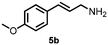
|
57c (84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16)d 16)d |
| 6 |
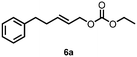
|
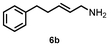
|
50c (55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45)d 45)d |
| 7 |
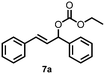
|
|
35 |
| 8 |
|
|
52e |
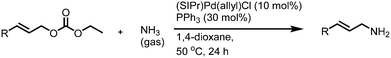
Next, the amination of allylic carbonates using ammonia gas was investigated. No reaction was observed when the reaction conditions used for aqueous ammonia were tried. After optimization, almost quantitative yield was achieved when the reaction was carried out using 30 mol% of PPh3 and 10 mol% of (SIPr)Pd-(allyl)Cl at 50 °C for 24 h (entry 1, Table 3). The scope of the reaction with ammonia gas was further investigated. When the reaction time was reduced to 12 h, the yield decreased dramatically (entry 2, Table 3). In contrast to the reaction with aqueous ammonia, the catalyst loading was critical for this reaction. No reaction took place when only 5 mol% of (SIPr)Pd(ally)Cl was used (entry 3, Table 3). Moderate to good yields were achieved for other substrates (entries 4–6, Table 3). For substrates 5a and 6a, a higher proportion of branched primary amines was obtained (entries 7 and 8, Table 3). In the case of substrate 7a, only 10% yield was obtained, which may be again due to steric hindrance around the allylic carbon (entry 9, Table 3). For the less reactive substrate 8a, no desired product was obtained even upon a prolonged reaction time (entry 10, Table 3).
| Entry | Substrate | Product | Yield (%)b |
|---|---|---|---|
| a Conditions: (SIPr)Pd(allyl)Cl (10 mol%) and PPh3 (30 mol%) were added to 1,4-dioxane (10 mL) and stirred for 30 min. Next the substrates (0.3 mmol) and liquid ammonia (4.4 mL, 500 equiv.) were added to the solution successively. The solution was stirred at 50 °C for 24 h. b Isolated yields containing 4% of the branched primary amines. c The reaction time was 12 h. d (SIPr)Pd(allyl)Cl (5 mol%) and PPh3 (15 mol%) were used. e The combined yield. f The ratio of the linear primary amine to the branched primary amine. g The reaction time was 40 h. | |||
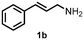
| 1 | 1a | 1b | 99 |
| 2 | 1a | 1b | 30c |
| 3 | 1a | 1b | —d |
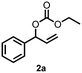
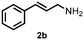
| 4 | 2a | 2b | 75 |
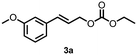
| 5 | 3a | 3b | 69 |
| 6 | 4a | 4b | 82 |
| 7 | 5a | 5b | 51e (81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19)f 19)f |
| 8 | 6a | 6b | 63e (51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 49)f 49)f |
| 9 | 7a | 7b | 10 |
| 10 | 8a | 8b | Traceg |
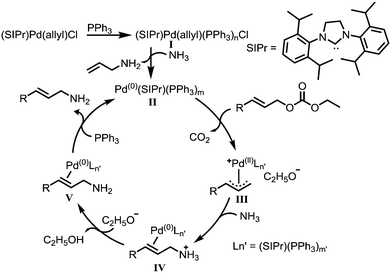
Recently, a number of reports pertaining to mechanistic studies of the Tsuji–Trost allylation reactions have been published.2,8 A plausible catalytic cycle is proposed (Scheme 1). It is suggested that the chloride ion in (SIPr)Pd(allyl)Cl is first replaced by PPh3 to generate complex I, which is subsequently reduced to complex II. Then, the complex II reacts with the carbonates with release of CO2. The resulting complex III reacts with ammonia to give the complex IV. After deprotonation by the base and dissociation from the palladium catalyst, the desired primary amine is obtained. Further detailed mechanistic studies are in progress.
Conclusions
The amination of allylic carbonates with aqueous and gaseous ammonia was successfully developed using a combination of (SIPr)Pd(allyl)Cl as the catalyst and PPh3. To the best of our knowledge, this is the first report of a Pd-catalyzed allylic amination with ammonia gas. It is important to note that PPh3 is crucial for this reaction. Further studies on the selectivity between linear and branched primary amines are in progress. In the future, it is anticipated that this new methodology will inspire others to develop a broad range of transition metal catalyzed reactions that utilize ammonia gas as the nitrogen source.Acknowledgements
We gratefully acknowledge the Nanyang Technological University and the Singapore Ministry of Education Academic Research Fund Tier 2 (MOE2010-T2-2-067 and MOE2011-T2-1-013) and the National Natural Science Foundation of China (21405043) for financial support.Notes and references
- (a) G. Petranyi, N. S. Ryder and A. Stutz, Science, 1984, 224, 1239 CAS; (b) A. Stutz, Angew. Chem., Int. Ed. Engl., 1987, 26, 320 CrossRef; (c) S. M. Nanavati and R. B. Silverman, J. Am. Chem. Soc., 1991, 113, 9341 CrossRef CAS; (d) B. M. Trost and M. L. Crawley, Chem. Rev., 2003, 103, 2921 CrossRef CAS PubMed; (e) S. Hayashi, H. Yorimitsu and K. Oshima, Angew. Chem., Int. Ed., 2009, 48, 7224 CrossRef CAS PubMed.
- For a general overview of allylic amination, see: (a) B. M. Trost and D. L. Van Vranken, Chem. Rev., 1996, 96, 395 CrossRef CAS PubMed; (b) M. Johannsen and K. A. Jorgensen, Chem. Rev., 1998, 98, 1689 CrossRef CAS PubMed; (c) J. Tsuji, New J. Chem., 2000, 24, 127 RSC; (d) Z. Lu and S. Ma, Angew. Chem., Int. Ed., 2008, 47, 258 CrossRef CAS PubMed; (e) M. L. Crawley, Sci. Synth., Stereosel. Synth., 2011, 3, 403 CAS; (f) B. Sundararaju, M. Achard and C. Bruneau, Chem. Soc. Rev., 2012, 41, 4467 RSC.
- For some examples, see: (a) S. Murahashi, Y. Taniguchi, Y. Imada and Y. Tanigawa, J. Org. Chem., 1989, 54, 3292 CrossRef CAS; (b) R. Weihofen, O. Tverskoy and G. Helmchen, Angew. Chem., Int. Ed., 2006, 45, 5546 CrossRef CAS PubMed; (c) C. Defieber, M. A. Ariger, P. Moriel and E. M. Carreira, Angew. Chem., Int. Ed., 2007, 46, 3139 CrossRef CAS PubMed.
- (a) T. Nagano and S. Kobayashi, J. Am. Chem. Soc., 2009, 131, 4200 CrossRef CAS PubMed; (b) M. J. Pouy, L. M. Stanley and J. F. Hartwig, J. Am. Chem. Soc., 2009, 131, 11312 CrossRef CAS PubMed; (c) K. Das, R. Shibuya, Y. Nakahara, N. Germain, T. Ohshima and K. Mashima, Angew. Chem., Int. Ed., 2012, 51, 150 CrossRef CAS PubMed; (d) R. Grigg, S. Akkarasamiyo, N. Chuanopparat, E. E. Elboray, M. F. Aly, H. H. Abbas-Temirek, B. Kongkathip and N. Kongkathip, Chem. Commun., 2013, 49, 2007 RSC.
- (a) J. I. Van Der Vlugt, Chem. Soc. Rev., 2010, 39, 2302 RSC and the corresponding citing references; (b) E. A. B. Kantchev, C. J. O'Brien and M. G. Organ, Angew. Chem., Int. Ed., 2007, 46, 2768 CrossRef CAS PubMed; (c) J. L. Klinkenberg and J. F. Hartwig, Angew. Chem., Int. Ed., 2011, 50, 86 CrossRef CAS PubMed; (d) T. Ohshima and K. Mashima, Yuki Gosei Kagaku Kyokaishi, 2012, 70, 1145 CrossRef CAS.
- B. M. Trost and E. Keinan, J. Org. Chem., 1979, 44, 3451 CrossRef CAS.
- (a) N-Heterocyclic Carbenes in Synthesis, ed. S. P. Nolan, Wiley-VCH, Weinheim, 2006 Search PubMed; (b) E. A. B. Kantchev, C. J. O'Brien and M. G. Organ, Angew. Chem., Int. Ed., 2007, 46, 2768 CrossRef CAS PubMed; (c) S. Diez-Gonzalez, N. Marion and S. P. Nolan, Chem. Rev., 2009, 109, 3612 CrossRef CAS PubMed; (d) J. A. Mata and M. Poyatos, Curr. Org. Chem., 2011, 15, 3309 CrossRef CAS; (e) A. Correa, S. P. Nolan and L. Cavallo, Top. Curr. Chem., 2011, 302, 131 CrossRef CAS; (f) G. C. Fortman and S. P. Nolan, Chem. Soc. Rev., 2011, 40, 5151 RSC; (g) C. Valente, S. Calimsiz, K. H. Hoi, D. Mallik, M. Sayah and M. G. Organ, Angew. Chem., Int. Ed., 2012, 51, 3314 CrossRef CAS PubMed; (h) S. Gaillard and J.-L. Renaud, Dalton Trans., 2013, 42, 7255 RSC.
- (a) H. Kurosawa, J. Organomet. Chem., 1987, 334, 243 CrossRef CAS; (b) N. Nomura, K. Tsurugi and M. Okada, Angew. Chem., Int. Ed., 2001, 40, 1932 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4qo00238e |
| This journal is © the Partner Organisations 2014 |