An efficient green chemistry protocol for the synthesis of novel spiropyrrolizidine compounds†
Krishnan
Revathy
and
Appaswami
Lalitha
*
Department of Chemistry, Periyar University, Salem-636011, Tamil Nadu, India. E-mail: lalitha2531@yahoo.co.in; Fax: +91 427 2345766; Tel: +91 427 2345271
First published on 7th November 2013
Abstract
An efficient method for the synthesis of spirooxindolopyrrolizidine/spirooxindolothiapyrrolizidine by the 1,3-dipolar cycloaddition of azomethine ylides generated via, decarboxylative condensation of 1,2-dicarbonyl compounds and L-proline/L-thiaproline/sarcosine with chalcones at ambient conditions with excellent yields has been described.
Introduction
Synthesis of organic compounds involving environment friendly greener techniques with lower energy costs has a significant potential for the reduction of by-products and waste materials.1–3 Conventional multistep reaction sequences are typically associated with lower yields, high cost, tedious isolation and purification processes of the resulting products. Recently, multicomponent reaction, a highly effective one-pot procedure with high atom economy has emerged as a valuable technique for the synthesis of biologically active molecules such as oxindole and pyrrolizidine derivatives.4–61,3-Dipolar cycloaddition of stabilized azomethine ylides (generated in situ by the reaction of isatin and secondary amino acids) to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C group, known as the Huisgen cycloaddition reaction serves as an efficient method for the formation of complex structures of spiro pyrrolizidines with multiple stereogenic centres in a single concerted step.7 Two π-electrons of the dipolarophile and four electrons of the dipolar compound participate in a concerted, pericyclic shift. The addition is stereo conservative (suprafacial) and the reaction is therefore a [2S + 4S] cycloaddition. This reaction occurs through the interaction between HOMO of azomethine ylide and LUMO of the alkene.8 The 1,3-dipolar cycloaddition of azomethine ylide to dipolarophile with exo cyclic double bond affords the spiro-heterocycles9–11 such as pyrrolizines, pyrrolizidines and pyrazolidines etc., which possess important biological activities.12
C group, known as the Huisgen cycloaddition reaction serves as an efficient method for the formation of complex structures of spiro pyrrolizidines with multiple stereogenic centres in a single concerted step.7 Two π-electrons of the dipolarophile and four electrons of the dipolar compound participate in a concerted, pericyclic shift. The addition is stereo conservative (suprafacial) and the reaction is therefore a [2S + 4S] cycloaddition. This reaction occurs through the interaction between HOMO of azomethine ylide and LUMO of the alkene.8 The 1,3-dipolar cycloaddition of azomethine ylide to dipolarophile with exo cyclic double bond affords the spiro-heterocycles9–11 such as pyrrolizines, pyrrolizidines and pyrazolidines etc., which possess important biological activities.12
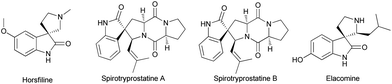
Spirooxindole ring systems shown in Fig. 1 exist as backbones for numerous alkaloids like horsfiline, spirotryprostatine A and B, elacomine,13etc., find applications as antimicrobial, antitumoral, antibiotic agents and inhibitors of human NK-1 receptor.14 Some of the spiropyrrolidines are potential antileukemic and anticonvulsant agents15 and also possess antiviral and local anaesthetic activities.16
Spirooxindolopyrrolizidine ring systems, formed by the joining of spirooxindole and pyrrolizidine rings at C-3 (spiro carbon), provide more opportunities for the development of a wide spectrum of biologically active compounds.17 Synthesis of spirooxindolopyrrolizidine derivatives using catalysts, such as K-10 montmorillonite18 and ZrOCl2·8H2O-ball clay19 have been carried out under solvent-free conditions using microwave irradiation.20 This synthetic method involved the reaction of an azomethine ylide with an electron-deficient alkene such as an α,β-unsaturated carbonyl compound. Most of the reported methods for the synthesis of these spiro compounds suffer from limitations, such as multi-step reactions, use of toxic solvents under reflux conditions and/or the formation of unwanted by-products etc.,21 In order to overcome the difficulties in the existing procedures, we report herein the synthesis of spirooxindolopyrrolizidine/spirooxindolothiapyrrolizidine derivatives through a simple experimental method with chalcone as a dipolarophile and a dipole (azomethine ylide) from isatin/acenaphthenequinone and secondary amino acids like L-proline/L-thiaproline/sarcosine providing good yields of the products.
Chalcones themselves have been reported to possess many useful properties, including anti-inflammatory, antimicrobial, antifungal, antioxidant, cytotoxic, antitumor and anticancer activities.22,23 So, inclusion of the chalcone moiety in the title compounds has been expected to enhance their biological activities. To the best of our knowledge, there are no reports of using chalcones for the above dipolar cycloaddition reactions.
Results and discussion
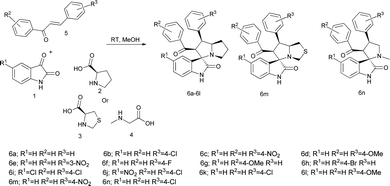
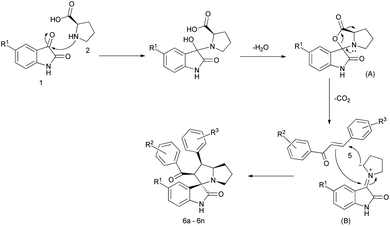
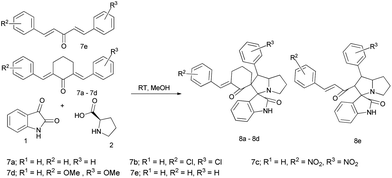
A series of spirooxindolopyrrolizidine/spirooxindolo thiapyrrolizidine and their derivatives have been synthesized in a simple and convenient method (Table 1) via, the dipolar cycloaddition reactions in methanol at room temperature, which resulted in products with excellent yields in shorter reaction time. The reaction of isatin (1) and L-proline (2) led to the formation of carboxylative cyclic ester (A), which on decarboxylation provided the azomethine ylide (B), a dipole as shown in the Fig. 2. The ylide thus formed added to the dipolarophile chalcone in a concerted manner to give the expected cycloaddition product and the only by-products formed were water and CO2. When the reaction was performed with L-thiaproline (entry 11–13; Table 1), the reaction time was little extended and when performed with sarcosine, an open chain amino acid (entry 14; Table 1) the reaction time was too longer compared to the other two amino acids studied.| S. no | R1 | R2 | R3 | 2/3/4 | Time (h) | Yield (%)b | Product |
|---|---|---|---|---|---|---|---|
| a Reactions were performed with 1 mmol of 1,2-dicarbonyl compound, 1 mmol of amino acid and 1 mmol of chalcone at room temperature. b Isolated Yield. | |||||||
| 1 | H | H | H | 2 | 12 | 80 | 6a (ref. 24) |
| 2 | H | H | 4-Cl | 2 | 4 | 87 | 6b |
| 3 | H | H | 4-NO2 | 2 | 3 | 88 | 6c |
| 4 | H | H | 4-OMe | 2 | 4 | 85 | 6d |
| 5 | H | H | 3-NO2 | 2 | 10 | 81 | 6e |
| 6 | H | H | 4-F | 2 | 7 | 83 | 6f |
| 7 | H | 4-OMe | H | 2 | 6 | 79 | 6g |
| 8 | H | 4-Br | H | 2 | 7 | 80 | 6h |
| 9 | Cl | H | 4-Cl | 2 | 6 | 86 | 6i |
| 10 | NO2 | H | 4-Cl | 2 | 5 | 87 | 6j |
| 11 | H | H | 4-Cl | 3 | 5 | 80 | 6k |
| 12 | H | H | 4-OMe | 3 | 8 | 77 | 6l |
| 13 | H | H | 4-NO2 | 3 | 6 | 79 | 6m |
| 14 | H | H | 4-Cl | 4 | 36 | 75 | 6n |
When the reaction was carried out with the non-natural amino acid D-proline, no changes were observed in the nature of the product and its yield. This may be due to the loss of the stereo centre in the proline moiety while forming the azomethine ylide as shown in the mechanism, resulting in the formation of spiropyrrolizidine product. The formation of single selective compound has also been evidenced from the crystal data.
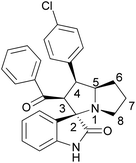
From 1H–1H COSY spectrum, we can conclude the structure for 6b as follows: The multiplet at 1.73–1.76 ppm corresponds to a proton at C-6, which correlates with a proton at C-5, ring fused carbon and with another proton at C-6. Similarly, another proton at C-6 is having same type of correlation with the protons at C-5 and another proton at C-6 i.e., possibly there is an internal cross coupling between the protons at C-6. The multiplet at 1.88–1.98 ppm corresponds to a proton at C-7, which is having a cross peak or correlation with the proton at C-8 and there is an internal cross couplings between the protons at C-8. Two multiplets at 2.59 and 2.68 ppm are having correlations with the proton at C-7. The triplet at 3.87–3.91 ppm (J = 12 Hz) corresponds to a proton at C-4, which is having correlation with C-3 and C-5 protons. The multiplet at 4.17–4.23 ppm corresponds to a proton at C-5 ring fused carbon, having correlations with the protons at C-4 and C-6. The sharp doublet at 4.85–4.88 ppm (J = 12 Hz) was due to the presence of a proton at C-3, benzoyl group bearing carbon, having correlation with a proton at C-4. The presence of three –CH2 groups represented in the proposed molecule was confirmed by DEPT-135 spectrum, which shows the three negative peaks for –CH2 and three positive peaks for –CH groups. From these spectral evidences, we can propose the structure as shown in Fig. 3.
In addition to this structural evidence, we have recorded the 1H–13C COSY spectra for 6b to confirm the above proposed structure. The signal at 27.28 ppm shows correlation with the protons at C-7 carbon. The signal at 30.70 ppm has two correlations with the two different protons at C-6 carbon. From this we confirmed the presence of two different environment natures of protons at C-6 carbon. The signal at 48.26 ppm has two correlations with two different environment protons at C-8 carbon. The signal at 52.36 ppm corresponds to the proton at C-4 carbon bearing chlorophenyl group. The signal at 64.51 ppm corresponds to a C-3 carbon bearing benzoyl moiety. The signal at 71.91 ppm corresponds to C-5 carbon, where two five membered rings get fused together. The signal at 73.77 ppm showing no correlation with any of the protons confirms the presence of a spiro carbon, where isatin and pyrrolizidine moieties get fused. The signal at 110.36–140.80 ppm shows correlation with the aromatic protons at 6.60–7.46 ppm. And finally, the peaks at 181.31 and 196.91 ppm are having no correlation with any protons and found to be –NHCO of isatin and –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of benzoyl group. Thus from the 1H, 13C, 1H–1H COSY, 1H–13C COSY, DEPT-135 spectra, we have confirmed the structure of compound 6b. In order to get more structural evidence, ORTEP diagram for a single crystal of compound 6b has been presented in Fig. 4 and that of compound 6n in Fig. 5, from which it can be inferred that the structure of all the products remains similar irrespective of the substituents present.
O of benzoyl group. Thus from the 1H, 13C, 1H–1H COSY, 1H–13C COSY, DEPT-135 spectra, we have confirmed the structure of compound 6b. In order to get more structural evidence, ORTEP diagram for a single crystal of compound 6b has been presented in Fig. 4 and that of compound 6n in Fig. 5, from which it can be inferred that the structure of all the products remains similar irrespective of the substituents present.
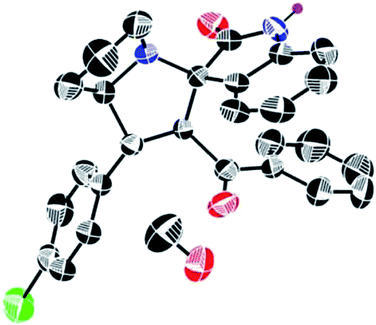 | ||
| Fig. 4 ORTEP representation of the crystal structure of compound 6b. Thermal ellipsoids are drawn at 50% probability and all H atoms are removed for clarity. | ||
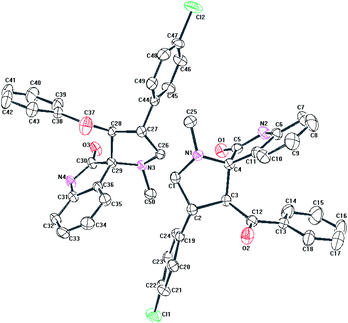 | ||
| Fig. 5 ORTEP representation of the crystal structure of compound 6n Thermal ellipsoids are drawn at 30% probability and all H atoms are removed for clarity. | ||
The same mild condition has been applied for the synthesis of spiropyrrolizidine compounds from dibenzylidine chalcone derivatives (Table 2) and we have obtained the expected products in good yields with lesser time. But for the substituted dibenzylidine chalcones, the reaction was carried out under reflux conditions, resulted in a mixture of products and was unstable while separating from the mixture.
| S. no | R1 | R2 | R3 | Time (h) | Yieldb (%) | Product |
|---|---|---|---|---|---|---|
| a Reactions were performed with 1 mmol of 1,2-dicarbonyl compound, 1 mmol of amino acid and 1 mmol of chalcone under reflux conditions (due to the insolubility of starting materials). b Isolated Yield. c Mixture of products were obtained. | ||||||
| 1 | H | H | H | 4 | 80 | 8a |
| 2 | H | 4-Cl | 4-Cl | 1a | 75 | 8b c |
| 3 | H | 4-NO2 | 4-NO2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30a 30a |
72 | 8c c |
| 4 | H | 4-OMe | 4-OMe | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30a 30a |
70 | 8d c |
| 5 | H | H | H | 4 | 78 | 8e (ref. 25) |
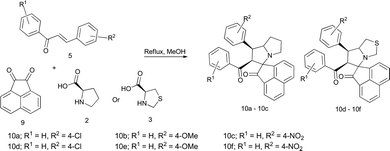
The same experimental procedures were extended to the synthesis of acenaphthenequinone containing spiropyrrolizidine/spirothiapyrrolizidine compounds under room temperature. But due to the insolubility of acenaphthenequinone, the reaction was carried out under reflux conditions, where the expected spiro product was obtained within 2–3 h in all the cases (Table 3). There was no substantial effect on the reaction by the presence of substituents on the chalcone for this spiro compound synthesis. Moreover most of the reactions were completed within shorter duration at reflux temperature compared to other reported procedures.26
| S. no | R1 | R2 | Amino acid | Time (h) | Yieldb (%) | Product |
|---|---|---|---|---|---|---|
| a Reactions were performed with 1 mmol of 1,2-dicarbonyl compound, 1 mmol of amino acid and 1 mmol of chalcone under reflux conditions (due to the insolubility of starting materials). b Isolated Yield. | ||||||
| 1 | H | 4-Cl | 2 | 2 | 85 | 10a |
| 2 | H | 4-OMe | 2 | 2 | 84 | 10b |
| 3 | H | 4-NO2 | 2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
86 | 10c |
| 4 | H | 4-Cl | 3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
84 | 10d |
| 5 | H | 4-OMe | 3 | 3 | 83 | 10e |
| 6 | H | 4-NO2 | 3 | 2 | 85 | 10f |
The structures of the products were confirmed by the 1H, 13C, 1H–1H, 1H–13C COSY NMR techniques. And also from these spectral data, we inferred that the structure of the product (10d) formed remains the same as that of 6b that has been confirmed by 2D NMR spectral studies. In all the cases, water and CO2 remained the only by-products, thereby fulfilling the desirable requirements of green chemistry. Hence, we can call it as an eco-friendlier greener synthesis of spiropyrrolizidine.
Conclusions
In Conclusion, we have developed a highly efficient method for the synthesis of spirooxindolopyrrolizidine/thiapyrrolizidine, acenaphthenequi-nonespiropyrrolizidine/spirothiapyrrolizidine derivatives in an eco-friendlier process without using any metallic reagents and hazardous solvents at ambient conditions.Experimental section
Chalcones have been prepared by the condensation of ketones with aldehydes in mild basic medium at cold conditions. Isatin, acenaphthenequinone, L-proline and L-thiaproline were purchased from Sigma-Aldrich and were used as such without further purification. The melting points of all compounds were determined with an electrothermal apparatus using capillary tube and are uncorrected. The purities of the compounds were checked by TLC using precoated silica gel plates with n-hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (6
ethyl acetate (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) as eluent. 1H, 13C and 2D NMR spectra were recorded on a Bruker Avance spectrophotometer at 400/100 and 500/125 MHz respectively using TMS as reference. High Resolution Mass Spectra of representative compounds were recorded on maXis 10138 Mass spectrometer at 70 eV. Elemental microanalyses were carried out on a Perkin-Elmer elemental analyzer Model 240C and a Thermo Finnigan analyser series Flash EA1112. Single crystal X-ray diffraction was performed on a Bruker-Nonius SMART APEX CCD area detector system using graphite monochromated, Mo-Kα (λ = 0.71073) radiation.
4) as eluent. 1H, 13C and 2D NMR spectra were recorded on a Bruker Avance spectrophotometer at 400/100 and 500/125 MHz respectively using TMS as reference. High Resolution Mass Spectra of representative compounds were recorded on maXis 10138 Mass spectrometer at 70 eV. Elemental microanalyses were carried out on a Perkin-Elmer elemental analyzer Model 240C and a Thermo Finnigan analyser series Flash EA1112. Single crystal X-ray diffraction was performed on a Bruker-Nonius SMART APEX CCD area detector system using graphite monochromated, Mo-Kα (λ = 0.71073) radiation.
General procedure for the synthesis of spiropyrrolizidine 6a–6n
To the solution of 0.5 mmol of isatin and 0.5 mmol of L-proline/L-thiaproline/sarcosine in 10 ml of methanol, 0.5 mmol mol of chalcone was added and stirred at room temperature until the completion of reaction as indicated by TLC. After the completion of reaction, solvent was removed in a rotary evaporator and the crystallized product was obtained in excellent yields and purity.General synthesis for the synthesis of spiropyrrolizidine 8a and 8e
To the solution of 0.5 mmol of isatin and 0.5 mmol of L-proline in 10 ml of methanol, 0.5 mmol of chalcone was added and stirred at reflux temperature until the completion of reaction as indicated by TLC. After the completion of reaction, solvent was removed in a rotary evaporator and the crystallized product was obtained in excellent yields and purity.General synthesis for the synthesis of spiropyrrolizidine 10a–10f
To the solution of 0.5 mmol of acenaphthenequinone and 0.5 mmol of L-proline/L-thiaproline in 10 ml of methanol, 0.5 mmol mol of chalcone was added and stirred at reflux temperature until the completion of reaction as indicated by TLC. After the completion of reaction, solvent was removed in a rotary evaporator and the crystallized product was obtained in excellent yields and purity.Acknowledgements
The authors are grateful to Prof. M. Periasamy and UGC Networking Resource Centre, University of Hyderabad, INDIA for providing the spectral facilities. K. R. thanks Jawaharlal Nehru Memorial Fund for Doctoral Studies for the financial support.Notes and references
- P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford, New York, 1998 Search PubMed.
- P. T. Anastas and T. Williamson, Green Chemistry, Frontiers in Benign Chemical Synthesis and Procedures, Oxford, 1998 Search PubMed.
- J. H. Clark, Green Chem., 1999, 1, 1–4 RSC.
- L. Weber, Drug Discovery Today, 2002, 7, 143–147 CAS.
- (a) F. Balkenhohl, C. von dem Bussche-Httnnefeld, A. Lansky and C. Zechel, Angew. Chem., Int. Ed. Engl., 1996, 35, 2289–2337 CrossRef; (b) L. A. Thompson and J. A. Ellman, Chem. Rev., 1996, 96, 555–600 CrossRef CAS PubMed.
- S. Fujimori, Jap. Pat. Appl., 88-2912, Chem. Abstr., 1990, 112, 98409.
- (a) A. P. Kozikowski and P. D. Stein, J. Am. Chem. Soc., 1982, 104, 4023–4024 CrossRef CAS; (b) S. F. Martin, M. S. Dappen, B. Dupre, C. J. Murphy and J. A. Colapret, J. Org. Chem., 1989, 54, 2209–2216 CrossRef CAS; (c) D. P. Curran, J. Am. Chem. Soc., 1983, 105, 5826–5833 CrossRef CAS; (d) V. Jager and I. Muller, Tetrahedron, 1985, 41, 3519–3528 CrossRef.
- K. N. Houk, J. Sims, C. R. Watts and L. J. Luskus, J. Am. Chem. Soc., 1973, 95, 7301–7315 CrossRef CAS.
- (a) Comprehensive Organic Synthesis, ed. A. Padwa, B. H. Trost and W. Flemming, Pergamon, Oxford, 1991, vol. 4, p. 1085 Search PubMed; (b) I. Fejes, M. Nyerges, A. Szollosy, G. Blasko and L. Toke, Tetrahedron, 2001, 57, 1129 CrossRef CAS.
- G. Broggini and G. Zecchi, Synthesis, 1999, 905 CrossRef CAS PubMed.
- (a) A. Amal Raj and R. Raghunathan, Tetrahedron, 2001, 57, 10293 CrossRef; (b) R. D. R. S. Manian, J. Jayashankaran and R. Raghunathan, Tetrahedron, 2006, 62, 12357 CrossRef CAS PubMed; (c) M. Poornachandran and R. Raghunathan, Tetrahedron, 2006, 62, 11274 CrossRef CAS PubMed.
- A. Amal Raj, R. Raghunathan, M. R. SrideviKumari and N. Raman, Bioorg. Med. Chem., 2003, 11, 407 CrossRef.
- S. T. Hilton, T. C. T. Ho, G. Pljevaljcic and K. Jones, Org. Lett., 2000, 2, 2639–2641 CrossRef CAS.
- (a) T. Okita and M. Isobe, Tetrahedron, 1994, 50, 11143–11152 CrossRef CAS; (b) P. Rosenmond, M. Hosseini-Merescht and C. Bub, Liebigs Ann. Chem., 1994, 2, 151–154 CrossRef.
- M. A. Abou-Gharbia and P. H. Doukas, Heterocycles, 1979, 12, 637–640 CrossRef CAS.
- M. J. Kornet and A. P. Thio, J. Med. Chem., 1976, 19, 892–898 CrossRef CAS.
- K. C. Joshi, R. Jain and P. Chand, Heterocycles, 1985, 23, 957 CrossRef CAS.
- A. R. Suresh Babu and R. Raghunathan, Tetrahedron Lett., 2007, 48, 305–308 CrossRef PubMed.
- J. Jayashankaran, R. D. R. S. Manian, R. Venkatesan and R. Raghunathan, Tetrahedron, 2005, 61, 5595–5598 CrossRef CAS PubMed.
- J. Jayashankaran, R. D. R. S. Manian and R. Raghunathan, Tetrahedron Lett., 2004, 45, 7303–7305 CrossRef CAS PubMed.
- (a) T. Coulter, R. Grigg, J. F. Malone and V. Sridharan, Tetrahedron Lett., 1991, 32, 5417 CrossRef CAS; (b) A. K. Ganguly, N. Seah, V. Popov, C. H. Wang, R. Kuang, A. K. Saksena, B. N. Pramanik, T. M. Chan and A. T. McPhail, Tetrahedron Lett., 2002, 43, 8981 CrossRef CAS.
- J. R. Dimmock, D. W. Elias, M. A. Beazely and N. M. Kandepu, Curr. Med. Chem., 1999, 6, 1125–1149 CAS.
- M. L. Go, X. Wu and X. L. Liu, Curr. Med. Chem., 2005, 12, 483–499 CrossRef CAS.
- (a) D. G. Powere, D. S. Casebier, D. Fokas, W. J. Ryan, J. R. Troth and D. L. Coffen, Tetrahedron, 1998, 54, 4085–4096 CrossRef; (b) D. Fokas, W. J. Ryan, D. S. Casebier and D. L. Coffen, Tetrahedron Lett., 1998, 39, 2235–2238 CrossRef CAS.
- S. Nirmala, R. Murugan, E. Theboral Sugi Kamala, L. Sudha and S. Sriman Narayanan, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2008, 64, o1817 CAS.
- A. Amal Raj and R. Ragunathan, Tetrahedron, 2001, 57, 10293–10298 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Copies of 1H- and 13C-NMR spectra of all compounds and 2D-NMR spectra for selected compounds and X-ray diffraction data for compounds 6b and 6n. CCDC 936044 and 955493. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3ra44627a |
| This journal is © The Royal Society of Chemistry 2014 |