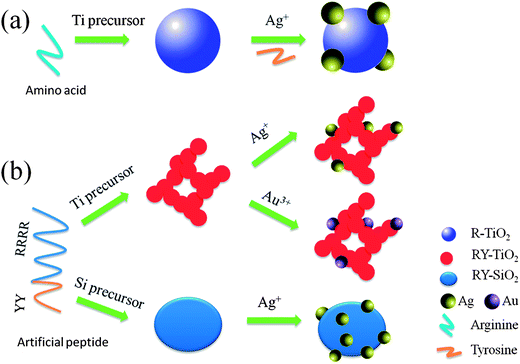
Biomimetic synthesis of inorganic nanocomposites by a de novo designed peptide
Chuang
Liu
ab,
Zhongyi
Jiang
ac,
Zhenwei
Tong
a,
Yixiao
Li
a and
Dong
Yang
*bc
aKey Laboratory for Green Technology of Ministry of Education, School of Chemical Engineering and Technology, Tianjin University, Tianjin 300072, China
bDepartment of Biochemical Engineering and Key Laboratory of Systems Bioengineering of Ministry of Education, School of Chemical Engineering and Technology, Tianjin University, Tianjin, 300072, China. E-mail: dongyang@tju.edu.cn; Fax: +86 22 2740 6642; Tel: +86 22 27406642
cSynergetic Innovation Center of Chemical Science and Engineering (Tianjin), Tianjin 300072, China
First published on 6th November 2013
Abstract
Inspired by the synthetic biology and biomineralization mechanism, a de novo designed biomimetic strategy is developed for the synthesis of metal oxide–metal nanocomposites (NCs) in aqueous solution under ambient conditions in this study. Via the screening of amino acids, it is found that arginine (R) can induce the formation of TiO2 and SiO2 nanoparticles (NPs), while tyrosine (Y) can reduce Ag+ and Au3+ ions into Ag and Au NPs, respectively. Subsequently, an artificial bifunctional peptide, arginine4tyrosine2 (R4Y2), as an example of this strategy, is designed to synthesize inorganic NCs including TiO2–Ag, TiO2–Au and SiO2–Ag, in which the positively charged moiety (R4) accelerates the polycondensation of negatively charged Ti or Si precursors, and the phenolic hydroxyl moiety (Y2) reduces Ag+ or Au3+ ions. This strategy may open an avenue for the green and controllable fabrication of a broad spectrum of inorganic NCs using artificial peptides as the inducer, designed rationally through encoding different parts of functional amino acids.
Introduction
Much effort has been devoted to inorganic nanocomposites (NCs) in recent years, since isolated or single component within a system is no longer sufficient to solve technological challenges involved in the development of multiple-function nanomaterials.1 For example, TiO2–Ag2–4 and TiO2–Au5 NCs are employed as visible light photocatalysts or antibacterial materials; SiO2–Ag6 NCs can be useful in sensors due to surface-enhanced Raman scattering properties etc. So far, the common fabrication strategies for inorganic NCs are physical mixing and sol–gel process, which often use harsh or toxic conditions including high temperature and pressure, organic solvents or strong acid/base catalysts. Therefore, a green and controllable synthesis strategy of inorganic NCs under mild conditions is urgently needed nowadays.Biomimetic approach, as a green synthesis strategy, which refers to utilize biomolecules like proteins/peptides and polysaccharides7 or bio-inspired polymers like polyamines8 to synthesize inorganic nanomaterials under mild conditions, has attracted much attention more recently. To date, three types of proteins/peptides have been employed as the catalyst and/or template to induce the formation of inorganic nanomaterials based on their source in the biomimetic method: (1) natural proteins extracted from organisms, such as silaffin,9 protamine,10 bovine serum albumin (BSA),11 and lysozyme;12 (2) artificial proteins/peptides derived from natural proteins like R5 peptide13 or selected by the phage display technique like peptides with affinity to Ti,14 Au,15 Ag,16 Pd,17 carbon nanotube,18 and graphene;19 (3) designed peptides originated from basic amino acids, such as poly-L-lysine,20 poly-L-tyrosine,21 cysteine–lysine,22 (lysine–leucine)8–PEG70,23 isoleucine3–lysine,24 alanine6–lysine,25 and amyloid-like peptide.26 Among these, the designed peptide is a future focal point since it can be rationally designed and construct different morphologies of inorganic nanomaterials inspired by the biomineralization mechanism. However, most of designed peptides have been used to prepare single-component inorganic nanomaterials, or to self-assemble into diverse morphologies till now.27 The synthesis of inorganic NCs by using rationally designed peptides as the inducer remains unexplored.
Whether a protein/peptide bears the ability to prepare inorganic nanomaterials is determined by its constituent amino acids. L-Arginine28 and L-lysine29 were reported as active amino acids to be able to induce TiO2 formation in aqueous conditions owing to the carriage of positive charges under neutral pH. L-Tyrosine,30,31L-tryptophan,32L-phenylalanine33 and L-cysteine34etc. can reduce metal ions into metal nanoparticles (NPs). Moreover, L-glutamic acid and L-aspartic acid are able to control the formation and growth process of inorganic nanocrystals.30 Therefore, the composition and content of amino acids are crucial for designing the peptide with capability to controllably induce the formation of inorganic NPs. In the last decade, the synthetic biology, which aims to create new biological systems by encoding different genetic parts to investigate natural biological phenomena or as a biofactory for a variety of bioprocessing applications, has triggered intense attention among bioengineers and chemists.35 Enlightened by such encouraged progress, the synthetic peptide composed of different amino acids with different functions may be theoretically powerful and versatile for producing inorganic NCs by a biomimetic approach.
In the present study, a de novo designed biomimetic strategy is developed for the synthesis of inorganic NCs by using a rationally designed peptide as the single inducer. In order to confirm the availability of this strategy, L-arginine (R) and L-tyrosine (Y) are screened to induce the formation of TiO2 or SiO2 NPs, and to reduce Ag+ or Au3+ into Ag or Au NPs, respectively. Subsequently, a six peptide, R4Y2, is designed to synthesize TiO2–Ag, TiO2–Au and SiO2–Ag NCs. This strategy is a facile, green and controllable technique, which may be applied for the fabrication of inorganic NCs with unique physical, chemical and biological properties in future.
Experimental
Chemicals
Titanium(IV) bis(ammonium lactato) dihydroxide (Ti-BALDH, a 50 wt% aqueous solution) was purchased from Sigma-Aldrich (Beijing, China). L-Arginine, L-tyrosine, silver nitrate (AgNO3), sodium silicate (Na2SiO3·9H2O) and chloroauric acid (HAuCl4·3H2O) were from Tianjin Guangfu Fine Chemical Research Institute. Artificial peptide arginine4tyrosine2 (R4Y2) (purity > 95%, obtained from HPLC analysis) was produced from China Peptides Co., Ltd. All chemicals were of analytic grade, and used as received without further purification. Deionized water was used throughout the study.Synthesis of inorganic nanoparticles by amino acids
A 0.3 mol L−1L-arginine solution (10 mL, pH = 7.0) was mixed with 1 mL of 0.25 mol L−1 Ti-BALDH solution under agitating vigorously for 5 min. After recovered by centrifugation and washed with deionized water, TiO2 NPs were obtained, which is denoted as R-TiO2. Similarly, Ag NPs (denoted as Y-Ag) were produced by mixing 10 mL of 2 mmol L−1L-tyrosine solution (pH = 9.0) with 1 mL of 10 mmol L−1 AgNO3 solution under stirring for about 48 h, separating by centrifugation, and washing with deionized water orderly. The synthetic process of TiO2–Ag NCs (denoted as R-TiO2-Ag(Y)) was the same as that of Ag NPs except that a certain amount of TiO2 NPs was added in the mixed solution of L-tyrosine and AgNO3 at the beginning.Synthesis of inorganic nanoparticles by artificial peptide R4Y2
Typically, 0.5 mL of 0.25 mol L−1 Ti-BALDH was added into 1 mL of 2 g L−1 peptide solution (pH = 7.0), and stirred for 5 min. TiO2 NPs, denoted as RY-TiO2, were obtained after centrifugated at 5000 rpm and rinsed with deionized water. Similarly, Ag NPs (denoted as RY-Ag) were fabricated by mixing 1 mL of 2 g L−1 peptide solution (pH = 7.0) with 1 mL of 10 mmol L−1 AgNO3 solution under stirring for about 48 h, separating by centrifugation, and washing with deionized water orderly. The TiO2 solution as prepared above was adjusted to pH 9.0 by NaOH, and then added into 3 mL of 10 mmol L−1 AgNO3 solution under stirring for 48 h. TiO2–Ag NCs were achieved after centrifugated at 5000 rpm and rinsed with deionized water, which is denoted as RY-TiO2-Ag. TiO2–Au NCs (denoted as RY-TiO2-Au) were prepared by the same procedure of TiO2–Ag NCs except that the AgNO3 solution is substituted for an 80 μL of 1 wt% chloroauric acid solution. SiO2–Ag NCs (denoted as RY-SiO2-Ag) were synthesized by the same procedure of TiO2–Ag NCs except that the Ti-BALDH solution is substituted for a 2 mL of 60 mM Na2SiO3 solution (Scheme 1)..Characterization
Transmission electron microscopy (TEM) analysis of the samples were performed on a JEM-100CX II transmission electron microscope at an accelerating voltage of 200 kV. High resolution transmission electron microscopy (HRTEM) and elemental mapping were conducted at 90 K using a JEOL-2011 equipped with a Bruker X-flash silicon drift detector (SDD) at 200 kV. X-ray photoelectron spectroscopy (XPS) of the samples was observed on a Perkin-Elmer PHI 1600 ESCA X-ray photoelectron spectroscope with a monochromatic Mg Kα radiation (1253.6 eV), and the binding energies were normalized to C1s peak at 284.6 eV. X-ray diffraction (XRD) patterns were obtained by a Rigaku D/max 2500V/PC X-ray diffractometer (Cu Kα, λ = 0.154 nm, 40 kV, 200 mA). Scattered radiation was detected in the angular range of 10–90° (2θ) with a scan rate of 4° min−1. The circular dichroism spectroscopy (CD) of R4Y2 and inorganic NPs induced by R4Y2 in water were recorded from 190 to 260 nm by using a JASCO J715 spectropolarimeter at 298 K with a resolution of 0.5 nm and a quartz cuvette with 5 mm path length.Results and discussion
Inorganic nanoparticles synthesized by amino acids
Recently, much effort has been devoted to the biomimetic synthesis of inorganic NPs by using diverse proteins/peptides, however, the involved mechanism, especially the role of specific amino acids, is not elucidated clearly. In order to design the peptide that is able to induce the formation of inorganic NCs, the function of individual amino acids is screened according to the reports in the literatures.28,33When an arginine solution at pH 7.0 was added into the Ti-BALDH solution under continually stirring, the mixed solution became turbid quickly. The SEM result (Fig. 1a) indicates that the forming precipitates are aggregated spherical NPs with diameter around 200–400 nm. As demonstrated in Fig. 2a, these NPs exhibit two weak broad peaks around 25.2° and 47.9°, suggesting that they are partially crystallized anatase TiO2 (JCPDS no. 21-2172), which is similar to the previous report.12 Arginine is a high isoelectric point amino acid (pI = 10.76) with positive charges at neutral pH, which can attract negatively charged Ti precursors and facilitate their dehydration and polycondensation process.
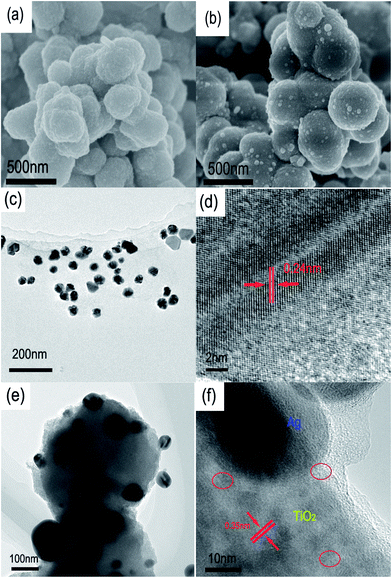 | ||
| Fig. 1 SEM images of R-TiO2 NPs (a) and R-TiO2-Ag(Y) NCs (b); a TEM picture of Y-Ag NPs (c) and its HRTEM image (d); a TEM picture of R-TiO2-Ag(Y) NCs (e) and its HRTEM image (f). | ||
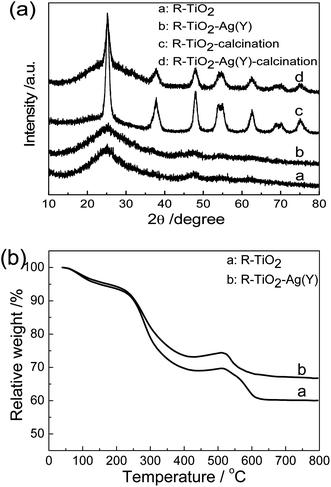 | ||
| Fig. 2 (a) XRD patterns of R-TiO2 and R-TiO2-Ag(Y) NPs before and after calcination; (b) TGA curves of R-TiO2 and R-TiO2-Ag(Y) NPs. | ||
As shown in Fig. 1c, some NPs without obvious aggregation can be produced by L-tyrosine under alkaline conditions, which are the combination of spherical-like, triangular and polygonal NPs with diameter of 36.0 ± 9.6 nm. HRTEM further confirms that these NPs possess a d-space of 0.24 nm, corresponding to (101) plane of face centered cubic Ag (Fig. 1d). Tyrosine is an aromatic amino acid with phenolic group, which can ionize under alkaline condition and transfer electrons to Ag+ ions, leading to the formation of Ag NPs and quinone compound.36 Moreover, it is reported that other amino acids with phenolic group, like tryptophan and phenylalanine, can also have the similar capability to reduce metal ions.33
TiO2–Ag NCs can be prepared easily by jointly using L-tyrosine and L-arginine as the reducer of Ag+ ions and the inducer of Ti precursors, respectively. From Fig. 1b and e, it can be seen that Ag NPs about 35 nm in diameter are deposited onto the surface of TiO2 NPs with the size about 400 nm. The corresponding HRTEM image (Fig. 1f) demonstrates that some nanoparticles exist near a Ag NP, and have a d-spacing of 0.35 nm, corresponding to (101) face of anatase TiO2. Furthermore, there are some organic substances around the Ag NP, which may be L-tyrosine. The similar phenomenon is also observed in the Au NPs synthesized by lysozyme and poly-tyrosine, in which Au NPs are entrapped in the protein.26,27 XRD measurements were carried out to analyze the crystallization behavior of R-TiO2-Ag(Y) NCs. As demonstrated in Fig. 2a, the XRD curve of R-TiO2-Ag(Y) is almost same as that of R-TiO2, indicating that the deposition of Ag NPs does not affect the crystallization of R-TiO2. There is no Ag peak in the XRD pattern, which can be assigned to the small amount of Ag in the NCs. Fig. 2b demonstrates the TG curves of R-TiO2 and R-TiO2-Ag(Y). There are three weight loss stages: 100–200, 200–400 and 500–600 °C, which can be assigned to the loss of bound water, the decomposition of amino acids and the phase transformation of TiO2 in the samples, respectively. The remaining weights account for 60% and 67% in R-TiO2 and R-TiO2-Ag(Y), which should be the inorganic phase.
The X-ray photoelectron spectroscopy (XPS) was conducted to further confirm the chemical state of Ti and Ag atoms in the TiO2–Ag NCs. Fig. 3a exhibits the high-resolution Ti2p XPS spectra of R-TiO2 and R-TiO2-Ag(Y). The Ti2p curve of R-TiO2 has two peaks at 458.2 and 464.2 eV, which belong to the Ti2p3/2 and Ti2p1/2 orbits, respectively, agreeing well with Ti(IV) in pure anatase TiO2. In comparison, these two peaks of R-TiO2-Ag(Y) shift to higher binding energy at 459.7 and 465.9 eV. This shift can be distributed to the strong interaction between Ag and TiO2, which results in a lower electron density of the TiO2 surface after the Ag NPs deposition.37 The high-resolution Ag3d XPS spectrum (Fig. 3b) has two peaks at 367.8 and 373.8 eV, which can be assigned to the Ag3d5/2 and Ag3d3/2 orbits, respectively. The peaks shift to lower binding energy compared to bulk Ag crystal (368.3 eV for Ag3d5/2, and 374.3 eV for Ag3d3/2), which can be explained by the electron transfer from TiO2 to Ag.37Fig. 3c and d are high-resolution O1s XPS spectra of R-TiO2 and R-TiO2-Ag(Y). It can be seen that three oxygen atom environments exist in R-TiO2: crystal lattice oxygen (OTi–O–Ti at 529.4 eV), surface hydroxyl group (OTi–O–H at 531.7 eV) and adsorbed O2 (at 533.5 eV). The O1s curve of R-TiO2-Ag(Y) NCs can be fitted to four peaks, in which three peaks locate at the same places as those in R-TiO2 despite different intensities. A new peak of Ti–O–Ag appears at binding energy of 530.1 eV, implying the covalent bonding between Ag and TiO2 NPs.
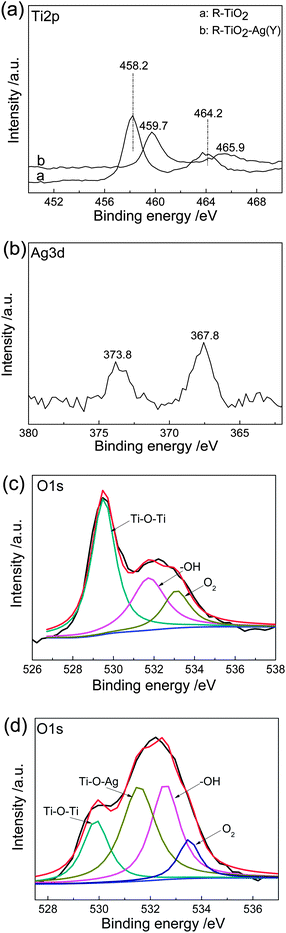 | ||
| Fig. 3 High-resolution XPS spectra: Ti2p of R-TiO2 and R-TiO2-Ag(Y) (a), Ag3d of R-TiO2-Ag(Y) (b), O1s of R-TiO2 (c) and R-TiO2-Ag(Y) (d). | ||
Inorganic nanocomposites synthesized by R4Y2
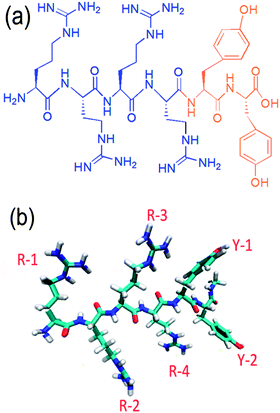
Although arginine and tyrosine can induce the formation of inorganic NCs like TiO2–Ag, they lack the complexity to control exquisitely the structure and morphology of inorganic materials. In nature, proteins which is built from 20 basic amino acids by peptide bonds, perform a vast array of functions within living organisms including cell structure, cell signaling, metabolic reaction catalysis etc. Particularly, some proteins like silaffin in diatom,9 matrix proteins in pearl oyster38 can elegantly induce the formation of different biominerals under physiological conditions. The complexity of proteins/peptides lies not only in the type, number and sequence of amino acids,39 but also in their secondary conformation,20 both of which contribute to the function of proteins/peptides. Therefore, the controllable synthesis of inorganic NCs can be realized by using de novo designed peptide as the inducer based on the functions of individual amino acids, which is named as the de novo designed biomimetic strategy. There are two critical requirements for the peptide design: one is that individual amino acids constituting the peptide have the interactions with formed inorganic materials, such as noncovalent, electrostatic, hydrogen bonding, hydrophobic, and aromatic stacking interactions; the other is the composition–structure–function relationship of the peptide, including the water-solubility, spatial structure and conformation etc. In order to confirm the feasibility of this strategy, an artificial hexapeptide R4Y2 (molecular weight, 969.13) composed of 4 arginines and 2 tyrosines has been designed and synthesized (Fig. 4). The short peptide containing 6 amino acids is favorable because it is easy to produce in large scale and to predict the structure–function relation.23 Moreover, in order to increase the water solubility, this peptide is designed with more arginine than tyrosine. It is assumed that two functional moieties in the artificial peptide R4Y2, R4 and Y2, may bear the ability to synthesize metal oxide and metal NPs, respectively.After adding R4Y2 into the Ti-BALDH solution, the transparent solution immediately became turbid, indicating the formation of inorganic precipitates as expected. These precipitates are highly interconnected network consisting of nanoparticles with the diameter of 15–30 nm (Fig. 5a), which are mainly composed of Ti and O elements (Fig. 5d), suggesting that they are TiO2 NPs. Compared to R-TiO2 NPs produced by arginine, RY-TiO2 NPs are smaller, however, they are similar with those induced by proteins like protamine10 or lysozyme.12 It is reported that the TiO2 precipitation can significantly be promoted by increasing the number of consecutive lysine in the peptide, because the sequence of consecutive lysine residues containing side-chain amine groups can attract anionic Ti precursors and thus, facilitating the nucleophilic attack between Ti precursors.29 It is deduced that the smaller size of TiO2 induced by R4Y2 results from the faster condensation of TiBALDH, since arginine has the side-chain guanidine group with higher electropositivity than amino group. Moreover, four consecutive arginine residues also possess the spatial proximity, which is believed to be a key element resulting in fast specific activity of Ti-mineralizing proteins.40 Unlike other designed peptide such as isoleucine3–lysine (I3K)23 and alanine6–lysine (V6K),24 R4Y2 doesn't self-assemble into supramolecular structure because of its high hydrophilicity. It directly acts as the catalyst to induce the TiO2 formation via the electrostatic and hydrogen bonding interactions with Ti-BALDH following by the polycondensation reaction.10 When R4Y2 is incubated with a Ag+ solution for about 24 to 48 h, the solution gradually turned brown, suggesting the reduction of Ag+ ions. TEM (Fig. 5b) and EDS (Fig. 5e) display that the formed sediment is spherical Ag NPs about 9.1 ± 2.3 nm in diameter, which are also much smaller than Y-Ag NPs synthesized by tyrosine, revealing the different influence of amino acid states (single molecule or amino acid residue in the peptide) on the Ag NP size.
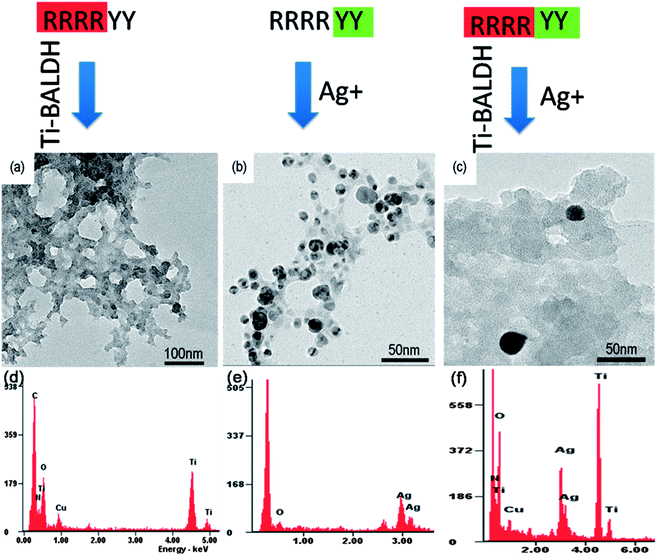 | ||
| Fig. 5 TEM images of (a) TiO2 NPs, (b) Ag NPs and (c) TiO2–Ag NCs synthesized by artificial peptide R4Y2 and their corresponding EDS spectra (d–f). | ||
In order to synthesize TiO2–Ag NCs by using R4Y2, interconnected TiO2 NPs were first produced, and then mixed with AgNO3 solution under stirring. As demonstrated in Fig. 5c and f, the obtained materials are TiO2–Ag NCs, in which Ag NPs are about 21.3 ± 6.7 nm in size. In order to confirm the R4Y2 universality for the biomimetic synthesis of metal oxide–metal NCs, TiO2–Au and SiO2–Ag NCs were also fabricated by the same method producing TiO2–Ag NCs. As shown in Fig. 6, the prepared TiO2–Au NCs are similar with TiO2–Ag NCs except that Au NPs are irregular aggregates; while the prepared SiO2–Ag NCs consist of quadrilateral-like SiO2 particles with diameter around 400–700 nm and spherical Ag NPs with diameter of 18.6 ± 9.5 nm.
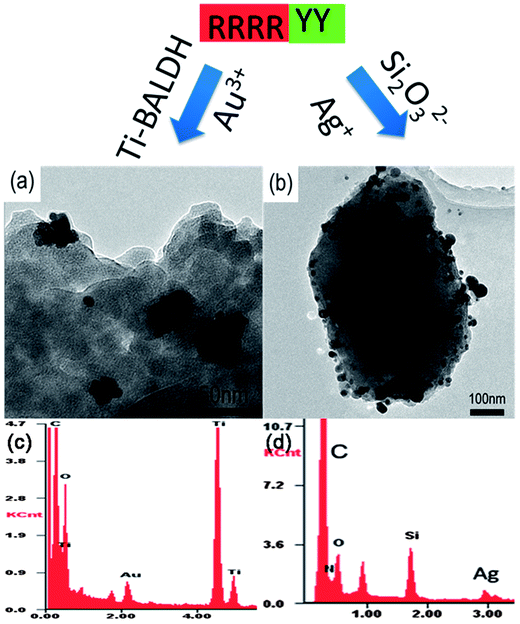 | ||
| Fig. 6 TEM images of TiO2–Au (a) and SiO2–Ag NCs (b) synthesized by artificial peptide R4Y2 and their corresponding EDS spectra (c and d). | ||
The circular dichroism (CD) spectra were employed to investigate the change of the peptide secondary structure during the biomimetic synthesis of inorganic NCs. The free peptide solution shows a β-turn structure with a minimum at 196 nm and maximum at 225 nm (Fig. 7a). When Ag+ was added to the peptide solution and reacted for 48 h, the CD spectrum yields a curve with three peaks around 195, 201 and 227 nm, indicating the overlap of β-turn and unordered conformation. After the addition of Ti-BALDH to the peptide solution, the solution yields a spectrum with minimum around 215 nm (Fig. 7b). This curve is indicative of a β-sheet conformation,20 which may be assigned to the electrostatic interaction between peptide and Ti precursor. After the addition of Ag+ or Au3+ to RY-TiO2 solution and incubation for about 48 hours, the solution yields a CD spectrum similar with the peptide–TiO2 solution. However, their minimum absorption shift to around 214 and 219 nm, respectively, which can be attributed to the interaction between peptide and Ag or Au NPs.15 The CD spectrum of RY-SiO2-Ag solution yields a complicated shape with a peak at 198 nm and a minimum at 217 nm, indicating the overlap of α-helix and β-sheet conformation.
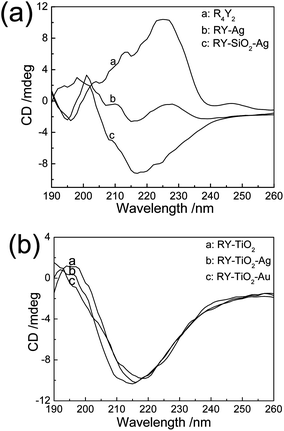 | ||
| Fig. 7 Circular dichroism spectra of R4Y2 solution, RY-Ag NPs, RY-SiO2-Ag (a) and RY-TiO2, RY-TiO2-Ag, RY-TiO2-Au (b). | ||
Based on the above results, a tentative mechanism that accounts for the role of R4Y2 in the biomimetic synthesis of inorganic NCs is deduced. Taking TiO2/Ag as an example, the R4 moiety of R4Y2 locally acts as acid–base catalyst during the formation of TiO2 NPs. The R4 moiety firstly triggers the hydrolysis/condensation reactions by its interaction with TiBALDH, and then promotes the nucleation and growth of titania. In this process, majority of R4Y2 molecules are not consumed, and locate on the surface of forming TiO2 NPs.14 When the AgNO3 solution is added in the reaction system subsequently, the Y2 segment of R4Y2 exposed on the surface of TiO2 NPs reduces Ag+ ions to form Ag NPs. Finally, the TiO2/Ag NCs are formed via the dual functions of R4Y2.
Conclusions
Artificial peptide R4Y2 is de novo designed to synthesize the metal oxide–metal NCs including TiO2–Ag, TiO2–Au and SiO2–Ag under mild conditions, based on the understanding of the function of each amino acid in the peptide. Specifically, the arginine (R) residues can induce the formation of metal oxide NPs through the electrostatic interaction with inorganic precursors, while the tyrosine (Y) residues can reduce metal ions into metal NPs via the electron transfer from phenolic groups to metal ions. CD spectra reveal that R4Y2 changes from β-turn to β-sheet upon the formation of metal oxide NPs, which subsequently induces the formation of metal NPs on the metal oxide surface. Through encoding different parts of functional amino acids to rationally design peptide, this strategy can enable the fabrication of a broad spectrum of inorganic NCs, which may find applications in antibacterial materials, photocatalyst, and sensors etc.Conflict of interest
The authors declare no competing financial interest.Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Acknowledgements
This research is supported by National Science Fund for Distinguished Young Scholars (21125627), the National Basic Research Program of China (2009CB724705) and the Program of Introducing Talents of Discipline to Universities (no. B06006).References
- P. Yang and J.-M. Tarascon, Nat. Mater., 2012, 11, 560–563 CrossRef CAS PubMed.
- L. Zhang, M. Han, O. K. Tan, M. S. Tse, Y. X. Wang and C. C. Sze, J. Mater. Chem. B, 2013, 1, 564–570 RSC.
- Q. Zhang, J. Ye, P. Tian, X. Lu, Y. Lin, Q. Zhao and G. Ning, RSC Adv., 2013, 3, 9739–9744 RSC.
- H. Zhang and G. Chen, Environ. Sci. Technol., 2009, 43, 2905–2910 CrossRef CAS.
- A. Tanaka, Y. Nishino, S. Sakaguchi, T. Yoshikawa, K. Imamura, K. Hashimoto and H. Kominami, Chem. Commun., 2013, 49, 2551 RSC.
- Z. Deng, M. Chen and L. Wu, J. Phys. Chem. C, 2007, 111, 11692–11698 CAS.
- Y. Yan, B. Hao and G. Chen, J. Mater. Chem., 2011, 21, 10755–10760 RSC.
- Y. Yan, B. Hao, X. Wang and G. Chen, Dalton Trans., 2013, 42, 12179–12184 RSC.
- N. Kröger, M. B. Dickerson, G. Ahmad, Y. Cai, M. S. Haluska, K. H. Sandhage, N. Poulsen and V. C. Sheppard, Angew. Chem., 2006, 118, 7397–7401 CrossRef.
- Y. Jiang, D. Yang, L. Zhang, L. Li, Q. Sun, Y. Zhang, J. Li and Z. Jiang, Dalton Trans., 2008, 4165–4171 RSC.
- M. Ramanathan, H. R. Luckarift, A. Sarsenova, J. R. Wild, E. K. Ramanculov, E. V. Olsen and A. L. Simonian, Colloids Surf., B, 2009, 73, 58–64 CrossRef CAS PubMed.
- C. Liu, D. Yang, Y. Jiao, Y. Tian, Y. Wang and Z. Jiang, ACS Appl. Mater. Interfaces, 2013, 5, 3824–3832 CAS.
- S. L. Sewell and D. W. Wright, Chem. Mater., 2006, 18, 3108–3113 CrossRef CAS.
- V. Puddu, J. M. Slocik, R. R. Naik and C. C. Perry, Langmuir, 2013, 29, 9464–9472 CrossRef CAS PubMed.
- J. Kim, M. J. Sadowsky and H.-G. Hur, Biomacromolecules, 2011, 12, 2518–2523 CrossRef CAS PubMed.
- R. R. Naik, S. J. Stringer, G. Agarwal, S. E. Jones and M. O. Stone, Nat. Mater., 2002, 1, 169–172 CrossRef CAS PubMed.
- R. Coppage, J. M. Slocik, B. D. Briggs, A. I. Frenkel, R. R. Naik and M. R. Knecht, ACS Nano, 2012, 6, 1625–1636 CrossRef CAS PubMed.
- M. J. Pender, L. A. Sowards, J. D. Hartgerink, M. O. Stone and R. R. Naik, Nano Lett., 2006, 6, 40–44 CrossRef CAS PubMed.
- Y. Cui, S. N. Kim, S. E. Jones, L. L. Wissler, R. R. Naik and M. C. McAlpine, Nano Lett., 2010, 10, 4559–4565 CrossRef CAS PubMed.
- S. V. Patwardhan, R. Maheshwari, N. Mukherjee, K. L. Kiick and S. J. Clarson, Biomacromolecules, 2006, 7, 491–497 CrossRef CAS PubMed.
- E. Kharlampieva, J. M. Slocik, T. Tsukruk, R. R. Naik and V. V. Tsukruk, Chem. Mater., 2008, 20, 5822–5831 CrossRef CAS.
- J. N. Cha, G. D. Stucky, D. E. Morse and T. J. Deming, Nature, 2000, 403, 289–292 CrossRef CAS PubMed.
- T. Nonoyama, T. Kinoshita, M. Higuchi, K. Nagata, M. Tanaka, K. Sato and K. Kato, J. Am. Chem. Soc., 2012, 134, 8841–8847 CrossRef CAS PubMed.
- H. Xu, Y. Wang, X. Ge, S. Han, S. Wang, P. Zhou, H. Shan, X. Zhao and J. R. Lu, Chem. Mater., 2010, 22, 5165–5173 CrossRef CAS.
- Q. Wang, J. Yu, X. Zhang, D. Liu, J. Zheng, Y. Pan and Y. Lin, RSC Adv., 2013, 3, 2784 RSC.
- H. Acar, R. Garifullin and M. O. Guler, Langmuir, 2011, 27, 1079–1084 CrossRef CAS PubMed.
- C.-L. Chen and N. L. Rosi, Angew. Chem., Int. Ed., 2010, 49, 1924–1942 CrossRef CAS PubMed.
- J. Shi, D. Yang, Z. Jiang, Y. Jiang, Y. Liang, Y. Zhu, X. Wang and H. Wang, J. Nanopart. Res., 2012, 14, 1–13 Search PubMed.
- S. Ahn, S. Park and S.-Y. Lee, J. Cryst. Growth, 2011, 335, 100–105 CrossRef CAS PubMed.
- J. Xie, J. Y. Lee, D. I. C. Wang and Y. P. Ting, ACS Nano, 2007, 1, 429–439 CrossRef CAS PubMed.
- Y. Wang, Y. Cui, Y. Zhao, R. Liu, Z. Sun, W. Li and X. Gao, Chem. Commun., 2012, 48, 871–873 RSC.
- S. Si and T. K. Mandal, Chem.–Eur. J., 2007, 13, 3160–3168 CrossRef CAS PubMed.
- Y. N. Tan, J. Y. Lee and D. I. C. Wang, J. Am. Chem. Soc., 2010, 132, 5677–5686 CrossRef CAS PubMed.
- M. Roy, P. Mukherjee, B. P. Mandal, R. K. Sharma, A. K. Tyagi and S. P. Kale, RSC Adv., 2012, 2, 6496 RSC.
- E. Andrianantoandro, S. Basu, D. K. Karig and R. Weiss, Mol. Syst. Biol., 2006, 2, 2006.0028 CrossRef PubMed.
- P. R. Selvakannan, A. Swami, D. Srisathiyanarayanan, P. S. Shirude, R. Pasricha, A. B. Mandale and M. Sastry, Langmuir, 2004, 20, 7825–7836 CrossRef CAS PubMed.
- C. Su, L. Liu, M. Zhang, Y. Zhang and C. Shao, CrystEngComm, 2012, 14, 3989–3999 RSC.
- M. Suzuki, K. Saruwatari, T. Kogure, Y. Yamamoto, T. Nishimura, T. Kato and H. Nagasawa, Science, 2009, 325, 1388–1390 CrossRef CAS PubMed.
- N. Choi, L. Tan, J.-r. Jang, Y. M. Um, P. J. Yoo and W.-S. Choe, J. Inorg. Biochem., 2012, 115, 20–27 CrossRef CAS PubMed.
- S. Park, H. Lee and S. Y. Lee, Dalton Trans., 2013, 42, 13817–13820 RSC.
| This journal is © The Royal Society of Chemistry 2014 |