A rhodamine-benzimidazole based sensor for selective imaging of acidic pH†
Zhongwei
Xue
a,
Mingliang
Chen
b,
Jianming
Chen
b,
Jiahuai
Han
c and
Shoufa
Han
*a
aDepartment of Chemical Biology, College of Chemistry and Chemical Engineering, The Key Laboratory for Chemical Biology of Fujian Province, The MOE Key Laboratory of Spectrochemical Analysis & Instrumentation and Innovation Center for Cell Biology, Xiamen University, Xiamen, 361005, China. E-mail: shoufa@xmu.edu.cn; Tel: +86-0592-2181728
bThe Key Laboratory of Marine genetic Resources, the Third Institute of Oceanography, State Oceanic Administration, 184 University Road, Xiamen, 361005, China
cState key Laboratory of Cellular Stress Biology, Innovation Center for Cell Biology, School of Life Sciences, Xiamen University, Xiamen, 361005, China
First published on 4th November 2013
Abstract
N-(Rhodamine B)-benzimidazole (RB-IM), featuring an intramolecular spiro-benzimidazole, was developed for selective sensing of protons via opening of the spiro-ring to give fluorescent and colored species. The utility of RB-IM was demonstrated by imaging of lysosomes in live cells and staining of the intestine of zebrafish.
Introduction
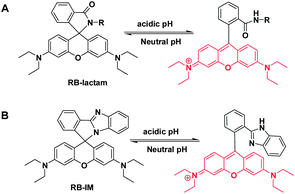
Biological systems often display organ- or organelle-specific pH homeostasis which is often fundamental for physiology. For instance, the acidic pH of digestive tracts is critical for food digestion meanwhile the lysosomal acidity mediates a variety of biological events such as endocytosis and autophagy.1 Alterations of the lysosomal acidity have been implicated in a number of pathological events,2 prompting extensive efforts in the development of pH reporting dyes for noninvasive imaging of lysosomal pH in live cells.Rhodamine dyes exhibit attractive fluorescence attributes such as high fluorescence quantum yields and high photo-stability, and thus are widely used for bioimaging. As conventional rhodamines remain emissive under physiological conditions (pH 4–8), substantial efforts have been devoted to the development of pH responsive rhodamine derivatives for reporting of endo-lysosomal acidity.3 For instance, rhodamine-lactams, a group of rhodamine derivatives hallmarked by the intramolecular spiro-lactams, have been employed for imaging of lysosomal pH by proton triggered opening of the intramolecular lactams (Scheme 1A).3b–e Albeit exhibiting fluorogenic signals to proton, the utility of these rhodamine-lactams are often compromised by their cross-reactivity to interfering cations, such as Fe3+.4 Herein we report the use of rhodamine B-benzimidazole diad (RB-IM) for selective imaging of the pH of lysosomes and staining the intestine of zebrafish.
Experimental procedure
Materials and methods
LysoTracker green DND-26 was purchased from Invitrogen. RB-EDA and RB-PDA were synthesized according to published procedures.5 All other chemicals were obtained from Alfa Aesar and used without further purification. Column chromatography was performed on silica gel (100–200 mesh). NMR spectra (1H-400 MHz and 13C-100 MHz) were recorded on a Bruker instrument using tetramethyl silane as the internal reference. Mass analysis was performed in Bruker En Apex ultra 7.0T FT-MS. Fluorescence spectra and UV-vis absorption spectra were recorded on a spectrofluorometer (SpectraMax M5, Molecular Device). L929 cells were obtained from American Type Culture Collection (ATCC). Fluorescence quantum yields of RB-IM and rhodamine B in Na2HPO4–NaH2PO4 buffer (200 mM, pH 4.5) were measured by Horiba Jobin Yvon HJY-FM4P-TCSPC. Confocal fluorescence microscopy images were obtained on Leica SP5 using the following filters: λex@543 nm and λem@565–625 nm for rhodamine B signal; λex@488 nm and λem@500–530 nm for DND-26 signal. The fluorescence of LysoTracker green and that of rhodamine B inside cells were merged by using Photoshop CS 5.0. Graph by GraphPad Prism5 software.Synthesis of RB-IM
RB-PDA (1.2 g) was added into anhydrous THF (20 ml) containing LiAlH4 (0.6 g). The solution was stirred at rt for 48 h. To the solution were added dropwisely 1-butanol (20 ml), and then the mixture was extracted by 1-butanol (100 ml) and water (100 ml). The organic layer was collected and dried over anhydrous sodium sulfate. The organic solution was concentrated by rotary evaporation and the residue was purified by silica gel column chromatography using ethyl acetate–petroleum ether–triethylamine (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v/v) as the eluent to give 0.6 g of solid as the desired product in 52% yield. 1H-NMR (400 MHz, CDCl3): δ: 8.13 (d, 1H, J = 7.56 Hz), 7.82 (d, 1H, J = 8.08 Hz), 7.49 (td, 1H, J1 = 7.52 Hz, J2 = 0.88 Hz), 7.40 (td, 1H, J1 = 7.50 Hz, J2 = 1.04 Hz), 7.25 (d, 1H, J = 7.64 Hz), 7.18 (m, 1H), 7.04 (m, 1H), 6.96 (d, 1H, J = 8.00 Hz) 6.52 (d, 2H, J = 2.52 Hz), 6.32 (d, 2H, J = 8.84 Hz), 6.19 (d, 1H, J = 2.56 Hz), 6.16 (d, 1H, J = 2.60 Hz), 3.33 (q, 8H, J = 7.06 Hz), 1.16 (t, 12H, J = 7.04 Hz); 13C-NMR (100 MHz, CDCl3): 156.63, 156.28, 153.07, 148.95, 130.90, 130.44, 128.61, 128.27, 127.92, 124.84, 122.54, 121.92, 121.37, 120.09, 110.21, 108.14, 106.28, 97.89, 63.86, 44.32, 12.60 ppm; MS (C34H34N4O): calculated (MH+): 515.27, found (MH+): 515.8.
1, v/v/v) as the eluent to give 0.6 g of solid as the desired product in 52% yield. 1H-NMR (400 MHz, CDCl3): δ: 8.13 (d, 1H, J = 7.56 Hz), 7.82 (d, 1H, J = 8.08 Hz), 7.49 (td, 1H, J1 = 7.52 Hz, J2 = 0.88 Hz), 7.40 (td, 1H, J1 = 7.50 Hz, J2 = 1.04 Hz), 7.25 (d, 1H, J = 7.64 Hz), 7.18 (m, 1H), 7.04 (m, 1H), 6.96 (d, 1H, J = 8.00 Hz) 6.52 (d, 2H, J = 2.52 Hz), 6.32 (d, 2H, J = 8.84 Hz), 6.19 (d, 1H, J = 2.56 Hz), 6.16 (d, 1H, J = 2.60 Hz), 3.33 (q, 8H, J = 7.06 Hz), 1.16 (t, 12H, J = 7.04 Hz); 13C-NMR (100 MHz, CDCl3): 156.63, 156.28, 153.07, 148.95, 130.90, 130.44, 128.61, 128.27, 127.92, 124.84, 122.54, 121.92, 121.37, 120.09, 110.21, 108.14, 106.28, 97.89, 63.86, 44.32, 12.60 ppm; MS (C34H34N4O): calculated (MH+): 515.27, found (MH+): 515.8.
pH titration
An aliquot of the stock solution of RB-IM, RB-PDA or RB-EDA in DMF was respectively added to Na2HPO4–NaH2PO4 buffer (200 mM) of various pH values (8.0, 7.5, 7.0, 6.5, 6.0, 5.5, 5.0, or 4.5) to a final concentration of 10 μM. The fluorescence emission@595 nm was recorded as a function of pH using λex@565 nm.An aliquot of the stock solution of RB-IM was spiked into Na2HPO4–NaH2PO4 buffer (200 mM, pH 5.0, 5.5, 6.0) to a final concentration of 10 μM. The fluorescence emission of the solutions was monitored over time by fluorescence emission intensity at 590 nm using Ex@560 nm.
Selectivity of RB-IM for pH over selected chemical species
A serial of solutions of RB-IM (10 μM) in water were prepared to contain one of the following anions (1) NaCl (1 mM), KCl (1 mM), CuSO4 (1 mM), MnCl2 (1 mM), MgCl2 (1 mM), ZnCl2 (1 mM), Fe(ClO4)3 (1 mM), Fe(ClO4)2 (1 mM), Hg(NO3)2 (1 mM), AgNO3 (1 mM), glycine (1 mM), alanine (1 mM), lysine (1 mM), tyrosine (1 mM), cysteine (1 mM), arginine (1 mM) and phenylalanine (1 mM). The samples were mixed and incubated at rt for 30 min. The fluorescence emission@595 nm was recorded using λex@565 nm.Imaging of lysosomal pH with RB-IM
L929 cells were respectively cultured in DMEM spiked with or without RB-IM (1 μg ml−1, 2 μM) and LysoTracker Green (1 μM) for 30 min. The cells were rinsed with DMEM and then analyzed by confocal fluorescence microscopy.Staining of intestine of zebrafish
Adult AB strain zebrafish were maintained at 28 °C with a 14 h/10 h light/dark cycle in a filtering system and fed twice daily with brine shrimps. Embryos were collected by natural spawning and raised in the E3 medium (1 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, and 0.33 mM MgSO4) at 28.5 °C. The staging of zebrafish embryo/larvae was expressed as days post fertilization (dpf). In the staining experiment, the larvae at 5 dpf were exposed to different concentrations of RB-IM diluted in E3 medium for 1 h. The embryos were then transferred to fresh medium for reversal staining. The embryos were rinsed three times in fresh medium before photograph capture using a Leica DM1L fluorescence microscope with a Leica camera DFC420.All experiments were conducted in accordance with guidelines of the Fujian Provincial Department of Science and Technology for the Administration of Affairs Concerning Experimental Animals.
Results and discussion
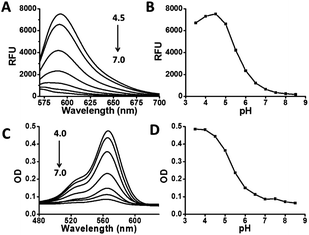
As the pKa of benzimidazole is at about 6, we anticipated that monovalent benzimidazole would be effectively protonated by lysosomal pH and yet exhibits low affinity towards metal cations. Hence RB-IM were prepared via treatment of N-(rhodamine B)-lactam-phenylethylenediamine (RB-PDA) with lithium aluminium hydride in anhydrous tetrahydrofuran for biological pH sensing by protonation of the benzimidazole moiety. To determine the pH sensitivity of RB-IM, the UV-vis absorption spectra and fluorescence emission spectra of RB-IM in buffers were recorded as a function of pH. As shown in Fig. 1, RB-IM, colorless and weakly fluorescent at or above pH 7, readily isomerizes into a red colored and fluorescent species in acidic media (pH 4–7) in a pH dependent manner. The fluorescence intensity of RB-IM at pH 4.5 was 10-fold brighter than that at pH 7.0 (Fig. 1). The fluorescence quantum yield of RB-IM at Na2HPO4–NaH2PO4 buffer (200 mM, pH 4.5) was determined using rhodamine B as the control. The quantum yield of RB-IM was 9.3% whereas that of rhodamine B was 19.9% under identical assay conditions, suggesting that RB-IM was of advantageous fluorescence property for cell imaging studies. Kinetic analysis showed that pH mediated turn-on fluorescence of RB-IM was instant in buffers of various pH (Fig. S1, ESI†). The optimal sensing range of RB-IM (pH 4.5–6.5) overlaps the pH window of lysosomes suggests its utility for imaging of lysosomal pH in live cells.To validate the proposed roles of the benzimidazole moiety of RB-IM in effective recognition of proton, RB-PDA and N-(rhodamine B)-lactam-ethylenediamine (RB-EDA), both containing intramolecular lactam, and were evaluated for their efficacy to sense acidic pH under identical conditions (Fig. 2). The fluorescence emission of RB-IM is 2-fold brighter than RB-EDA in the range of pH 4–8 (Fig. 2). In contrast, RB-PDA is almost nonfluorescent under identical assay conditions. Collectively, these results support the critical role of benzimidazole moiety in effective sensing of acidic pH over rhodamine-lactams.
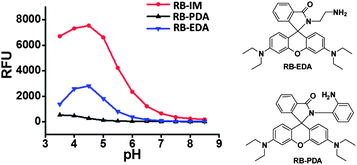 | ||
| Fig. 2 pH profiles of RB-IM, RB-PDA and RB-EDA. The emission@565 nm of the substrates (10 μM) in NaH2PO4–Na2HPO4 buffer (200 mM, pH 3.5–8.5) was recorded over pH using λem@595 nm. | ||
Lysosomes are the major acidic intracellular compartments and the acidic intralysosomal pH is critical for a number of biological pathways ranging from endocytosis, autophagy to cancer metathesis. In addition, lysosomes are the key regulators of intracellular Fe3+ homeostasis.6 The release of Fe3+ from endocytosized transferrin is triggered by the acidic lysosomal pH and the released Fe3+ is then in situ reduced into Fe2+,6 suggesting that lysosomes might contain high levels of free Fe3+. Rhodamine-lactams often display dramatic fluorogenic responses to various metal ions. For instance, N-(rhodamine 6G)-lactam-ethylenediamine functionalized nanoparticles have been employed to detect intracellular Fe3+.7 Apart from being responsive to Fe3+, rhodamine 6G-lactam also exhibits turn-on fluorescence under acidic pH.3b As the cytosol is slightly alkaline (pH 7.2), free Fe3+ is unlikely to be present at biologically significant levels at extra-lysosomal settings owing to the liability of Fe3+ to hydrolysis at neutral or alkaline conditions. It is not clear at this stage whether exogenously administered Fe3+ could lead to alterations of intracellular pH. Accordingly, there are controversial reports on the identity of the species recognized by rhodamine-lactams within cells.8 As such, sensors that could overcome the promiscuity of rhodamine-lactams towards H+ and Fe3+ are useful for studies of lysosomal pH mediated cell signaling pathways.
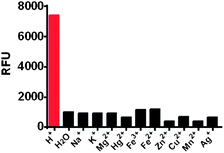
The selectivity of RB-IM towards a broad range of biologically relevant species was determined. It was shown that RB-IM did not respond to all the cations and anions tested at concentration up to 1 mM in, which included Fe2+, Fe3+, Na+, K+, Mg2+, Hg2+, Cu2+, Mn2+, Ag+ (Fig. 3), and representative amino acids (Fig. S2, ESI†). The stringent sensitivity of RB-IM to pH over Fe2+, Fe3+, and many other biologically relevant interfering species indicates that RB-IM is useful for selective imaging of pH in the presence of other biologically relevant cations.
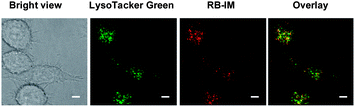
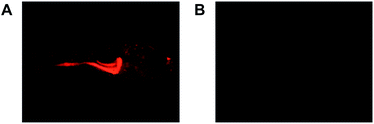
With the demonstrated pH responsiveness and the stringent selectivity, RB-IM was then evaluated for its efficacy to stain lysosomes. L929 cells were co-cultured with RB-IM and LysoTracker Green DND-26 in Dulbecco's Modified Eagle's Medium (DMEM) and then analyzed by confocal fluorescence microscopy. The images show that the intracellular rhodamine fluorescence is superimposable to that of LysoTracker Green (Fig. 4) which is a lysosome specific dye. Analysis showed that colocalization coefficient of RB-IM and Lysotracker Green was 0.69, proving the utility of RB-IM for acidic pH triggered fluorogenic staining of lysosomes.
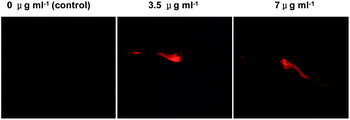
Acidic pH is critical for the functions of digestive tracts of vertebrate animals, e.g. activation of digestive enzymes by gastric milieu. Zebrafish is widely used for studying vertebrate development9 and cancers.10 We proceeded to evaluate the applicability of RB-IM to stain the digestive tract of zebrafish. Larval zebrafish at 5 days post fertilization (dpf) were maintained in water spiked with or without RM-IM for 1 h and then imaged by fluorescence microscopy. Fig. 5 revealed robust fluorescence signals in the intestine of zebrafish treated with RB-IM whereas no signal could be identified in control zebrafish, demonstrating effective staining of the digestive tracts of zebrafish with RB-IM. To probe if the staining is reversal, zebrafish pre-cultured with RB-IM were then maintained in fresh E3 medium, and the fluorescence localized in intestine was monitored over time. As shown in Fig. 6, the fluorescence emission faded to negligible levels after 24 h incubation, suggesting that the in vivo accumulated RM-IM could be dissipated under normal culturing conditions. Taken together, the results show that RB-IM is applicable for staining of the intestines of zebrafish. The demonstrated reversible and pH dependent staining of the digestive tract of zebrafish suggests RB-IM might be useful to study the digestive tracts of zebrafish in pathogen infection and food metabolism.11
Conclusions
RB-IM, the diad of rhodamine B and benzimidazole, is featured by an intramolecular spiro-ring connected by the imidazole nitrogen and the cationic rhodamine moiety. RB-IM is poised to proton mediated fluorogenic opening of the spiro-ring and yet remains unreactive to Fe3+ and other biologically relevant ions, enabling selective imaging of acidic lysosomes in L929 cells and staining of the digestive tract of zebrafish. Given the prevalence of Fe3+ in lysosomes in mammalian cells, RB-IM would be of utility for discerning the roles of lysosomal acidity in related cell signalling events or imaging the digestive tract of zebrafish.Acknowledgements
Dr S. Han was supported by grants from NSF China (21272196, 21072162), 973 program 2013CB93390, PCSIRT, and the Fundamental Research Funds for the Central Universities (2011121020); Dr J. Chen was supported by grants DY125-15-T-03 from COMRA and 3502Z20111051 of Xiamen Science and Technology Plan; Dr J. Han was supported by grants from NSF China (30830092, 30921005, 91029304, 81061160512).Notes and references
- (a) F. Lang, G. Busch, H. Volkl and D. Haussinger, Biochem. Soc. Trans., 1994, 22, 502 CAS; (b) J. P. Luzio, P. R. Pryor and N. A. Bright, Nat. Rev. Mol. Cell Biol., 2007, 8, 622 CrossRef CAS PubMed.
- (a) A. Di, M. E. Brown, L. V. Deriy, C. Li, F. L. Szeto, Y. Chen, P. Huang, J. Tong, A. P. Naren, V. Bindokas, H. C. Palfrey and D. J. Nelson, Nat. Cell Biol., 2006, 8, 933 CrossRef CAS PubMed; (b) E. S. Trombetta, M. Ebersold, W. Garrett, M. Pypaert and I. Mellman, Science, 2003, 299, 1400 CrossRef CAS PubMed; (c) C. Nilsson, K. Kagedal, U. Johansson and K. Ollinger, Methods Cell Sci., 2003, 25, 185 CrossRef.
- (a) H. S. Lv, S. Y. Huang, B. X. Zhao and J. Y. Miao, Anal. Chim. Acta, 2013, 788, 177 CrossRef CAS PubMed; (b) Z. Li, S. Wu, J. Han and S. Han, Analyst, 2011, 136, 3698 RSC; (c) G. Men, G. Zhang, C. Liang, H. Liu, B. Yang, Y. Pan, Z. Wang and S. Jiang, Analyst, 2013, 138, 2847 RSC; (d) W. Zhang, B. Tang, X. Liu, Y. Liu, K. Xu, J. Ma, L. Tong and G. Yang, Analyst, 2009, 134, 367 RSC; (e) T. Hasegawa, Y. Kondo, Y. Koizumi, T. Sugiyama, A. Takeda, S. Ito and F. Hamada, Bioorg. Med. Chem., 2009, 17, 6015 CrossRef CAS PubMed; (f) S. Wu, Z. Li, J. Han and S. Han, Chem. Commun., 2011, 47, 11276 RSC; (g) Z. Li, Y. Song, Y. Yang, L. Yang, X. Huang, J. Han and S. Han, Chem. Sci., 2012, 3, 2491 Search PubMed; (h) Z. Hu, M. Li, M. Liu, W. Zhuang and G. Li, Dyes Pigm., 2013, 96, 71 CrossRef CAS PubMed; (i) M. Tian, X. Peng, J. Fan, J. Wang and S. Sun, Dyes Pigm., 2012, 95, 112 CrossRef CAS PubMed; (j) H. Li, H. Guan, X. Duan, J. Hu, G. Wang and Q. Wang, Org. Biomol. Chem., 2013, 11, 1805 RSC; (k) J. Wang, Q. Yang, H. Song and W. Zhang, Org. Biomol. Chem., 2012, 10, 7677 RSC; (l) H. S. Lv, J. Liu, J. Zhao, B. Zhao and J. Miao, Sens. Actuators, B, 2013, 956 CrossRef CAS PubMed; (m) D. Aigner, S. M. Borisov, F. J. Fernandez, J. F. Fernandez Sanchez, R. Saf and I. Klimant, Talanta, 2012, 99, 194 CrossRef CAS PubMed; (n) Q. A. Best, R. Xu, M. E. McCarroll, L. Wang and D. J. Dyer, Org. Lett., 2010, 12, 3219 CrossRef CAS PubMed; (o) H. Zhu, J. Fan, Q. Xu, H. Li, J. Wang, P. Gao and X. Peng, Chem. Commun., 2012, 48, 11766 RSC.
- H. N. Kim, M. H. Lee, H. J. Kim, J. S. Kim and J. Yoon, Chem. Soc. Rev., 2008, 37, 1465 RSC.
- (a) Z. Li, Z. Xue, Z. Wu, J. Han and S. Han, Org. Biomol. Chem., 2011, 9, 7652 RSC; (b) S. Wu, Y. Song, Z. Li, Z. Wu, J. Han and S. Han, Anal. Methods, 2012, 4, 1699 RSC.
- G. Papanikolaou and K. Pantopoulos, Toxicol. Appl. Pharmacol., 2005, 202, 199 CrossRef CAS PubMed.
- B. Wang, J. Hai, Z. Liu, Q. Wang, Z. Yang and S. Sun, Angew. Chem., Int. Ed. Engl., 2010, 49, 4576 CrossRef CAS PubMed.
- J. D. Chartres, M. Busby, M. J. Riley, J. J. Davis and P. V. Bernhardt, Inorg. Chem., 2011, 50, 9178 CrossRef CAS PubMed.
- W. Driever, D. Stemple, A. Schier and L. Solnica-Krezel, Trends Genet., 1994, 10, 152 CrossRef CAS.
- (a) S. Liu and S. D. Leach, Annu. Rev. Pathol.: Mech. Dis., 2011, 6, 71 CrossRef CAS PubMed; (b) H. M. Stern and L. I. Zon, Nat. Rev. Cancer, 2003, 3, 533 CrossRef CAS PubMed; (c) J. F. Amatruda, J. L. Shepard, H. M. Stern and L. I. Zon, Cancer Cell, 2002, 1, 229 CrossRef CAS.
- (a) J. W. Walters, J. L. Anderson, R. Bittman, M. Pack and S. A. Farber, Chem. Biol., 2012, 19, 913 CrossRef CAS PubMed; (b) M. B. Levanti, M. C. Guerrera, M. G. Calavia, E. Ciriaco, G. Montalbano, J. Cobo, A. Germana and J. A. Vega, Neurosci. Lett., 2011, 494, 24 CrossRef CAS PubMed; (c) I. Semova, J. D. Carten, J. Stombaugh, L. C. Mackey, R. Knight, S. A. Farber and J. F. Rawls, Cell Host Microbe, 2012, 12, 277 CrossRef CAS PubMed.
Footnote |
| † Electronic Supplementary Information (ESI) available: On selectivity of RB-IM to selected amino acids and kinetic profiles of fluorogenic opening of the intramolecular ring of RB-IM in buffers of different pH values. See DOI: 10.1039/c3ra45329d |
| This journal is © The Royal Society of Chemistry 2014 |