Zebrafish based strategy for the identification of a potential pharmacophore for apoptosis: a greener CuAAC approach for novel 1,2,3-triazoles derived from mefenamic acid†
P. Vijaya Babuab,
Soumita Mukherjeea,
Dhilli Rao Gorjaa,
Swapna Yellankiac,
Raghavender Medisettiac,
Pushkar Kulkarniac,
K. Mukkantib and
Manojit Pal*a
aDr. Reddy's Institute of Life Sciences, University of Hyderabad Campus, Gachibowli, Hyderabad 500046, India. E-mail: manojitpal@rediffmail.com; Tel: +91 40 6657 1500
bChemistry Division, Institute of Science and Technology, JNT University, Kukatpally, Hyderabad 500072, India
cZephase Therapeutics (an incubated company at the DRILS), University of Hyderabad Campus, Gachibowli, Hyderabad 500046, India
First published on 4th November 2013
Abstract
Prompted by the potential of a screening strategy in zebrafish for the identification of valuable pharmacophores, a series of triazole substituted mefenamic acid derivatives were designed and synthesized via a CuAAC under green conditions. A variety of terminal alkynes were reacted with the azide obtained from mefenamic acid to give the expected products in good to excellent yields. When screened for apoptosis, teratogenicity and hepatotoxicity in zebrafish embryos, one of these compounds showed encouraging apoptotic properties and safety profiles and seemed to have medicinal value.
The synthesis of a library of novel small molecules and their screening in zebrafish for early indications of their possible utility in therapeutic regimes and potential toxic side-effects is of immense importance. Apart from identifying useful chemical probes, this strategy can also help in identifying valuable pharmacophores for the design and discovery of potential drugs to combat deadly diseases.
Zebrafish (or Danio rerio), a small pet-shop fish, has attracted considerable interest as an emerging model for understanding human biology and is being pursued as a tool for enhancing interdisciplinary studies in biology and chemistry as well as in drug discovery.1 For example, zebrafish provide an inexpensive, reliable and efficient first-level screening model for testing toxicity, efficacy, and tissue-targeting for a large number of new chemical entities (NCEs). The use of zebrafish embryos is also being considered as a one-step strategy to perform developmental screens to identify compounds with bioactivities relevant to vertebrates.2,3 Indeed, pharmacologically active African plant extracts and subsequently their active components, possessing anti-angiogenic properties, were identified using this strategy.4 The structure activity relationship (SAR) studies centered on pharmacologically active scaffolds have also been reported using zebrafish embryos.5 Our continued interest6–11 in zebrafish as a screening model for NCEs prompted us to design and synthesise a library of triazole based small organic molecules derived from mefenamic acid to assess their potential pharmacological effects in zebrafish and/or their embryos.
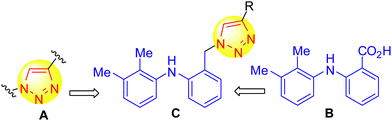
Triazoles, one of the extensively studied 5-membered nitrogen heterocycles, have found a wide range of applications especially as drugs and agrochemicals. Besides anti-HIV,12 antiallergic,13 antifungal,14 and antimicrobial15 activities, the 1,2,3-triazole derivatives (A, Fig. 1) have shown p38 MAP kinase and PfPK7 protein kinase inhibitory properties.16 These moieties can also be tuned to form valuable pharmacophores17 and play an important role in bio-conjugation. Mefenamic acid (B, Fig. 1) on the other hand is a well known non-steroidal anti-inflammatory drug (NSAID) used to treat pain and is a non-selective inhibitor of COX-1 and 2.18 The presence of the –CO2H moiety is known to be responsible for its gastrointestinal side effects in addition to COX-1 inhibition. We therefore designed the basic template C by replacing the –CO2H group of B with a –CH2-linked triazole moiety and introducing an “R” group to create diversity around this framework. In view of the reported apoptotic activities of both 1,2,3-triazoles19 and mefenamic acid,20 we anticipated that a library of molecules generated based on C would show similar pharmacological properties.
1,2,3-Triazoles are generally prepared using a click chemistry approach due to its specificity, efficiency, simple reaction workup procedure, and quantitative yield of products.21,22 We adopted a similar but greener approach to prepare our target compounds C (or 5, Scheme 1).
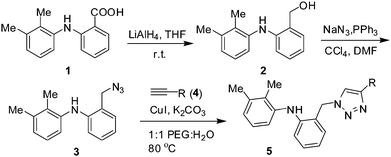
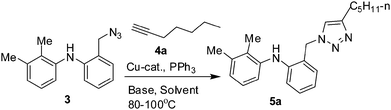
The key starting material i.e. the azide 3 was prepared via the reduction of the carboxylic acid moiety of mefenamic acid (1) to the corresponding alcohol 2, which was then converted to the azide 3 (Scheme 1). The Cu-catalysed azide–alkyne cycloaddition (CuAAC) of the azide 3 and alkyne 4a was performed initially with a catalytic amount of CuI in the presence of diisopropyl ethyl amine (DIPEA) in DMF. After 2 h, the desired triazole 5a was obtained in 95% yield (entry 1, Table 1). The use of an inorganic base e.g. K2CO3 (entry 2, Table 1) decreased the product yield. The reaction did not proceed in the absence of CuI (entry 3, Table 1) or DIPEA (entry 4, Table 1), indicating the key role played by the catalyst and the base. The reaction however proceeded well in the presence of other copper catalysts e.g. CuCl (entry 5, Table 1) or CuBr (entry 6, Table 1). While all these reactions were performed in DMF, the use of other solvents such as 1,4-dioxane, acetonitrile, DMSO and PEG400 were also successful (entries 7–10, Table 1). To make this method more economic, we replaced PEG with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PEG400–H2O (entry 11, Table 1) and to make it more environmentally benign we replaced DIPEA with K2CO3 (entry 12, Table 1). To our delight 5a was obtained in 96 and 95% yield in both the cases. In order to avoid the use of hazardous organic amine bases we opted for the reaction conditions of entry 12 of Table 1 to perform further reactions.
1 PEG400–H2O (entry 11, Table 1) and to make it more environmentally benign we replaced DIPEA with K2CO3 (entry 12, Table 1). To our delight 5a was obtained in 96 and 95% yield in both the cases. In order to avoid the use of hazardous organic amine bases we opted for the reaction conditions of entry 12 of Table 1 to perform further reactions.
| Entry | Solvent | Catalyst | Base | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction was carried out using 3 (1 mmol) and 4a (1 mmol) in presence of catalyst, base and a solvent at 80–100 °C.b Isolated yield.c PPh3 was not added. | |||||
| 1 | DMF | CuI | DIPEA | 2 | 95 |
| 2 | DMF | CuI | K2CO3 | 2.5 | 82 |
| 3 | DMF | No catalyst | DIPEA | 3 | No product |
| 4 | DMF | CuI | No base | 4 | No reaction |
| 5 | DMF | CuCl | DIPEA | 2 | 89 |
| 6 | DMF | CuBr | DIPEA | 2 | 85 |
| 7 | 1,4-Dioxane | CuI | DIPEA | 2.5 | 90 |
| 8 | Acetonitrile | CuI | DIPEA | 2.5 | 92 |
| 9 | DMSO | CuI | DIPEA | 2.5 | 93 |
| 10 | PEG 400 | CuI | DIPEA | 1.5 | 98c |
| 11 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PEG–H2O 1 PEG–H2O |
CuI | DIPEA | 1.5 | 96c |
| 12 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PEG–H2O 1 PEG–H2O |
CuI | K2CO3 | 1.5 | 95c |
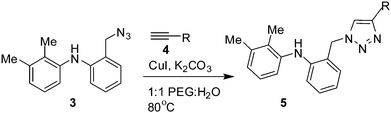
We then prepared a range of 1,2,3-triazole derivatives (5) using this greener methodology (Table 2). A variety of terminal alkynes containing alkyl, hydroxyl alkyl, aryl, cyclic alkyl and heterocyclic moieties were reacted with the azide 3 to give the corresponding products 5 in good to excellent yields.
| Entry | Alkyne; R = (4) | Productb (5) | Yieldb (%) |
|---|---|---|---|
a Reaction was carried out using 3 (1 mmol), 4 (1 mmol), CuI (0.05 mmol) and K2CO3 (3 mmol) in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PEG–H2O (5 mL) at 80 °C.b Isolated yield. 1 PEG–H2O (5 mL) at 80 °C.b Isolated yield. |
|||
| 1 | –(CH2)4CH3 (4a) | 5a | 88 |
| 2 | –(CH2)2CH3 (4b) | 5b | 98 |
| 3 | –(CH2)3CH3 (4c) | 5c | 90 |
| 4 | –(CH2)5CH3 (4d) | 5d | 85 |
| 5 | –(CH2)7CH3 (4e) | 5e | 98 |
| 6 | –(CH2)9CH3 (4f) | 5f | 80 |
| 7 | –(CH2)3CN (4g) | 5g | 95 |
| 8 | –(CH2)3Cl (4h) | 5h | 97 |
| 9 | –CH2OH (4i) | 5i | 95 |
| 10 | –(CH2)2OH (4j) | 5j | 80 |
| 11 | –(CH2)3OH (4k) | 5k | 98 |
| 12 | –C(CH3)3 (4l) | 5l | 95 |
| 13 | –C(CH3)2OH (4m) | 5m | 85 |
| 14 | –CH(CH3)OH (4n) | 5n | 80 |
| 15 | –Ph (4o) | 5o | 98 |
| 16 | –C6H4CH3-p (4p) | 5p | 97 |
| 17 | 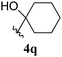 |
5q | 91 |
| 18 | –CH(OH)Ph (4r) | 5r | 90 |
| 19 | 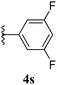 |
5s | 96 |
| 20 | 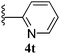 |
5t | 95 |
All the mefenamic acid based 1,2,3-triazole derivatives 5 prepared were characterized using NMR, IR and MS spectroscopy. In most cases the appearance of a singlet at ∼7.40 δ (this singlet was merged with another aromatic proton in some cases) in the 1H NMR and a signal at 122.6 ppm in the 13C NMR confirmed the presence of a triazole ring.
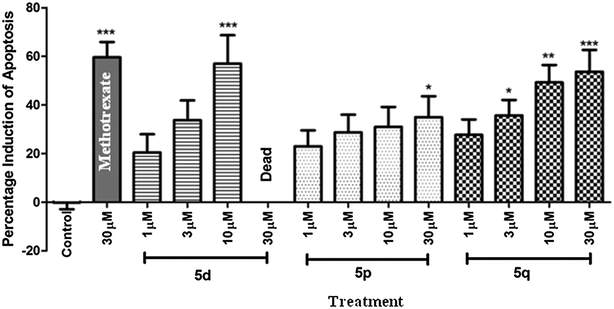
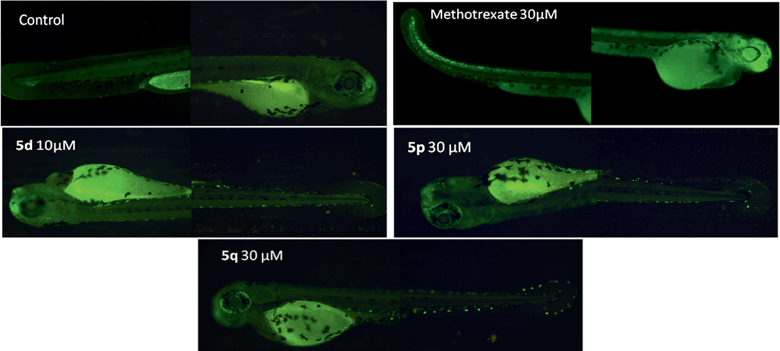
These compounds (only solids) were tested for their apoptotic activities initially at 30 μM using zebrafish embryos. Apoptosis23 is an ordered and orchestrated cellular process that occurs under physiological and pathological conditions. Its complex mechanism involves many pathways. Defects in apoptotic pathways are thought to contribute to a number of human diseases, ranging from neurodegenerative disorders to malignancy.23 For example, cancer is associated with a lower degree of apoptosis and most cytotoxic anticancer agents are known to induce apoptosis. The most active compounds i.e. 5d, 5p and 5q were tested at 1, 3, 10 and 30 μM along with a known drug methotrexate24 at 30 μM. The percentage induction of apoptosis caused by these three compounds at different concentrations is shown in Fig. 2 and the representative images of embryos are also shown in Fig. 3. It is evident from Fig. 2 that all these compounds showed considerable effects in the present apoptosis assay. While compound 5d showed milder apoptotic activities at 1 and 3 μM, a substantial increase in activity was observed when tested at 10 μM. Notably, the embryos were found dead at a higher concentration i.e. 30 μM. The compound 5p showed a consistent increase in activity with a dose that reached a maximum value at 30 μM. Like 5d, the compound 5q also showed minimal apoptotic activities at 1 and 3 μM that increased at 30 μM. The EC50 values of 5d, 5p and 5q were found to be 8.23, 2.28 and 3.64 μM, respectively.
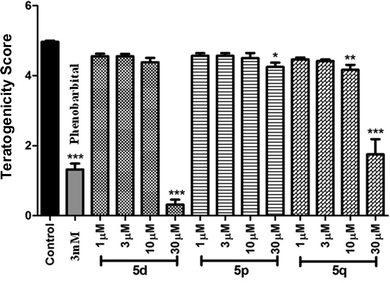
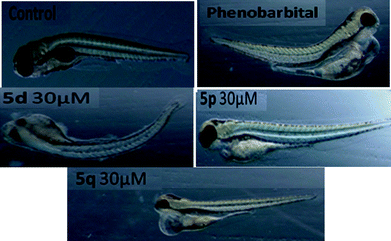
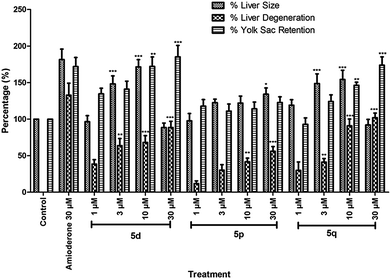
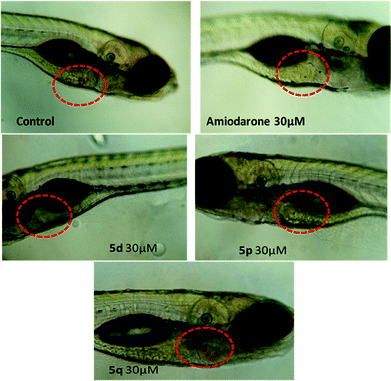
In view of their promising apoptotic activities the safety profile of 5d, 5p and 5q were evaluated in zebrafish embryos in the range of 1.0–30 μM. The transparency of the developing zebrafish embryos and their similar toxicity profiles to humans made it a promising model for various toxicity assessments like teratogenicity, hepatotoxicity etc. In the teratogenicity assay (Fig. 4 and 5, see also Table S-1 in ESI†), compound 5p was found to be safe up to 30 μM with No Observed Adverse Effect Level (NOAEL) ≥ 10 μM, while compounds 5d and 5q were non-toxic at 10 μM with NOAEL ≥ 10 and 3 μM, respectively. However, in the hepatotoxicity assay (Fig. 6 and 7), the NOAEL for 5d, 5p and 5q was found to be ≤3 μM, ≤10 μM and ≤1 μM, respectively. The overall results of the zebrafish based pharmacological evaluation of compounds 5d, 5p and 5q are summarized in Table 3 (see also Fig. S-1 in the ESI†). It is evident from Table 3 that compound 5p showed encouraging apoptotic properties and safety profiles with a superior therapeutic index (ratio of NOAEL/EC50) of 4.38, compared to other compounds e.g. 5d (therapeutic index = 0.12) and 5q (therapeutic index = 0.27).
| Pharmacological evaluations | Test compounds data | |||||
|---|---|---|---|---|---|---|
| Tests | Endpoint | Positive control | Parameters | 5d | 5p | 5q |
| Apoptosis | Acridine orange staining of apoptotic cells | Methotrexate | EC50 (μM) | 8.231 | 2.278 | 3.642 |
| Hepatotoxicity | % Liver size, % liver degeneration, % yolk sac retention | Amiodarone | NOAEL (μM) | 1 | 10 | 1 |
| Teratogenicity | Morphological assessment of phenotypic changes | Phenobarbital | NOAEL (μM) | 10 | 10 | 3 |
| Overall therapeutic index | Ratio of NOAEL/EC50 (overall NOAEL = lowest NOAEL) | — | Therapeutic index | 0.121 | 4.389 | 0.274 |
In conclusion, we have demonstrated the potential of a screening strategy in zebrafish for the identification of a new pharmacophore for apoptosis. A series of novel triazole substituted mefenamic acid derivatives were designed and subsequently synthesized via a greener Cu-catalyzed azide–alkyne cycloaddition (CuAAC). A variety of terminal alkynes containing alkyl, hydroxyl alkyl, aryl, cyclic alkyl and heterocyclic moieties were reacted with the azide obtained from mefenamic acid to give the expected products in good to excellent yields. When screened for apoptosis, teratogenicity and hepatotoxicity in zebrafish embryos, compound 5p showed encouraging apoptotic properties and safety profiles. In view of the fact that most cytotoxic anticancer agents are known to induce apoptosis, compound 5p seemed to have medicinal value. The present class of compounds therefore represents a new pharmacophore for the design and discovery of potential new drugs.
PVB thanks CSIR, India for a Research Fellowship. SM thanks CSIR, India for a Research Associateship.
Notes and references
- For an informative review, see: S. Basu and C. Sachidanandan, Chem. Rev., 2013, 113, 7952 CrossRef CAS PubMed.
- N. Mandrekar and N. L. Thakur, Biotechnol. Lett., 2009, 31, 171 CrossRef CAS PubMed.
- I. Torregroza, T. Evans and B. C. Das, Chem. Biol. Drug Des., 2009, 73, 339 CAS.
- A. D. Crawford, S. Liekens, A. R. Kamuhabwa, J. Maes, S. Munck, R. Busson, J. Rozenski, C. V. Esguerra and P. A. de Witte, PLoS One, 2011, 6, e14694 CAS.
- (a) J. Hao, J. N. Ho, J. A. Lewis, K. A. Karim, R. N. Daniels, P. R. Gentry, C. R. Hopkins, C. W. Lindsley and C. C. Hong, ACS Chem. Biol., 2010, 5, 245 CrossRef CAS PubMed; (b) T. V. Bowman and L. I. Zon, ACS Chem. Biol., 2010, 5, 159 CrossRef CAS PubMed.
- P. V. Babu, S. Mukherjee, G. S. Deora, K. S. Chennubhotla, R. Medisetti, S. Yellanki, P. Kulkarni, S. Sripelly, K. V. L. Parsa, K. Chatti, K. Mukkanti and M. Pal, Org. Biomol. Chem., 2013, 11, 6680 CAS.
- A. Nakhi, S. Archana, G. P. K. Seerapu, K. S. Chennubhotla, K. L. Kumar, P. Kulkarni, D. Haldar and M. Pal, Chem. Commun., 2013, 49, 6268 RSC.
- A. Nakhi, Md. S. Rahman, G. P. K. Seerapu, R. K. Banote, K. L. Kumar, P. Kulkarni, D. Haldar and M. Pal, Org. Biomol. Chem., 2013, 11, 4930 CAS.
- B. Dulla, K. T. Kirla, V. Rathore, G. S. Deora, S. Kavela, S. Maddika, K. Chatti, O. Reiser, J. Iqbal and M. Pal, Org. Biomol. Chem., 2013, 11, 3103 CAS.
- D. R. Gorja, S. Mukherjee, C. L. T. Meda, G. S. Deora, K. L. Kumar, A. Jain, G. H. Chaudhari, K. S. Chennubhotla, R. K. Banote, P. Kulkarni, K. V. L. Parsa, K. Mukkanti and M. Pal, Org. Biomol. Chem., 2013, 11, 2075 CAS.
- P. M. Kumar, K. S. Kumar, C. L. T. Meda, G. R. Reddy, P. K. Mohakhud, K. Mukkanti, G. R. Krishna, C. M. Reddy, D. Rambabu, K. S. Kumar, K. K. Priya, K. S. Chennubhotla, R. K. Banote, P. Kulkarni, K. V. L. Parsa and M. Pal, Med. Chem. Commun., 2012, 3, 667 RSC.
- R. Alvarez, S. Velázquez, A. San-Félix, S. Aquaro, E. De Clerq, C. F. Perno, A. Karlsson, J. Balzarini and M. J. Camarasa, J. Med. Chem., 1994, 37, 4185 CrossRef CAS.
- D. R. Buckle, C. J. Rockell, H. Smith and B. A. Spicer, J. Med. Chem., 1986, 29, 2262 CrossRef CAS.
- (a) C. B. Vicentini, V. Brandolini, M. Guarneri and P. Giori, Farmaco, 1992, 47, 1021 CAS; (b) J. C. Fung-Tomc, H. Elizabeth, M. Beatrice and D. P. Bonner, Antimicrob. Agents Chemother., 1998, 42, 313 CAS.
- M. J. Genin, D. A. Allwine, D. J. Anderson, M. R. Barbachyn, D. E. Emmert, S. A. Garmon, D. R. Graber, K. C. Grega, J. B. Hester, D. K. Hutchinson, J. Morris, R. J. Reischer, C. W. Ford, G. E. Zurenko, J. C. Hamel, R. D. Schaadt, D. Stapert and B. H. Yagi, J. Med. Chem., 2000, 43, 953 CrossRef CAS PubMed.
- (a) P. Diner, T. Andersson, J. Kjellén, K. Elbing, S. Hohmann and M. Grøtli, New J. Chem., 2009, 33, 1010 RSC; (b) M. Klein, P. Dinér, D. Dorin-Semblat, C. Doerig and M. Grøtli, Org. Biomol. Chem., 2009, 7, 3421 RSC.
- Y. Bourne, H. C. Kolb, Z. Radić, K. B. Sharpless, P. Taylor and P. Marchot, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 1449 CrossRef CAS PubMed.
- F. P. Trinus, N. A. Mokhort, L. M. Yagupol'skii, A. G. Fadeicheva, V. S. Danilenko, T. K. Ryabukha, Yu. A. Fialkov, L. M. Kirichek, É. S. Endel'man and G. A. Get'man, Pharm. Chem. J., 1977, 11, 1706 CrossRef.
- R. Majeed, P. L. Sangwan, P. K. Chinthakindi, I. Khan, N. A. Dangroo, N. Thota, A. Hamid, P. R. Sharma, A. K. Saxena and S. Koul, Eur. J. Med. Chem., 2013, 63, 782 CrossRef CAS PubMed.
- D. H. Woo, I.-S. Han and G. Jung, Life Sci., 2004, 75, 2439 CrossRef CAS PubMed.
- H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004 CrossRef CAS.
- H. C. Kolb and K. B. Sharpless, Drug Discovery Today, 2003, 8, 1128 CrossRef CAS.
- C. B. Thompson, Science, 1995, 267, 1456 CrossRef CAS.
- Y.-X. Chen, W.-G. Lv, H.-Z. Chen, F. Ye and X. Xie, Eur. J. Obstet. Gynecol. Reprod. Biol., 2009, 142, 107 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, spectral data for all new compounds, results of in vitro/in vivo study. See DOI: 10.1039/c3ra46185h |
| This journal is © The Royal Society of Chemistry 2014 |