A novel core-concentration gradient-shelled LiNi0.5Co0.2Mn0.3O2 as high-performance cathode for lithium-ion batteries
Peiyu Houa,
Xiaoqing Wangb,
Dongge Wanga,
Dawei Song*a,
Xixi Shia,
Lianqi Zhang*a,
Jian Guoc and
Jun Zhangc
aSchool of Materials Science and Engineering, Tianjin University of Technology, Tianjin 300384, P. R. China. E-mail: songdw2005@mail.nankai.edu.cn; tianjinzhanglq@163.com; Fax: +86 22 60214028; Tel: +86 22 60214578
bSchool of Environment and Chemical Engineering, Tianjin Polytechnic University, Tianjin 300387, P. R. China
cPylon Technologies Co., Ltd, Shanghai 201203, P. R. China
First published on 11th February 2014
Abstract
A novel core-concentration gradient-shelled LiNi0.5Co0.2Mn0.3O2 was successfully synthesized for the first time by a simple method from core-shelled precursors [(Ni0.6Co0.2Mn0.2)1/2(Ni0.4Co0.2Mn0.4)1/2](OH)2 that were synthesized via a co-precipitation route. Particle size increase of hydroxide precursors from core to shell, in combination with subsequent investigations of Energy Disperse X-ray Spectrum (EDS) on precursors, supported the formation of a core-shelled structure. To obtain concentration gradient layer between core and shell, a high calcined temperature of 900 °C was selected as high temperature calcination gave rise to diffusion of cations in the core-shelled structure. Thus, the prepared precursor powders were then calcined with stoichiometric ratio lithium carbonate (Li/M = 1.05) at 900 °C in air, which resulted in core-concentration gradient-shelled (CCGS) LiNi0.5Co0.2Mn0.3O2. The compositions of core and shell separately were LiNi0.60Co0.20Mn0.20O2 and LiNi0.44Co0.20Mn0.36O2, between which was a concentration gradient layer. X-ray diffraction (XRD) studies show that the prepared material was indexed to a typical layered structure with a R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group. Compared to LiNi0.5Co0.2Mn0.3O2, the CCGS-LiNi0.5Co0.2Mn0.3O2 presented remarkably improved cycling performance and thermal stability, which can be ascribed to LiNi0.44Co0.20Mn0.36O2 shell providing structural and thermal stability.
m space group. Compared to LiNi0.5Co0.2Mn0.3O2, the CCGS-LiNi0.5Co0.2Mn0.3O2 presented remarkably improved cycling performance and thermal stability, which can be ascribed to LiNi0.44Co0.20Mn0.36O2 shell providing structural and thermal stability.
During the past decades, lithium-ion batteries have been the major power source for a wide range of electronic consumer products since their initial commercialization by Sony in the early 1990s. LiCoO2 has been widely used as the commercial cathode material because of its easy synthesis and high charge–discharge reversibility.1 However, it also has some limitations such as toxicity, high cost and instability at high voltages (above 4.3 V),2,3 which has led to the study of other candidate cathode materials for lithium-ion batteries. Then LiNi1−x−yCoxMnyO2 and LiFePO4 have been used as alternative cathode materials for lithium-ion batteries in an attempt to solve those problems.4–12 Among the potential materials, LiNi0.5Co0.2Mn0.3O2 was considered as one of the most promising materials and commercialized recently in respect to its comparatively lower cost, lesser toxicity, and larger reversible capacity.13 The Ni rich LiNi1−xMxO2 (M = transition metal) materials have much higher specific capacity than other NCM materials in the same voltage range (3.0–4.3 V). However, the electrodes based on the chemical formula LiNi1−xMxO2 are structurally unstable during thermal runaway reactions due to the oxygen release from the host structure. The released oxygen can then react with the organic electrolyte and carbon anode causing fire and severe safety problems. These problems have hindered their wide commercial use in a lithium battery system.14–16
From our previous study of double shelled structure17 and some recent reports by Yang-Kook Sun,18,19 it is found that core–shell or concentration-gradient structure could indeed enhance the cyclability and safety property. The high manganese content Li[(Ni1/2Mn1/2)1−xCox]O2 (x = 0–0.333) was commonly used as a shell or outer layer due to good cycling performance, and thermal stability.6,20–27 The chemical valence of this material is interesting, and it is different from LiNiO2 (with an average Ni oxidation state of +3) and forms the formal charges of Ni (+2), Co (+3) and Mn (+4) in Li[(Ni1/2Mn1/2)1−xCox]O2.28 In this case, the average oxidation state of Mn is tetravalent, so that the electrochemically inactive tetravalent Mn provides significant structural stability and results in a simple topotactic reaction maintaining the hexagonal phase during electrochemical cycling, even at the high voltage cutoff limit of 4.6 V.29–31 However, structural mismatch in interface was found in prepared core–shell powders after sufficient cycling due to a different shrinkage ratio between the core and shell. The core material surrounded by a concentration-gradient outer layer was reported to overcome this problem.19 As for the synthesis of concentration-gradient hydroxide precursors, the co-precipitation condition is difficult to control and meanwhile the improved performances were not as obvious as core–shell material because of lack of a thick stable shell.
In view of it, we hope to synthesize a novel core-concentration gradient-shelled (CCGS) LiNi0.5Co0.2Mn0.3O2 via a simple route, which would have advantages of core–shell and concentration-gradient structure, to improve its performances. Considering high temperature calcination could give partial diffusion of cation between core and shell, we only need firstly synthesize core-shelled hydroxide precursors via a co-precipitation route. Then the prepared precursor powders were calcined with stoichiometric ratio lithium sources at appropriate temperature, which will result in core-concentration gradient-shelled structure. Therefore, in this paper, LiNi0.6Co0.2Mn0.2O2 which could deliver high capacity and LiNi0.4Co0.2Mn0.4O2 which could provide structural and thermal stability were used as the core and shell, respectively. In addition, unlike the previous reports,18,19 lithium carbonate was chosen in our study because it was widely used in commercial production.
To synthesize the spherical core, stoichiometric NiSO4·6H2O, CoSO4·7H2O, and MnSO4·H2O (cationic ratio of Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co
Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn = 3
Mn = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were dissolved with a concentration of 2.5 mol dm−3 as the starting materials. Then the starting aqueous solution were pumped into a continuously stirred tank reactor (CSTR, capacity of 170 L) under a nitrogen atmosphere and reacted with 10 mol L−1 NaOH solution (aq) as the pH control agent and 1.5 mol L−1 NH4OH solution (aq) as the chelating agent in the reactor under 50 °C and constant pH (11) with 500 rpm stirring rate, leading to [Ni0.6Co0.2Mn0.2](OH)2. Ni, Co and Mn-containing aqueous solution (cationic ratio of Ni
1) were dissolved with a concentration of 2.5 mol dm−3 as the starting materials. Then the starting aqueous solution were pumped into a continuously stirred tank reactor (CSTR, capacity of 170 L) under a nitrogen atmosphere and reacted with 10 mol L−1 NaOH solution (aq) as the pH control agent and 1.5 mol L−1 NH4OH solution (aq) as the chelating agent in the reactor under 50 °C and constant pH (11) with 500 rpm stirring rate, leading to [Ni0.6Co0.2Mn0.2](OH)2. Ni, Co and Mn-containing aqueous solution (cationic ratio of Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co
Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn = 2
Mn = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) was continuously pumped into the coprecipitation reactor one after another to encapsulate the [Ni0.6Co0.2Mn0.2](OH)2 core completely, leading to core-shelled [(Ni0.6Co0.2Mn0.2)1/2(Ni0.4Co0.2Mn0.4)1/2](OH)2. [Ni0.5Co0.2Mn0.3](OH)2 was also synthesized via a co-precipitation route. Then, the obtained particles were filtered, washed, and dried for 24 h in air. The mixture of the obtained precursor particles with stoichiometric ratio Li2CO3 powders were preheated to 750 °C and subsequently calcined at 900 °C in a furnace under air to form lithiated layered core-concentration gradient-shelled powders.
2) was continuously pumped into the coprecipitation reactor one after another to encapsulate the [Ni0.6Co0.2Mn0.2](OH)2 core completely, leading to core-shelled [(Ni0.6Co0.2Mn0.2)1/2(Ni0.4Co0.2Mn0.4)1/2](OH)2. [Ni0.5Co0.2Mn0.3](OH)2 was also synthesized via a co-precipitation route. Then, the obtained particles were filtered, washed, and dried for 24 h in air. The mixture of the obtained precursor particles with stoichiometric ratio Li2CO3 powders were preheated to 750 °C and subsequently calcined at 900 °C in a furnace under air to form lithiated layered core-concentration gradient-shelled powders.
Precursors products were periodically collected during the co-precipitation experiment and the particle size distribution was measured with a particle size analyzer (OMEC, LS-POP(6), China). X-ray diffractometry (XRD, Rigaku D/MAX-2500 Japan) was employed to characterize structure of the prepared materials. XRD data were obtained at 2θ = 10–80° with a step size of 0.02°, using Cu Kα radiation. The morphology of synthesized materials was observed by a scanning electron microscope (SEM, JMS-6700F, JEOL, Japan). Energy dispersive X-ray spectroscopy (EDS, Hiroba EDX) was used to analyse surface chemical composition of precursors and lithiated materials. The total chemical compositions of precursors were analyzed by an inductively coupled plasma spectrometer (ICP, SPS 7800, Seiko Instruments, Japan).
Thermal gravimetric analysis (TGA, EXSTAR6000, SEIKO, Japan) under an oxygen flow was conducted on 10 mg of [(Ni0.6Co0.2Mn0.2)1/2(Ni0.4Co0.2Mn0.4)1/2](OH)2 powders that had been placed inside platinum pans. The reference pan in the TGA furnace was consisted of an empty platinum pan. The data were collected with Seiko Exstar 6000 instrument at a scan rate of 10 °C min−1 in the temperature range of room temperature to 800 °C.
For fabrication of cathode electrodes, the prepared materials were mixed with acetylene black and PVDF (83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 in weight) in NMP. The obtained slurry was coated onto Al foil and dried at 80 °C for a day, followed by a roll-pressing. Prior to use, the electrodes were dried again at 120 °C for half a day in a vacuum oven. The electrodes were electrochemically characterized using a 2032 type of coin cell with lithium foil as the anode and 1 M LiPF6 in ethylene carbonate diethyl carbonate (1
7 in weight) in NMP. The obtained slurry was coated onto Al foil and dried at 80 °C for a day, followed by a roll-pressing. Prior to use, the electrodes were dried again at 120 °C for half a day in a vacuum oven. The electrodes were electrochemically characterized using a 2032 type of coin cell with lithium foil as the anode and 1 M LiPF6 in ethylene carbonate diethyl carbonate (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in volume) as the electrolyte. The cells were preliminarily charged and discharged in the voltage range of 3.0–4.3 V (versus Li) at 25 °C at a constant current density of 20 mA g−1 for initial three cycles and 150 mA g−1 for subsequent cycles.
1 in volume) as the electrolyte. The cells were preliminarily charged and discharged in the voltage range of 3.0–4.3 V (versus Li) at 25 °C at a constant current density of 20 mA g−1 for initial three cycles and 150 mA g−1 for subsequent cycles.
For Differential Scanning Calorimetry (DSC) experiments, cells were finally charged to 4.3 V and opened carefully in the Ar-filled dry box. After opening the cells, the electrode materials were recovered from the current collector. The DSC data were collected in a differential scanning calorimeter (NETZSCH 204F1, Germany) using a temperature scan rate of 4 °C min−1 in the temperature range of 25–360 °C.
Fig. 1 shows the particle size distribution of core [Ni0.6Co0.2Mn0.2](OH)2, and core–shell [(Ni0.6Co0.2Mn0.2)1/2(Ni0.4Co0.2Mn0.4)1/2](OH)2 particles collected during co-precipitation. Both curves had a single distribution peak, which meant the particle distribution of precursors was expectedly peak. The formation of new crystal nuclear was not observed while shell was co-precipitated. The average particle diameter (D50) grown from 6.92 μm (core) to 8.81 μm (core–shell) in the process of co-precipitation. These suggested the possible formation of core-shelled [(Ni0.6Co0.2Mn0.2)1/2(Ni0.4Co0.2Mn0.4)1/2](OH)2, with the thickness of shell 0.95 μm.
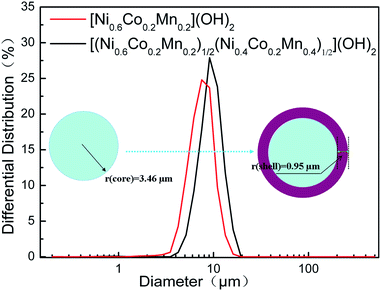 | ||
| Fig. 1 Change in particle size of core [Ni0.6Co0.2Mn0.2](OH)2 and core–shell [(Ni0.6Co0.2Mn0.2)1/2(Ni0.4Co0.2Mn0.4)1/2](OH)2. | ||
Fig. 2 shows XRD patterns of as-prepared [(Ni0.6Co0.2Mn0.2)1/2(Ni0.4Co0.2Mn0.4)1/2](OH)2 precursors as well as its core and shell. As observed in Fig. 2, XRD patterns of core-shelled structured [(Ni0.6Co0.2Mn0.2)1/2(Ni0.4Co0.2Mn0.4)1/2](OH)2 overlapped its core and shell components, which further supported the formation of core-shelled structure in precursors.
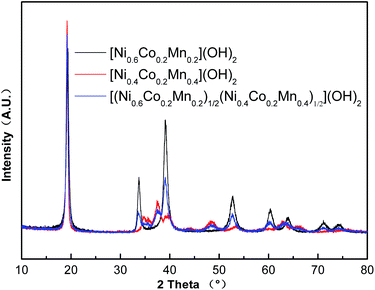 | ||
| Fig. 2 XRD patterns of as-prepared [Ni0.6Co0.2Mn0.2](OH)2, [Ni0.4Co0.2Mn0.4](OH)2 and [(Ni0.6Co0.2Mn0.2)1/2(Ni0.4Co0.2Mn0.4)1/2](OH)2. | ||
Fig. 3 shows the SEM images of the precursors prepared by co-precipitation route. It is obvious that the block-like shaped primary particles of [(Ni0.6Co0.2Mn0.2)1/2(Ni0.4Co0.2Mn0.4)1/2](OH)2 and [Ni0.5Co0.2Mn0.3](OH)2 are seen in Fig. 3(a) and (c). At the same time, the low-magnification SEM images of core–shell and bulk powders were revealed in Fig. 3(b) and (d), both of which showed spherical shape. The surface chemical compositions of core and core–shell of precursors separately were [Ni0.602Co0.199Mn0.199](OH)2 and [Ni0.401Co0.200Mn0.399](OH)2 by EDS in Fig. 4, which matched what we designed. In addition, the average chemical compositions of bulk and core–shell of precursors separately were [Ni0.503Co0.199Mn0.298](OH)2 and [Ni0.506Co0.196Mn0.298](OH)2 by ICP, which also is consistent with the intended [Ni0.5Co0.2Mn0.3](OH)2.
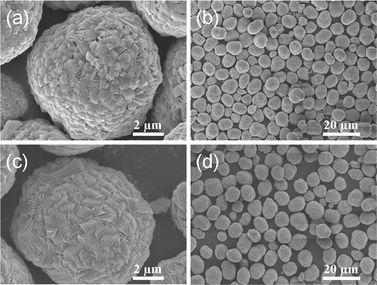 | ||
| Fig. 3 SEM images of (a) high- and (b) low-magnification of core-shelled [(Ni0.6Co0.2Mn0.2)1/2(Ni0.4Co0.2Mn0.4)1/2](OH)2, (c) high- and (d) low-magnification of [Ni0.5Co0.2Mn0.3](OH)2. | ||
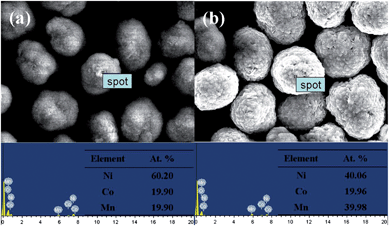 | ||
| Fig. 4 EDS of (a) [Ni0.6Co0.2Mn0.2](OH)2 and (b) [(Ni0.6Co0.2Mn0.2)1/2(Ni0.4Co0.2Mn0.4)1/2](OH)2 precursors. | ||
Fig. 5 compares the DTG curve of the as-prepared precursors recorded between room temperature and 800 °C under a purified oxygen atmosphere. It was obvious that the [(Ni0.6Co0.2Mn0.2)1/2(Ni0.4Co0.2Mn0.4)1/2](OH)2 precursors had two sharp endothermic peaks which were combined with the peak of [Ni0.6Co0.2Mn0.2](OH)2 and [Ni0.4Co0.2Mn0.4](OH)2. The similar phenomenon was also found in XRD patterns of as-prepared [(Ni0.6Co0.2Mn0.2)1/2(Ni0.4Co0.2Mn0.4)1/2](OH)2 precursor as well as its core and shell in Fig. 2. It was surely believed that [(Ni0.6Co0.2Mn0.2)1/2(Ni0.4Co0.2Mn0.4)1/2](OH)2 precursors contained core [Ni0.6Co0.2Mn0.2](OH)2 and shell [Ni0.4Co0.2Mn0.4](OH)2, no matter formed core-shelled structure or the two individual core and shell compositions in precursors. However, well spherical shape in Fig. 3 in combination with particle size increase from core to shell in Fig. 1 as well as accurately designed composition of core and shell by EDS data in Fig. 4 can confirm the formation of core-shelled structure in final precursors.
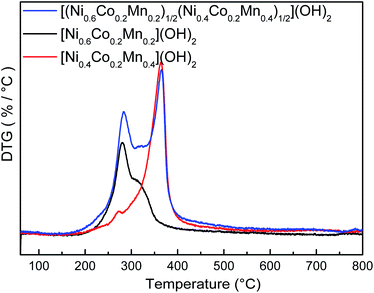 | ||
| Fig. 5 DTG curves of [Ni0.6Co0.2Mn0.2](OH)2, [Ni0.4Co0.2Mn0.4](OH)2 and [(Ni0.6Co0.2Mn0.2)1/2(Ni0.4Co0.2Mn0.4)1/2](OH)2. | ||
The prepared core-shelled precursor powders [(Ni0.6Co0.2Mn0.2)1/2(Ni0.4Co0.2Mn0.4)1/2](OH)2 were calcined with stoichiometric ratio lithium carbonate at a high temperature of 900 °C. To determine the composition change in the core-concentration gradient-shelled LiNi0.5Co0.2Mn0.3O2 oxide, EDS was used to analyze the composition on the cross-section of a single particle. The concentrations of Ni, Co and Mn were plotted as a function of distance from the core center to the particle surface. As can be seen in Fig. 6(a), the Ni, Co and Mn concentrations remained almost constant (Ni, Co and Mn were about 60, 20 and 20%, respectively) from the core center to about 1 μm, which meant the core was LiNi0.6Co0.2Mn0.2O2. After this point, the Ni concentration decreased gradually from 60% to 44%, while the Mn concentration increased steadily from 20% to 36% at the particle, which was called concentration-gradient layer. After this concentration-gradient layer, the Ni, Co and Mn concentrations remained almost constant (Ni, Co and Mn were 44, 20 and 36%, respectively) from this layer to the outer surface about 1 μm, resulting in a shell composition of LiNi0.44Co0.20Mn0.36O2. Therefore, based on those EDS results, we believed that a novel core-concentration gradient-shelled LiNi0.5Co0.2Mn0.3O2 as revealed in Fig. 6(b) was successfully synthesized via a simple method from the core-shelled precursor powders.
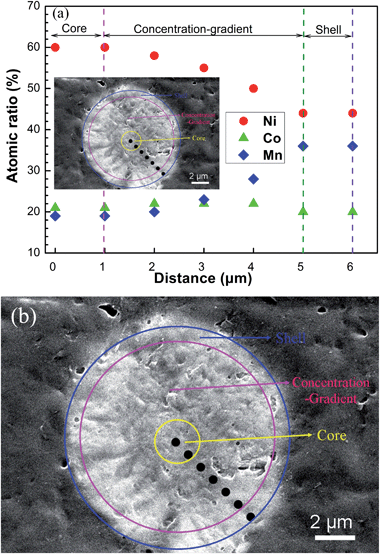 | ||
| Fig. 6 (a) EDS compositional change from (b) the cross-section of the core-concentration gradient-shelled LiNi0.5Co0.2Mn0.3O2 particle. | ||
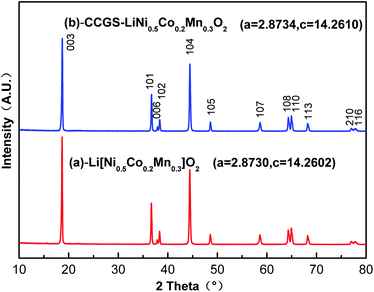
Fig. 7 shows XRD patterns of LiNi0.5Co0.2Mn0.3O2 and CCGS-LiNi0.5Co0.2Mn0.3O2 calcined at 900 °C. Both materials could be indexed to a well-defined hexagonal α-NaFeO2-type structure with a space group of R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m. No impurities were observed in the XRD patterns. The lattice parameters were calculated in Fig. 7, indicating good similarity. Therefore, differences between core-concentration gradient-shelled and bulk LiNi0.5Co0.2Mn0.3O2 materials were hardly detected.
m. No impurities were observed in the XRD patterns. The lattice parameters were calculated in Fig. 7, indicating good similarity. Therefore, differences between core-concentration gradient-shelled and bulk LiNi0.5Co0.2Mn0.3O2 materials were hardly detected.
SEM images of the lithiated powders obtained at 900 °C are shown in Fig. 8. Similarly, there was no disruption of the particle morphology for the CCGS-LiNi0.5Co0.2Mn0.3O2 in Fig. 8(a) and (b), since the two different hydroxide compositions would cause a different shrinkage ratio during the high temperature calcination process reported by Sun,18 but formed core-concentration gradient-shelled structure based on previous EDS results. As can be seen in Fig. 8(a) and (c), the primary particle morphologies of the CCGS-LiNi0.5Co0.2Mn0.3O2 and LiNi0.5Co0.3Mn0.2O2 were very similar.
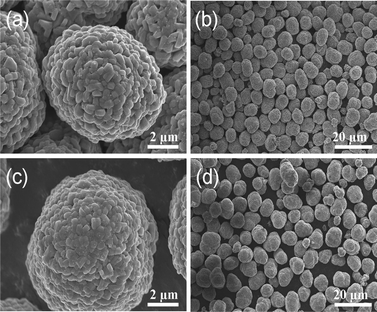 | ||
| Fig. 8 SEM images of (a) high- and (b) low-magnification of CCGS-LiNi0.5Co0.2Mn0.3O2, (c) high- and (d) low-magnification LiNi0.5Co0.2Mn0.3O2. | ||
As can be seen in Table 1, tap density of core-shelled [(Ni0.6Co0.2Mn0.2)1/2(Ni0.4Co0.2Mn0.4)1/2](OH)2 precursors was higher than [Ni0.5Co0.2Mn0.3](OH)2. Meanwhile, compared with tap density of LiNi0.5Co0.2Mn0.3O2 (2.59 g cm−3), the tap density of the prepared lithiated CCGS-LiNi0.5Co0.2Mn0.3O2 powders was as high as 2.60 g cm−3, and close to that of commercialized LiCoO2 (2.70 g cm−3).32 So the core-concentration gradient-shelled structure could not decrease the tap density of material if design and preparation conditions are well controlled.
| Tap density | |
|---|---|
| [(Ni0.6Co0.2Mn0.2)1/2(Ni0.4Co0.2Mn0.4)1/2](OH)2 | 2.13 g cm−3 |
| [Ni0.5Co0.2Mn0.3](OH)2 | 2.09 g cm−3 |
| CCGS-LiNi0.5Co0.2Mn0.3O2 | 2.60 g cm−3 |
| LiNi0.5Co0.2Mn0.3O2 | 2.59 g cm−3 |
Fig. 9(a) shows the first charge–discharge curves and (b) cycling performances of CCGS-LiNi0.5Co0.2Mn0.3O2 and LiNi0.5Co0.2Mn0.3O2. The electrochemical performance was measured at 25 °C applied over voltage ranges 3.0–4.3 V versus Li+/Li. The initial discharge capacity of the CCGS-LiNi0.5Co0.2Mn0.3O2 electrodes was 173.4 mA h g−1, which was similar to the 172.1 mA h g−1 of LiNi0.5Co0.2Mn0.3O2. As expected, CCGS-LiNi0.5Co0.2Mn0.3O2 had much improved Li intercalation stability in the voltage range of 3.0 to 4.3 V, which is shown in Fig. 9(b). The CCGS-LiNi0.5Co0.2Mn0.3O2 has capacity retention of 93.87% (153.1 mA h g−1) after 200 cycles. By contrast, the LiNi0.5Co0.2Mn0.3O2 electrode suffered from a severe capacity fading, leading to a capacity retention of only 82.41% (133.5 mA h g−1) over the same cycling period. The formal charges for Ni, Co and Mn of shell layer LiNi0.44Co0.20Mn0.36O2 are 2+ and 3+, 3+, and 4+, respectively. The electrochemically inactive tetravalent Mn provides significant structural stability and results in a simple topotactic reaction maintaining the hexagonal phase during electrochemical cycling. In addition, the high concentration of unstable Ni4+ occurs if this material was charged to high voltages, which will result in high interfacial cell impedance and poor electrochemical performances because NiO phase generates at the cathode surface, which indicates the stability of material is related to surface layers among Ni-rich materials. Therefore, the core-concentration gradient-shelled sample revealed more excellent cycling performance compared with normal samples due to more stable shell layer.
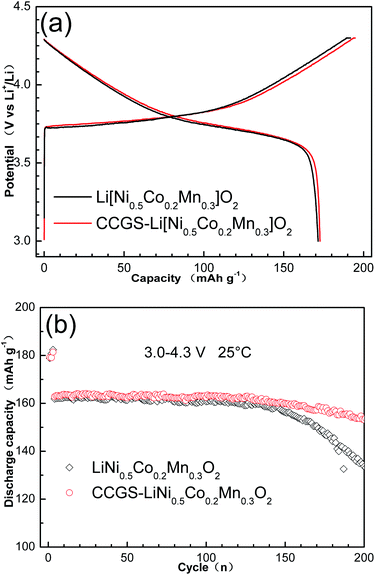 | ||
| Fig. 9 (a) The initial charge–discharge curves and (b) cycle performance of LiNi0.5Co0.2Mn0.3O2 and CCGS-LiNi0.5Co0.2Mn0.3O2 at 25 °C. | ||
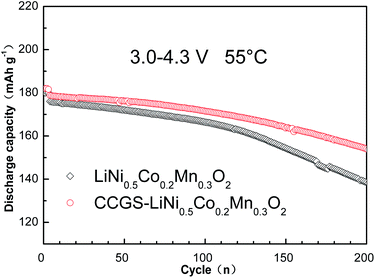
To evaluate the stability of the CCGS-LiNi0.5Co0.2Mn0.3O2 material, a constant current density of 100 mA g−1 and 55 °C were selected as testing conditions (Fig. 10). When the operating temperature increased to 55 °C, the initial discharge capacity of the CCGS-LiNi0.5Co0.2Mn0.3O2 and LiNi0.5Co0.2Mn0.3O2 increased by 10 mA h g−1 in the voltage range of 3.0 to 4.3 V compared to the initial discharge capacity at 25 °C. The lithium intercalation stability of the CCGS-LiNi0.5Co0.2Mn0.3O2 was remarkably improved by complete encapsulation with the stable shell, still maintaining discharge capacity of 154.1 mA h g−1 (capacity retention of 86.13%) after 200 cycles. By contrast, the LiNi0.5Co0.2Mn0.3O2 showed a rapid decrease in capacity, leading to a capacity retention of only 78.69% (138.5 mA h g−1) over the same cycling period. The core-concentration gradient-shelled structure gave rise to a significant improvement in the low voltage cycling performance, which was probably due to the impact of the shell LiNi0.44Co0.20Mn0.36O2 providing structural stability.
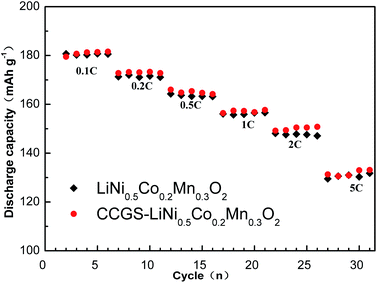
Fig. 11 shows the discharge capacity of LiNi0.5Co0.2Mn0.3O2 and CCGS-LiNi0.5Co0.2Mn0.3O2 cells at various rates between 3.0 and 4.3 V. Each cell was charged at the same density of 8 mA g−1 (0.05 C rate) and then discharged at different density of 16 mA g−1 (0.1 C rate), 32 mA g−1 (0.2 C rate), 80 mA g−1 (0.5 C rate), 160 mA g−1 (1 C rate), 320 mA g−1 (2 C rate) and 800 mA g−1 (5 C rate). It revealed that the CCGS-LiNi0.5Co0.2Mn0.3O2 electrode has similar rate capability with the LiNi0.5Co0.2Mn0.3O2 electrode.
Fig. 12 shows DSC profiles of Li1−δNi0.5Co0.2Mn0.3O2 and CCGS-Li1−δNi0.5Co0.2Mn0.3O2 electrodes charged to 4.3 V. Each material showed different tendencies for heat generation. A large exothermic peak in LiNi0.5Co0.2Mn0.3O2 material appeared at 266.8 °C. In contrast, the exothermic peak for the CCGS-LiNi0.5Co0.2Mn0.3O2 material moved to higher temperatures of 276.8 °C. Furthermore, the amounts of heat generation also decreased at the same time. As a result, the core-concentration gradient-shelled LiNi0.5Co0.2Mn0.3O2 material can enhance the thermal stability of the material.
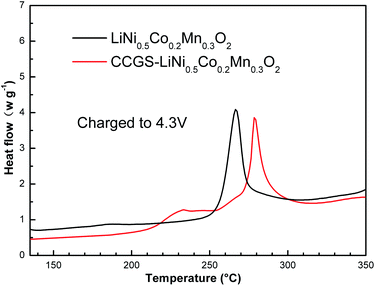 | ||
| Fig. 12 DSC results of the CCGS-Li1−δNi0.5Co0.2Mn0.3O2 and Li1−δNi0.5Co0.2Mn0.3O2 charged to 4.3 V at 25 °C. | ||
In summary, a novel core-concentration gradient-shelled LiNi0.5Co0.2Mn0.3O2 was successfully synthesized via a simple method from the core-shelled precursors. The compositions of core and shell separately were LiNi0.60Co0.20Mn0.20O2 and LiNi0.44Co0.20Mn0.36O2, between which was a concentration gradient layer. XRD studies show that the prepared materials LiNi0.5Co0.2Mn0.3O2 and CCGS-LiNi0.5Co0.2Mn0.3O2 could be indexed to a typical layered structure with R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group, having similar lattice constant. CCGS-LiNi0.5Co0.2Mn0.3O2 delivered more excellent cycling performance than LiNi0.5Co0.2Mn0.3O2 in the voltage ranges 3.0–4.3 V versus Li+/Li at 25 °C and 55 °C. Besides, DSC experiments demonstrated remarkably improved thermal stability compared to LiNi0.5Co0.2Mn0.3O2, which was possibly ascribed to the shell LiNi0.44Co0.20Mn0.36O2 providing structural and thermal stability.
m space group, having similar lattice constant. CCGS-LiNi0.5Co0.2Mn0.3O2 delivered more excellent cycling performance than LiNi0.5Co0.2Mn0.3O2 in the voltage ranges 3.0–4.3 V versus Li+/Li at 25 °C and 55 °C. Besides, DSC experiments demonstrated remarkably improved thermal stability compared to LiNi0.5Co0.2Mn0.3O2, which was possibly ascribed to the shell LiNi0.44Co0.20Mn0.36O2 providing structural and thermal stability.
This work was financially supported by the National 863 Program of China (2011AA11A234, 2013AA050906), NSFC (51272175, 20901058, 21301127), Program for New Century Excellent Talents in University of China (NCET-10-0952).
Notes and references
- J. M. Tarascon and M. Armand, Nature, 2001, 414, 359 CrossRef CAS PubMed.
- Z. Lu, D. D. MacNeil and J. R. Dahn, Electrochem. Solid-State Lett., 2001, 4, 200 CrossRef.
- D. MacNeil, Z. Lu and J. R. Dahn, J. Electrochem. Soc., 2002, 149, 1332 CrossRef.
- S. Jouanneau, D. D. Macneil and J. R. Dahn, J. Electrochem. Soc., 2003, 150, 1299 CrossRef.
- S. Jouanneau, K. W. Eberman and J. R. Dahn, J. Electrochem. Soc., 2003, 150, 1637 CrossRef.
- T. Ohzuku and Y. Makimura, Chem. Lett., 2001, 7, 642 CrossRef.
- J. K. Ngala, N. A. Chernova and M. S. Whittingham, J. Mater. Chem., 2004, 14, 214 RSC.
- K. S. Lee, S. T. Myung and Y. K. Sun, Chem. Mater., 2007, 19, 2727 CrossRef CAS.
- S. H. Kang, J. Kim and M. E. Stoll, J. Power Sources, 2002, 112, 41 CrossRef CAS.
- J. Choi and A. Manthiram, J. Power Sources, 2006, 162, 667 CrossRef CAS.
- L. Wang, H. B. Wang, Z. H. Liu, C. Xiao, S. M. Dong, P. X. Han, Z. Y. Zhang, X. Y. Zhang, C. F. Bi and G. L. Cui, Solid State Ionics, 2010, 181, 1685 CrossRef CAS.
- C. J. Zhang, X. He, Q. S. Kong, H. Li, H. Hu, H. B. Wang, L. Gu, L. Wang, G. L. Cui and L. Q. Chen, CrystEngComm, 2012, 14, 4344 RSC.
- D. C. Li, Y. Sasaki, M. Kageyama, K. Kobayakawa and Y. Sato, J. Power Sources, 2005, 148, 85 CrossRef CAS.
- H. Arai, S. Okada, Y. Sakurai and J. Yamaki, Solid State Ionics, 1998, 109, 295 CrossRef CAS.
- D. P. Abraham, R. D. Twesten, M. Balasubramanian, I. Petrov, J. McBreen and K. Amine, Electrochem. Commun., 2002, 4, 620 CrossRef CAS.
- S. U. Woo, C. S. Yoon, K. Amine, I. Belharouak and Y. K. Sun, J. Electrochem. Soc., 2007, 154, 1005 CrossRef.
- P. Y. Hou, J. Guo, D. W. Song, J. Zhang, E. L. Zhou and L. Q. Zhang, Chem. Lett., 2012, 41, 1712 CrossRef CAS.
- Y. K. Sun, S. T. Myung, M. H. Kim, J. Prakash and K. Amine, J. Am. Chem. Soc., 2005, 127, 13411 CrossRef CAS PubMed.
- Y. K. Sun, S. T. Myung, B. C. Park, J. Prakash, I. Belharouak and K. Amine, Nat. Mater., 2009, 8, 320 CrossRef CAS PubMed.
- G. H. Kim, J. H. Kim, S. T. Myung, C. S. Yoon and Y. K. Sun, J. Electrochem. Soc., 2005, 152, 1707 CrossRef.
- W. Lu, I. Belharouak, D. Vissers and K. Amine, J. Electrochem. Soc., 2006, 153, 2147 CrossRef.
- S. T. Myung, S. Komaba, K. Hosoya, Y. Miura, N. Hirosaki and N. Kumagai, Chem. Mater., 2005, 17, 2427 CrossRef CAS.
- S. T. Myung and Y. K. Sun, Electrochim. Acta, 2005, 50, 4800 CrossRef CAS.
- T. Ohzuku and Y. Makimura, Chem. Lett., 2001, 8, 744 CrossRef.
- S. M. Park, T. H. Cho and M. Yoshio, Chem. Lett., 2004, 33, 6 CrossRef.
- M. H. Lee, Y. J. Kang, S. T. Myung and Y. K. Sun, Electrochim. Acta, 2004, 50, 939 CrossRef CAS.
- S. W. Oh, S. H. Park, C. W. Park and Y. K. Sun, Solid State Ionics, 2004, 171, 167 CrossRef CAS.
- W. S. Yoon, Y. Paik, X. Q. Yang, M. Balasubramanian, J. McBreen and C. P. Grey, Electrochem. Solid-State Lett., 2002, 5, 263 CrossRef.
- X. Q. Yang, J. McBreen, W. S. Yoon and C. P. Grey, Electrochem. Commun., 2002, 4, 649 CrossRef CAS.
- S. T. Myung, S. Komaba, K. Hosoya, N. Hirosaki, U. Miura and N. Kumagai, Chem. Mater., 2005, 17, 2427 CrossRef CAS.
- Z. Lu, L. Y. Beaulieu, R. A. Donaberger, C. L. Thomas and J. R. Dahn, J. Electrochem. Soc., 2002, 149, 778 CrossRef.
- J. R. Ying, C. Y. Jiang and C. R. Wan, J. Power Sources, 2004, 129, 264 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |