Antibacterial activity of adamantyl substituted cyclohexane diamine derivatives against methicillin resistant Staphylococcus aureus and Mycobacterium tuberculosis†
Beenaa,
Deepak Kumara,
Widuranga Kumbukgollab,
Sampath Jayaweerab,
MaiAnn Baileyc,
Torey Allingc,
Juliane Ollingerc,
Tanya Parishc and
Diwan S. Rawat*a
aDepartment of Chemistry, University of Delhi, Delhi-110007, India. E-mail: dsrawat@chemistry.du.ac.in; Fax: +91-11-27667501; Tel: +91-11-27662683
bFaculty of Medicine and Allied Sciences, Rajarata University of Sri Lanka, Mihinthale, Sri Lanka
cInfectious Disease Research Institute, 1124 Columbia Street, Suite 400, Seattle, Washington 98104, USA
First published on 18th February 2014
Abstract
A series of forty two adamantyl based cyclohexane diamine derivatives were synthesized and the antibacterial activities of these compounds were assessed against 29 strains of methicillin resistant Staphylococcus aureus (MRSA) and a virulent strain of Mycobacterium tuberculosis. The compounds showed potent to moderate activity against MRSA while moderate to weak activity against the virulent strain of M. tuberculosis. The compound 8e showed the most potent activity against MRSA having minimum inhibitory concentration (MIC) values in the range of 8–64 μg mL−1 against 26 MRSA strains out of the 29 strains examined. It exhibited improved inhibitory activity compared to oxacillin. Compound 8i with an MIC value of 13.7 μM against M. tuberculosis, was bactericidal with rapid kill kinetics demonstrating a 4 log reduction in viability of M. tuberculosis within 7 days.
Introduction
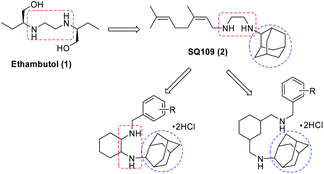
Methicillin resistant Staphylococcus aureus (MRSA) causes blood stream infections, skin infections, respiratory tract infections, surgical wound infections and bone infections. In intensive care units (ICUs), MRSA is a predominant causative agent of sepsis which is a life threatening condition.1 Many MRSA isolates are resistant to several antibiotics. It has been found, 47.6% of MRSA which were isolated from inpatients, are multidrug-resistant. They are resistant to erythromycin, ciprofloxacin, clindamycin and gentamicin.2 In controlling the multidrug-resistant strains of MRSA, vancomycin and linezolid are considered as the most effective antibiotics. However, resistant strains against vancomycin and linezolid have been detected recently, which indicates that MRSA would become fully resistant to vancomycin and linezolid, eventually.3 The presence of microbial resistance in the hospital and the community has a clear effect on health costs. The results of several studies have shown that MRSA infections are more costly to manage because of increased cost for infection prevention, extensive investigations and expensive therapies.4 Therefore, effective control of MRSA infections is urgently required.In recent years, a large number of analogues of the first line antitubercular drug ethambutol (EMB, 1) have been synthesized and screened for their anti-TB activity,5,6 such as SQ109 or ([N-geranyl-N′-(2-adamantyl)ethane-1,2-diamine]) (2).7,8 SQ109 is an important drug currently in phase II clinical trial for the treatment of tuberculosis.9–13
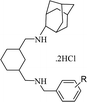
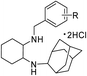
In the attempts to synthesize new antimicrobial agents,14–16 we reported antimicrobial activity of various series of cyclohexane diamine derivatives17–21 and in continuation with our efforts herein we report the synthesis and antimicrobial activity of another series of novel cyclohexane diamine derivatives (Fig. 1). The phenyl ring incorporated in this series provides structural diversity to these compounds for structure–activity relationship (SAR) study.
Chemistry
In order to synthesize the titled compounds one of the NH2 group of cyclohexane-1,2-diamine (3) or C-(3-aminomethyl-cyclohexyl)-methylamine (9) was protected with Boc group (Schemes 1 and 2).22,23 The free NH2 group of Boc protected compound 4 (Scheme 1) or 10 (Scheme 2) was treated with 2-adamantanone to give Schiff's base (5 or 11) in quantitative yield and it was reduced using NaBH4 in dry MeOH–THF to give substituted amine (6 or 12) containing one NH2 group protected with Boc, which on treatment with 35% aq. H3PO4 in dichloromethane leads to removal of Boc group (7 or 13).23 The free NH2 group was then reacted with different substituted benzaldehydes in dry MeOH and resulting imines were reduced by NaBH4 in situ. The amines were purified by column chromatography using 2% MeOH–CHCl3 as eluent. All these adamantyl ring containing amines were converted to their respective hydrochloride salts by passing dry HCl gas to their solution in chloroform. Resulting, hydrochloride salts (8a–u and 14a–u) were characterized by spectroscopic methods.Biological activity
(a) In vitro anti-MRSA activity
(b) In vitro anti-tuberculosis assay
For compounds 8a–u and 14a–u the minimum inhibitory concentration (MIC) was determined using a 96-well plate format assay in Middlebrook 7H9 medium containing 0.05% w/v Tween 80 and 10% v/v OADC supplement (oleic acid, albumin, dextrose, catalase: Becton Dickinson). Compounds were dissolved in DMSO and tested using a 10-point, two-fold serial dilution curve with the highest concentration of 20 μM and a final DMSO concentration in the assay of 2% v/v. Growth was measured after 5 days in the presence of compound. Two readouts of growth were used (OD590 and fluorescence) to calculate % growth. MICs were calculated after curve fitting MICs to the Gompertz model; two points of maximum (100%) and minimum (0%) growth were required to generate a complete curve and record an accurate MIC. For active compounds, MICs were conducted at least twice using independent cultures.Results and discussion
(a) In vitro anti-MRSA activity
All the forty two compounds (8a–u and 14a–u) of the two series, examined by agar well method, exhibited a clear zone in varying diameters. From the series 8a–u with cyclohexane-1,2-diamine moiety, five compounds (8a, 8d, 8e, 8m and 8q) which showed the largest zone diameters were selected to determine their MIC value, using agar plate dilution method. Oxacillin, the standard antibiotic was used to compare the activity. All five compounds were more potent than oxacillin against MRSA. Compounds with alkyl substituent at para-position (8d and 8e) of phenyl ring displayed an enhanced activity against most of the MRSA strains having MIC values in the range of 16–64 μg mL−1 (Table 1). Compounds 8d and 8e could inhibit 25 strains of MRSA in within 16–64 μg mL−1 concentration range. Amongst the five active compounds, 8e was found to be the most active; it was active at a concentration 8 μg mL−1, against one MRSA strain and another 10 strains were inhibited at 16 μg mL−1 concentration (Table 1).From the series 14a–u, three compounds were active (14i, 14k and 14o) and three compounds exhibits moderate activity (14f, 14n and 14t). Compounds 14i and 14k exhibited extra large clear zones. Compound 14o showed clear zone inhibition and compound 14t displayed very small clear zone while 14n and 14f moderate clear zones. Most active is compound 14k with chloro group at meta-position of the phenyl ring and active at 32 μg mL−1 concentration against most of the strains used. From the series, compounds 8m, 8q, 14k, 14n and 14o with halo substituents in the phenyl ring inhibited various strains of MRSA.
(b) In vitro antituberculosis activity
Table 2 shows the anti-tubercular activity of forty two SQ109 analogues (8a–u and 14a–u) against M. tuberculosis H37Rv. Ethambutol was used as the standard reference with a MIC of 2–3 μM. Nine compounds (8a, 8e, 8g, 8h, 8i, 8r, 8t, 14h and 14i) from both the series were active at concentration below 20 μM. Twelve compounds in series 8a–u showed MIC ≤ 20 μM. Compound 8i was active with MIC = 13.7 μM. The halo substituted derivatives were either weakly or partially active. But, an ortho-CF3 substituted derivative (8r) was active with MIC 16.7 μM. The unsubstituted derivative (8a) was also active (MIC = 16.4 μM). Derivatives with 4-Et (8e), 4-i-Pr (8g) and 4-n-Bu (8h) substituent's were active with MIC values 16.8, 15.8 and 16.4 μM, respectively. Compound 8t with dimethyl substitution also showed significant activity (MIC = 16.5 μM).| Compd | MIC (μM) | Compd | MIC (μM) | ||
|---|---|---|---|---|---|
| R | R | ||||
| 8a | H | 16.4 ± 4.2 | 14a | H | NA |
| 8b | 2-Me | >20.0 | 14b | 2-Me | NA |
| 8c | 3-Me | >20.0 | 14c | 3-Me | >20 |
| 8d | 4-Me | 20.0 | 14d | 4-Me | >20 |
| 8e | 4-Et | 16.8 ± 3.7 | 14e | 4-Et | >20 |
| 8f | 4-n-Pr | NA | 14f | 4-n-Pr | >20 |
| 8g | 4-i-Pr | 15.8 ± 4.9 | 14g | 4-i-Pr | >20 |
| 8h | 4-n-Bu | 16.4 ± 4.1 | 14h | 4-n-Bu | 7.5 |
| 8i | 4-t-Bu | 13.7 ± 7.3 | 14i | 4-t-Bu | 6.1 |
| 8j | 2-Cl | 20.0 | 14j | 2-Cl | >20 |
| 8k | 3-Cl | 20.0 | 14k | 3-Cl | >20 |
| 8l | 4-Cl | 20.0 | 14l | 4-Cl | >20 |
| 8m | 2-Br | >20.0 | 14m | 2-Br | >20 |
| 8n | 3-Br | >20.0 | 14n | 3-Br | >20 |
| 8o | 4-Br | >20.0 | 14o | 4-Br | >20 |
| 8p | 2-F | >20.0 | 14p | 2-F | NA |
| 8q | 4-F | >20.0 | 14q | 4-F | NA |
| 8r | 2-CF3 | 16.7 ± 3.9 | 14r | 3-F | NA |
| 8s | 4-CF3 | >20.0 | 14s | 4-OCH3 | NA |
| 8t | 2,6-Me | 16.5 ± 4.1 | 14t | 3-NO2 | >20 |
| 8u | 3,4-Me | 20.0 | 14u | 4-NO2 | >20 |
| Ethambutol | 2–3 | ||||
From the series 14a–u, two compounds 14h and 14i with n-butyl and tert-butyl groups at para-position of the benzene ring showed very good activity against M. tuberculosis with MIC values of 7.5 and 6.1 μM, respectively. Compounds with n-butyl and t-butyl groups at para-position (8h, 8i, 14h and 14i) were most active from both the series of compounds (8a–u and 14a–u).
Overall series 8a–u showed better activity against M. tuberculosis than the series 14a–u. The most active compound (8i) was selected for bactericidal activity (Fig. 2). The bactericidal activity was monitored over 7 days in different concentrations of compound. Using standard methods for generating kill curves at 10× MIC, compound 8i rapidly killed M. tuberculosis. The compound was rapidly bactericidal, effectively sterilizing cultures within 7 days (limit of detection 10 CFU mL−1). This study confirmed that the compounds were bactericidal in nature rather than bacteriostatic.
Conclusions
In conclusion we have synthesized forty two cyclohexane diamine derivatives which showed promising activity against MRSA and exhibited moderate to weak activity against M. tuberculosis. Compound 8i, 14h and 14i showed promising activity against M. tuberculosis amongst the series and compound 8i exhibited a rapid bactericidal effect. Compounds 8a, 8e and 14i showed good activity against M. tuberculosis as well as resistant strains of S. aureus.Acknowledgements
DSR acknowledge the Council of Scientific and Industrial Research (02(0049)/12/EMR-II), New Delhi, India and DU-PURSE, University of Delhi, Delhi, India for financial support. Beena and DK are thankful to Council for Scientific and Industrial Research, New Delhi, India for the award of JRF and SRF. Authors are also thankful to CIF-USIC, University of Delhi, Delhi for NMR spectral data and RSIC, CDRI, Lucknow for mass data. We thank Alfredo Blakeley, Julie early, Stephanie Florio, and David Roberts for technical assistance. Work at IDRI was funded in part by the Lilly TB Drug Discovery Initiative.Notes and references
- L. Mihaljevic, S. Mihaljevic, I. Vasilj, S. Cavaljuga, F. Serdarevic and I. Soldo, Bosnian J. Basic Med. Sci., 2007, 7, 266 Search PubMed.
- D. Styers, D. J. Sheehan, P. Hogan and D. F. Sahm, Ann. Clin. Microbiol. Antimicrob., 2006, 5, 2 CrossRef PubMed.
- (a) V. Cafiso, T. Bertuccio, D. Spina, F. Campanile, D. Bongiorno, M. Santagati, A. Sciacca, C. Sciuto and S. Stefani, Eur. J. Clin. Microbiol. Infect. Dis., 2010, 29, 1277 CrossRef CAS PubMed; (b) F. Campanile, S. Borbone, M. Perez, D. Bongiorno, V. Cafiso, T. Bertuccio, S. Purrello, D. Nicolosi, C. Scuderi and S. Stefani, Int. J. Antimicrob. Agents, 2010, 36, 415 CrossRef CAS PubMed.
- D. Nathwani, J. Antimicrob. Chemother., 2003, 51(suppl. S2), ii37–ii44F CAS.
- A. Pilheu and A. Catrangolo, Rev. Asoc. Med. Argent., 1962, 76, 513 Search PubMed.
- Beena and D. S. Rawat, Med. Res. Rev., 2013, 33, 693 CrossRef CAS PubMed.
- M. Protopopova, C. Hanrahan, B. Nikonenko, R. Samala, P. Chen, J. Gearhart, L. Einck and C. A. Nacy, J. Antimicrob. Chemother., 2005, 56, 968 CrossRef CAS PubMed.
- R. E. Lee, M. Protopopova, E. Crooks, R. A. Slayden, M. Terrot and C. E. Barry III, J. Comb. Chem., 2003, 5, 172 CrossRef CAS PubMed.
- K. A. Sacksteder, M. Protopopova, C. E. Barry, K. Andries and C. A. Nacy, Future Microbiol., 2012, 7, 823 CrossRef CAS PubMed.
- Anonymous, Tuberculosis, 2008, 88, 159 CrossRef.
- P. Chen, J. Gearhart, M. Protopopova, L. Einck and C. A. Nacy, J. Antimicrob. Chemother., 2006, 58, 332 CrossRef CAS PubMed.
- B. V. Nikonenko, M. Protopopova, R. Samala, L. Einck and C. A. Nacy, Antimicrob. Agents Chemother., 2007, 51, 1563 CrossRef CAS PubMed.
- K. Tahlan, R. Wilson, D. B. Kastrinsky, K. Arora, V. Nair, E. Fischer, S. W. Barnes, J. R. Walker, D. Alland, C. E. Barry and H. I. Boshoff, Antimicrob. Agents Chemother., 2012, 56, 1797 CrossRef CAS PubMed.
- M. C. Joshi, G. S. Bisht and D. S. Rawat, Bioorg. Med. Chem. Lett., 2007, 17, 3226 CrossRef CAS PubMed.
- G. S. Bisht, D. S. Rawat, A. Kumar, R. Kumar and S. Pasha, Bioorg. Med. Chem. Lett., 2007, 17, 4343 CrossRef CAS PubMed.
- Beena, N. Kumar, R. K. Rohilla, N. Roy and D. S. Rawat, Bioorg. Med. Chem. Lett., 2009, 19, 1396 CrossRef CAS PubMed.
- D. Kumar, S. Joshi, R. K. Rohilla, N. Roy and D. S. Rawat, Bioorg. Med. Chem. Lett., 2010, 20, 893 CrossRef CAS PubMed.
- D. Kumar, R. K. Rohilla, N. Roy and D. S. Rawat, Chem.-Biol. Interface, 2011, 1, 263 CAS.
- M. Sharma, P. Joshi, N. Kumar, S. Joshi, R. K. Rohilla, N. Roy and D. S. Rawat, Eur. J. Med. Chem., 2011, 46, 480 CrossRef CAS PubMed.
- Beena, S. Joshi, N. Kumar, S. Kidwai, R. Singh and D. S. Rawat, Arch. Pharm. Chem. Life Sci., 2012, 345, 896 CrossRef CAS PubMed.
- D. Kumar, K. K. Raj, M. Bailey, T. Alling, T. Parish and D. S. Rawat, Bioorg. Med. Chem. Lett., 2013, 23, 1365 CrossRef CAS PubMed.
- L. H. Uppadine, F. R. Keene and P. D. Beer, J. Chem. Soc., Dalton Trans., 2001, 14, 2188 RSC.
- F. J. Quijada, J. G. Sabin, F. Rebolledo and V. Gotor, Tetrahedron, 2009, 65, 8028 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Yield, Mp, 1H NMR, 13C, mass, CHN. See DOI: 10.1039/c4ra00224e |
| This journal is © The Royal Society of Chemistry 2014 |