Thermally tunable amplified spontaneous emission from Cu2ZnSnS4 quantum dot doped photonic cavity by a soft solvothermal route†
L. J. Chen*a,
Y. J. Chuangb and
C. Chenc
aInstitute of Electro-Optical Science and Engineering, National Cheng Kung University, Tainan, 701, Taiwan. E-mail: linjerchen@hotmail.com
bDepartment of Materials Science and Engineering, National Cheng Kung University, Tainan, 701, Taiwan
cDepartment of Chemistry, National Cheng Kung University, Tainan, 701, Taiwan
First published on 11th April 2014
Abstract
In this article, non-toxic Cu2ZnSnS4 quantum dots (CZTSQDs) were synthesized by the solvothermal method and then embedded into a photonic cavity to tune the amplified spontaneous emission of the device. The amplified spontaneous mechanism was investigated based on the liquid crystal cavity structure and fluorescence behaviours. The results clearly indicate that employing CZTSQDs in creating novel composites allows for the fabrication of active medium materials with thermally tunable emission properties.
Semiconductor nanocrystals, known as quantum dots (QDs), have been intensely investigated because of their size dependent optical and electrical properties.1–10 A quaternary semiconductor of Cu2ZnSnS4 (CZTS), which is a direct band gap semiconductor having a high absorption coefficient of the order of 104 cm−1, has been regarded as one of the most promising materials for light-absorbing materials in optical–electrical devices because of its content of only low-toxicity elements that are abundant in the earth's crust.11–13 They are promising for laser devices due to their spectral size tunability and temperature stability. Absorption of any photons with wavelengths above this specific wavelength causes the formation of an electron–hole pair, the recombination of which results in photon emission. Quantum dots are characterized by a number of unique properties that originate from the quantum confinement effect of their reduced dimension. Especially, quantum dots possess a unique property of optical gain media that holds potential advantages, such as high efficiency, sharp emission, long term photostability, low lasing threshold and temperature insensitivity.14–18
In recent years there has been intensely interest for soft materials. Liquid crystals (LCs) incorporating optically active moieties show periodic helicoidal molecular structures such as cholesteric LC (CLC), blue phase, smectic C* and twist grain boundary phases as a result of their intrinsic twisting ability.19,20 Among those chiral-LC composites, CLC is most known to all because of its easy-fabrication via the molecular self-organization, highly flexible tunability of their photonic properties, and wide industrial applicability in display technologies, optical modulation devices, and lasers.21–23 Tunability in cholesteric liquid crystal (CLC) has been demonstrated by utilizing the external field sensitivity of the LC molecules or the change of the pitch in these systems was easily achieved by the change of the chiral dopant concentration.24,25 Since the photon group velocity approaches zero and photon density of states (DOS) is enhanced at the edge of the photonic band gap. Most bandedge lasing of the CLC has only been studied with doping laser dye to date.26–30 The disadvantages of semiconductor laser and dye-doped CLC laser are the need for the high temperature annealing process and lack of high tunability of externality and fatally thermal instability, respectively. Moreover, an anomalous strong absorption occurs at the band edge of an absorbing CLC.
Recent interest in photonic crystals is due to their ability to control the propagation of light, as the semiconductor nanocrystal control the propagation of electrons.31–33 While there have been a few reports of photoluminescence emission from self-assembled quantum-dot-doped CLCs (QDCLCs).17,18,34,35 However, current states for extraction and harvesting lasing from QDCLC are generally not well controlled. The timing and parameter of the emitted lasing are difficult to manipulate, presenting a contemporary challenge. Indeed, while summarizing our results for this work, it has come to our attention that a step toward this goal was made, where directional lasing from QDs was demonstrated by embedding CLC cells. The QDCLC lasers are available in a wide range of periodic helicoidally and selective reflection due to the chiral dopant. This combination of the QDCLC offers the potential for devices with low cost, highly thermal stability and high tunability. Another interesting section in this regard is to understand the physical mechanism of the confinement of QDs induced lasing emission from the bandedge of the CLC.
CZTS QDs were successfully synthesized by a novel soft-chemical solvothermal method with ethylenediamine template (see the Experimental section). Fig. 1 shows the (a) TEM image and (b) XRD patterns (JCPDS 26-0575) of the kesterite quaternary Cu2ZnSnS4 QDs prepared in ethylenediamine for 2 h. The corresponding QDs size distribution histograms indicate that these particles have diameters ranging from 3 to 5 nm are shown in Fig. 1 inset. The absorption and fluorescence emission spectra of the QDs solved in a toluene solution with a molar concentration of ∼0.2 mmol are pre-measured using a UV-visible spectrometer (U-4100, HITACHI) in Fig. S2.† Fig. 2(a) shows the measured lasing spectrum at E = 40 μJ per pulse and reflection spectrum in the sample, represented by the red and black curves, respectively. A sharp lasing emission with a narrow bandwidth of ∼1.5 nm occurs at λ = 580 nm, which is coincidentally located at the short wavelength edge (SWE) of the CLC stop band. If the CLC in the cell is heated to isotropic phase, no lasing emission can be detected from the cell, as stated by the blue curve. To understand this lasing formation, we make a spectral comparison of the CZTS QD embed CLC and isotropic phase in Fig. 2(a). Here, the lasing peak emission of the QDs doped in the CLC and isotropic phase overlaps with the wavelength edge region of the CLC stop band. As the sample is cooled and the CLC phase forms, we observe that the lasing emission overlaps with the short wavelength edge of the CLC stop band.
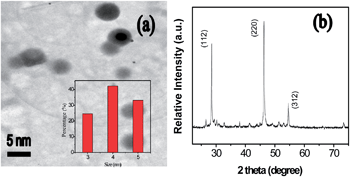 | ||
| Fig. 1 TEM images of (a) and (b) XRD patterns of as-synthesized quaternary Cu2ZnSnS4 quantum dots by the solvothermal process for 2 h. Inset shows the corresponding dots size distribution histogram. | ||
When there is an overlap, the lasing emission occurred at the band edges of the CLC, but when there is no overlap there is no any lasing peak. This is contributed by the band-edge effect. At the edges of the photonic band gap, the DOS and photon dwell time are significantly enhanced because of the multiple internal reflections of the CLC. Therefore, when the band edge is within the absorption spectrum of the quantum dots, the excitation of the semiconductor is dramatically enhanced, which means much more electron are elevated to the excited state by absorption of the pumping photons. As a result, the laser efficiency is dramatically enhanced. Fig. S3† shows the lasing spectra of the QDCLC samples with various concentrations of quantum dots. We can see from this figure that lasing intensity increases with quantum dots concentrations increasing (ESI Fig. S4†).
We investigated the wavelength band edge lasing for right-handed circularly polarized (RCP) or left-handed circularly polarized (LCP) incident light in Fig. 2(b). The linear polarized light beam was converted into RCP and LCP light by a half-wave plate and was focused by a lens onto the sample. The spot diameter on the sample was measured to be about 100 μm. The left-handed band edge lasing has been demonstrated by RCP and LCP incident light. This is because a circularly polarized light in the opposite handedness to the CLC helix could be to pass through with the CLC medium. Note that the lasing emission intensity is significantly decreased for the RCP excitation beam. Moreover, the emission intensity for the RCP excitation beam is around 8 times smaller than that for the LCP one. This is contributed by the above-mentioned the reflection induced by interference effects.36 From the result, the circularly polarized excitation is remarkably effective to increase the lasing efficiency.
Fig. S5† further demonstrates the variation of the fluorescence emission spectrum of the QDCLC with increasing the pumped energy. Manifestly, the strength of the fluorescence output increases with increasing the pumped energy. An energy threshold exists at around Eth = 40 μJ per pulse such that the fluorescence emission strength abruptly enhances and the FWHM decreases sharply as the pumped energy just exceeds the Eth. These observations clearly signify the occurrence of the QDCLC bandedge laser. Fig. 3(a) shows the photoluminescence (PL) spectra for QDCLC with different temperature. As can be seen, the continuous blue shift of the Bragg peak was observed with increasing temperature.37 The PL toward the shorter wavelength region as the temperature increases, which is attributed to the temperature dependence of the helical pitch length of the CLC. At 25.0 °C, the band edge lasing occur at the wavelength edge of the QDCLC, as Fig. 3(b) shows. When the temperature is increased to 70 °C, the PL is shifted spectral range from 580 to 568 nm. For the resulting, the spectrum position gradually decreases with the blue-shift of the band edge. This stems from the emission spectrum of the cholesteric liquid crystal medium, which determines the phase has been transferred from CLC to isotropic phase.
 | ||
| Fig. 3 (a) Temperature-dependent PL spectra of QDCLC cell. (b) The lasing intensity of the QDCLC sample with various temperature conditions. | ||
The optical properties of the QD doped cholesteric systems can be controlled by utilizing temperature when the pump energy is 40 μJ per pulse. The quantum dots used in this experiment exhibits its maximum spontaneous emission at λ = 580 nm. Therefore, the lasing intensity reaches the lowest when the temperature occurs at 70 °C and then gradually disappear when the temperature achieve above 70 °C. For all the samples, independent of the temperature, the peak intensity decreases with increasing temperature.38 This behaviour can be attributed to the effect of the cholesteric liquid crystal structure and electron–photon interaction. As a result, higher optical gain is achieved in the sample at 25 °C than that at 70 °C. Consequently, higher laser efficiency is obtained when the sample temperature is controlled at 25 °C. The first observations we make are an overall blue shift of the lasing emission and an increase in intensity when the sample is cooled into the cholesteric phase.
Conclusions
We have demonstrated a thermally controlled lasing emission in a cholesterics doped with CZTS QDs. This kind of the semiconductor materials could be further optimized in order to improve their performances. The lasing is evident as a small full width at half maximum of 1.5 nm, an abrupt increase of emitted intensity with the increase of pump energy. We have provides available clues to design and develop the semiconductor embedded in the soft matter for the bandedge induce laser action. In our first demonstration of light amplification in semiconductor combine photonic crystals. Also, future work will address the issue of how to reduce the threshold pumping energy for laser action with QDCLC cells and changing the quantum dots could be possible to have emission in different ranges of the optical spectrum.Acknowledgements
Present study has been supported in by Top 100 University Advancement and the collaboration of Center for Micro/Nano Science and Technology of National Cheng Kung University.Notes and references
- A. K. Viswanath, M. B. Krogh-Jespersen, J. Vetuskey, C. Baker, W. D. Ellenson and H. H. Patterson, Mol. Phys., 1981, 42, 1431 CrossRef CAS.
- A. K. Viswanath, J. Vetuskey, R. Leighton, M. B. Krogh-Jespersen and H. H. Patterson, Mol. Phys., 1983, 48, 567 CrossRef.
- W. D. Ellenson, A. K. Viswanath and H. H. Patterson, Inorg. Chem., 1981, 20, 780 CrossRef CAS.
- A. K. Viwanath and H. H. Patterson, Chem. Phys. Lett., 1981, 82, 25 CrossRef.
- A. P. Alivisatos, Science, 1996, 271, 933 CAS.
- L. E. Brus, J. Chem. Phys., 1984, 80, 4403 CrossRef CAS PubMed.
- D. Englund, D. Fattal, E. Waks, G. Solomon, B. Zhang, T. Nakaoka, Y. Arakawa, Y. Yamamoto and J. Vuckovic, Phys. Rev. Lett., 2005, 95, 013904 CrossRef.
- P. Lodahl, A. F. van Driel, I. S. Nikolaev, A. Irman, K. Overgaag, D. Vanmaekelbergh and W. L. Vos, Nature, 2004, 430, 654 CrossRef CAS PubMed.
- I. Fushman, D. Englund and J. Vuckovic, Appl. Phys. Lett., 2005, 87, 241102 CrossRef PubMed.
- A. K. Viswanath, J. Nanosci. Nanotechnol., 2014, 14, 1253 CrossRef CAS PubMed.
- L. J. Chen and Y. J. Chuang, J. Power Sources, 2013, 241, 259 CrossRef CAS PubMed.
- J. Wang, X. Xin and Z. Lin, Nanoscale, 2011, 3, 3040 RSC.
- L. J. Chen and Y. J. Chuang, J. Cryst. Growth, 2013, 376, 11 CrossRef CAS PubMed.
- J. Chen, W. Lei and W. Q. Deng, Nanoscale, 2011, 3, 674 RSC.
- M. A. Foster, A. C. Turner, M. Lipson and A. L. Gaeta, Opt. Express, 2008, 16, 1300 CrossRef.
- L. J. Chen, Mater. Lett., 2013, 101, 83 CrossRef CAS PubMed.
- R. Zhang, H. Yu and B. Li, Nanoscale, 2012, 4, 5856 RSC.
- A. L. Rodarte, C. Gray, L. S. Hirst and S. Ghosh, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 85, 035430 CrossRef.
- V. I. Kopp, Z. Q. Zhang and A. Z. Genack, Phys. Rev. Lett., 2001, 86, 1753 CrossRef CAS.
- J. Hwang, M. H. Song, B. Park, S. Nishimura, T. Toyooka, J. W. Wu, Y. Takanishi, K. Ishikawa and H. Takezoe, Nat. Mater., 2005, 4, 383 CrossRef CAS PubMed.
- J. P. Dowling, M. Scalora, M. J. Bloemer and C. M. Bowden, J. Appl. Phys., 1994, 75, 1896 CrossRef CAS PubMed.
- Y. Huang, Y. Zhou, C. Doyle and S. T. Wu, Opt. Express, 2006, 14, 1236 CrossRef CAS.
- J. Schmidtke, S. Kniesel and H. Finkelmann, Macromolecules, 2005, 38, 1357 CrossRef CAS.
- V. I. Kopp, B. Fan, H. K. M. Vithana and A. Z. Genack, Opt. Lett., 1998, 21, 1707 CrossRef.
- H. Coles and S. Morris, Nat. Photon., 2010, 4, 676 CrossRef CAS.
- M. Y. Jeong, H. Choi and J. W. Wu, Appl. Phys. Lett., 2008, 92, 051108–051110 CrossRef PubMed.
- G. Petriashvili, M. A. Matranga, M. P. De Santo, G. Chilaya and R. Barberi, Opt. Express, 2009, 17, 4553 CrossRef CAS.
- S. Murai, K. Fujita, T. Hirao, K. Nakanishi and K. Hirao, Opt. Mater., 2007, 9, 949 CrossRef PubMed.
- Q. Song, S. Xiao, X. Zhou, L. Liu, L. Xu, Y. Wu and Z. Wang, Opt. Lett., 2007, 32, 373 CrossRef.
- J. M. Winkler, S. G. Lukishova and L. J. Bissell, J. Phys.: Conf. Ser., 2013, 414, 012006 CrossRef.
- V. I. Kopp, B. Fan, H. K. M. Vithana and A. Z. Genack, Opt. Lett., 1998, 23, 1707 CrossRef CAS.
- R. Bose, X. Yang, R. Chatterjee, J. Gao and C. W. Wong, Appl. Phys. Lett., 2007, 90, 111117 CrossRef PubMed.
- C. J. Barrelet, J. Bao, M. Lončar, H. G. Park, F. Capasso and C. M. Lieber, Nano Lett., 2006, 6, 11 CrossRef CAS PubMed.
- A. L. Rodarte, G. V. Shcherbatyuk, L. Shcherbatyuk, L. S. Hirst and S. Ghosh, RSC Adv., 2012, 2, 12759 RSC.
- J. Mirzaei, M. Reznikov and T. Hegmann, J. Mater. Chem., 2012, 22, 22350 RSC.
- H. K. Lee, K. Doi, H. Harada, O. Tsutsumi, A. Kanazawa, T. Shiono and T. Ikeda, J. Phys. Chem. B, 2000, 104, 7023 CrossRef CAS.
- G. Wu, Y. Jiang, D. Xu, H. Tang, X. Liang and G. Li, Langmuir, 2011, 27(4), 1505 CrossRef CAS PubMed.
- X. Tong, J. W. Chung, S. Y. Park and Y. Zhao, Langmuir, 2009, 25(15), 8532 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental setup, PL spectra, polarizing microscopy image, lasing spectra, experimental section. See DOI: 10.1039/c4ra01361a |
| This journal is © The Royal Society of Chemistry 2014 |

