Hexagonal phase β-NaGdF4:Yb3+/Er3+ thin films with upconversion emission grown by electrodeposition
Linlin Tian,
Ping Wang,
Hui Wang and
Run Liu*
Department of Chemistry, Zhejiang University, Hangzhou, Zhejiang 310027, China. E-mail: runliu@zju.edu.cn; Tel: +86 571 87953390
First published on 23rd April 2014
Abstract
Hexagonal phase β-NaGdF4:Yb3+/Er3+ thin films composed of hexagonal prism structures were obtained from aqueous solution at low temperature by a facile electrodeposition. The annealed electrodeposited films exhibit intense green and red emission excited by a 980 nm laser and a two photon upconversion mechanism is involved.
Upconversion (UC) phosphors,1 which can convert two or more low energy photons to one higher energy photon, have attracted great attention due to their numerous potential applications, such as color 3D displays,2 solid-state lasers,3 bioimaging,4,5 and solar cells that capture sub-bandgap solar radiation.6–8 Among upconversion materials, fluorides doped with lanthanide ions are regarded as the most efficient phosphor materials due to their low phonon energy which can reduce the non-radiation transition and then result in long lifetimes of their excited states and high luminescence quantum yields even in the case of IR emitting ions.9
The fluorides with composition MGdF4, where M = Li, Na, or K, are of particular interest, since MGdF4 materials doped with Eu3+ ions can display down conversion emission under deep UV excitation with an internal quantum efficiency of 190%.10,11 Normally, NaReF4 with less symmetric hexagonal phase exhibits a higher upconversion luminescence efficiency than that of the corresponding more symmetric cubic phase.12
Recently, the synthesis of highly efficient NaReF4 nanocrystals doped with lanthanide ions through solution procedures has been well developed.5,13–17 However, for photonic applications, the upconversion materials have to be made with thin films or be embedded into a transparent matrix. By far, the most commonly reported upconversion thin films are produced by sol–gel techniques18 or embedded the upconversion nanocrystals into polymer matrix.19–21 These methods normally require organic polymer matrix which is difficult to avert degradation if exposed to intense short wavelength radiation and heat. Pulsed laser deposition has also been used to successfully produce upconversion thin films,22 however, it is a relatively expensive and complex technique.
In this work, we introduce a facile anodic electrodeposition process which can obtain high quality hexagonal phase β-NaGdF4:Yb3+/Er3+ thin films with a visible strong upconversion luminescence from aqueous solutions at low temperature. The advantages of electrodeposition compared with other techniques include low process temperature, low cost, and capability of controlling grain size, orientation of the deposited films.23–26
The electrochemical deposition of NaGdF4:Yb3+/Er3+ films was conducted in a solution containing 7.9 mM Gd(NO3)3, 2.0 mM Yb(NO3)3, 0.1 mM Er(NO3)3, and 10 mM EDTA. The solution containing 100 mM NaF and 100 mM sodium ascorbates was added into beaker after the forming of stable Re–EDTA complex and the final pH was adjusted to 7.00 using 0.01 M NaOH. The solution was then transferred into a four-neck flask. The deposition was performed in a three-electrode cell with a platinum (Pt) plate as counter electrode, tin doped indium oxide (ITO) glass as working electrode and Ag/AgCl electrode as reference electrode. The films were deposited at temperatures from 50 °C to reflux. The temperature was maintained with a heating mantle and monitored by a thermometer. A condenser was attached to one of the necks of the flask to prevent electrolyte losses due to evaporation. All potential values reported in this study were against Ag/AgCl reference electrode unless otherwise stated. Before the deposition, the ITO electrodes were cleaned with acetone in an ultrasonic bath for 15 min and then rinsed with distilled water. The deposition was carried out on CHI 650D Electrochemical Workstation (Shanghai, China) using anode potential with 0.8 V vs. Ag/AgCl for 2 h without stirring, and the total passed charge is about 5 C. The electrodeposited films were further annealed in air at 300 °C for 2 hours.
X-ray diffraction (XRD) measurements were carried out with a D/max-2550/PC diffractometer (Rigaku) using CuKα radiation at step of 0.02 degree per second. The surface morphology of the electrodeposited films was observed by a Hitachi SU-70 scanning electron microscope (SEM) with incident electron energy of 25 kV. In order to increase conductivity of films, gold powders were sputtered on the surface of the films before the measurement. The photo luminescent (PL) spectra of the films were recorded on Edinburgh FLS920 luminescence spectrophotometer equipped with a 980 nm laser as the excitation source.
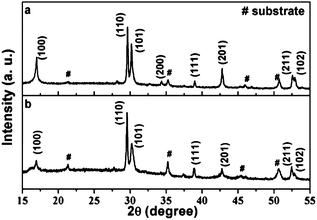
Fig. 1 shows the X-ray diffraction results of as prepared NaGdF4 thin films containing 1% mol Er3+ and 20% mol Yb3+ and the same film annealed at 300 °C for two hours. As shown in Fig. 1, the XRD pattern is in accordance with literature data for the hexagonal phase β-NaGdF4 (JCPDS: 27-0699), besides the XRD pattern of ITO substrate signed out, there is no other impure phase observed. The sharp and strong peaks indicate that the as prepared and annealed NaGdF4:Yb3+/Er3+ thin films are well crystallized. There are also a little [110] preferred orientation of the electrodeposited NaGdF4:Yb3+/Er3+ films.
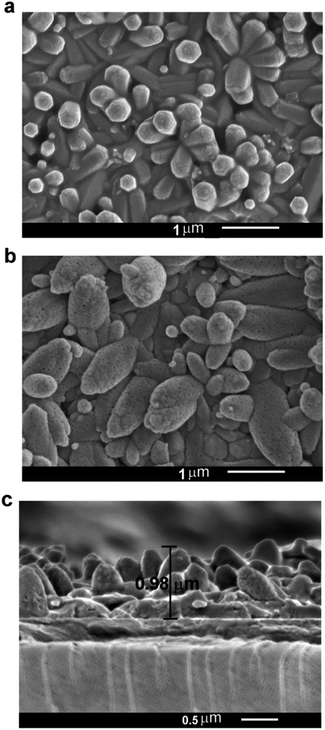
The morphology of the as prepared and annealed NaGdF4:Yb3+/Er3+ thin film were observed by SEM. Fig. 2a shows that the as obtained β-NaGdF4:Yb3+/Er3+ films are composed of interlaced hexagonal prism structures with size of the 1.5 μm in length and 200 nm in diameter and they adhere to each other to form dense film. After annealing, the morphology of the film changes from interlaced hexagonal prism structures to dense short shuttle like structures and there are some pores on top of the crystals which might come from the evaporation of water (Fig. 2b). The cross-section image of the film (Fig. 2c) shows that the thickness of the film is about 1 μm and also consists of columns features.
The electrodeposition of NaGdF4:Yb3+/Er3+ films conducted in an aqueous solution containing lanthanide ions–EDTA complexes and sodium ascorbates. It is been reported that stability of metal ion–EDTA complex was decreased by acid generated through anodic oxidation of water.27 Under the current applied anode potentials, sodium ascorbate was first oxidized to generate H+ ions instead of water, which is beneficial to cut down the oxidation potential.28 The released H+ ions will lower the pH at the surface of the ITO working electrode where the lanthanide ions are released from lanthanide ions–EDTA complex and then react with Na+ and F− ions to form NaGdF4:Yb3+/Er3+ deposits. The possible reaction mechanism of anodic electrodeposition of mechanism of anodic electrodeposition of NaGdF4:Yb3+/Er3+ can be explained by the following expressions:
| Re3+ + EDTA → Re3+–EDTA complex | (1) |
| C6H7O6− → C6H6O6 + H+ + 2e | (2) |
| Re3+–EDTA complex + xH+ → HxEDTAx−4 + Re3+ | (3) |
| Na+ + 4F− + Re3+ → NaReF4 | (4) |
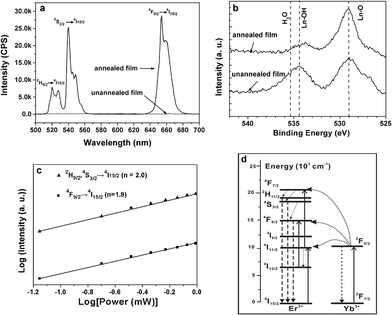
The upconversion luminescence spectra of as electrodeposited and annealed NaGdF4:Yb3+/Er3+ films under excitation by 980 nm laser were investigated. As is shown in Fig. 3a, there is little emission from as electrodeposited films. The emission might be annihilated by absorbed water or O2 on the surface of the NaGdF4:Yb3+/Er3+ crystals, as shown in Fig. 3b. The similar results have also been observed in other works.29 The absolute quantum efficiency of the film is measured by the method developed by Zhang and Zhao30 and is about 0.0055 ± 0.0001%. The improvement of the absolute quantum efficiency of the films is under working. For the annealed films, there are strong emission peaks at 524, 539 and 654 nm. The emission band at around 524 nm is corresponding to the 2H9/2 → 4I15/2 transition, the intense green emission peak at around 539 nm accounts for the 4S3/2 → 4I15/2 transition and the red luminescence centered at 654 nm is ascribed to the 4F9/2 → 4I15/2 transition of Er3+, respectively. In order to investigate the upconversion mechanism of NaGdF4:Yb3+/Er3+, the intensities of the upconversion emissions were recorded as a function of the 980 nm laser excitation intensity. For an unsaturated upconversion process, the emission intensity (I) will be proportional to power (n) of laser power (P):
| I ∝ Pn | (5) |
Therefore, the energy level diagrams of Er3+ and Yb3+ ions as well as the upconversion mechanism are presented in Fig. 3d. Firstly, Yb3+ ion is excited to 2F2/5 state by absorbing a near-infrared photon, and then energy transfer occurred from Yb3+ ion to Er3+ ion populating the 4I11/2 level. Besides, Er3+ ion can also absorb a 980 nm photon directly from the laser. Another energy transfer between Yb3+ and Er3+ can populate the 4F7/2 level of the Er3+ ion. Then Er3+ ion relax non-radiatively to the 2H11/2 and 4S3/2 levels. When 2H11/2 → 4I15/2 and 4S3/2 → 4I15/2 transitions of Er3+ ions occur, bright green light can be observed. As for red light emission, it comes from 4F9/2 → 4I15/2 transition. There are two pathways for the population of 4F9/2 level. One is the relaxation of 2F7/2 and the other is excitation from 4I13/2 by the energy transfer of Yb3+ or absorption of a 980 nm photon.
In summary, we introduce a facile anodic electrodeposition method to grow hexagonal phase NaGdF4:Yb3+/Er3+ thin films that exhibit intense green and red emissions excited by a 980 nm laser. It is expected that the NaGdF4:Yb3+/Er3+ thin films on transparent ITO substrate will be suitable for applications in optical display devices and solar cells. The method we outline here should work for growing other lanthanide ions doped fluoride films with up/down conversion luminescence.
Acknowledgements
This work was financially supported by the National Basic Research Program of China (Grant no. 2011CB936003), National Natural Science Foundation of China (21373183), and Zhejiang Provincial Natural Science Foundation of China (LY12B07001).Notes and references
- F. Auzel, Chem. Rev., 2004, 104, 139 CrossRef CAS PubMed.
- E. Downing, L. Hesselink, J. Ralston and R. Macfarlane, Science, 1996, 273, 1185 CAS.
- T. Sandrock, H. Scheife, E. Heumann and G. Huber, Opt. Lett., 1997, 22, 808 CrossRef CAS.
- B. E. Cohen, Nature, 2010, 467, 407 CrossRef CAS PubMed.
- G. Wang, Q. Peng and Y. Li, Acc. Chem. Res., 2011, 44, 322 CrossRef CAS PubMed.
- A. Shalav, B. S. Richards, T. Trupke, K. W. Krämer and H. U. Güdel, Appl. Phys. Lett., 2005, 86, 013505 CrossRef PubMed.
- T. Trupke, A. Shalav, B. S. Richards, P. Wurfel and M. A. Green, Sol. Energy Mater. Sol. Cells, 2005, 90, 3327 CrossRef PubMed.
- B. M. van der Ende, L. Aarts and A. Meijerink, Phys. Chem. Chem. Phys., 2009, 11, 11081 RSC.
- N. Menyuk, K. Dwight and J. W. Pierce, Appl. Phys. Lett., 1972, 21, 159 CrossRef CAS PubMed.
- R. T. Wegh, H. Donker, K. D. Oskam and A. Meijerink, Science, 1999, 283, 663 CrossRef CAS.
- P. Gosh, S. Tang and A.-V. Mudring, J. Mater. Chem., 2011, 21, 8640 RSC.
- K. W. Krämer, D. Biner, G. Frei, H. U. Güdel, M. P. Hehlen and S. R. Lüthi, Chem. Mater., 2004, 16, 1244 CrossRef.
- M. Haase and H. Schäfer, Angew. Chem., Int. Ed., 2011, 50, 5808 CrossRef CAS PubMed.
- S. Heer, K. Kömpe, M. Haase and H. U. Güdel, Adv. Mater., 2004, 16, 2102 CrossRef CAS.
- G. Yi, H. Lu, S. Zhao, Y. Ge, W. Yang, D. Chen and L.-H. Guo, Nano Lett., 2004, 4, 2191 CrossRef CAS.
- H. X. Mai, Y. W. Zhang, L. D. Sun and C. H. Yan, J. Phys. Chem. C, 2007, 111, 13721 CAS.
- F. Wang, Y. Han, C. S. Lim, Y. Lu, J. Wang, J. Xu, H. Chen, C. Zhang, M. Hong and X. Liu, Nature, 2010, 463, 1061 CrossRef CAS PubMed.
- S. Sivakumar, F. C. J. M. van Veggel and M. J. Raudsepp, J. Am. Chem. Soc., 2005, 127, 12464 CrossRef CAS PubMed.
- C. Lin, M. T. Berry, R. Anderson, S. Smith and P. S. May, Chem. Mater., 2009, 21, 3406 CrossRef CAS.
- J. C. Boyer, N. J. J. Johnson and F. C. J. M. van Veggel, Chem. Mater., 2009, 21, 2010 CrossRef CAS.
- R. Chai, H. Lian, Z. Hou, C. Zhang, C. Peng and J. Lin, J. Phys. Chem. C, 2010, 114, 610 CAS.
- D. M. Bubb, D. Cohen and S. B. Qadri, Appl. Phys. Lett., 2005, 87, 131909 CrossRef PubMed.
- J. A. Switzer and G. Hodes, MRS Bull., 2010, 35, 743 CAS.
- M. J. Siegfried and K.-S. Choi, Angew. Chem., Int. Ed., 2005, 44, 3218 CrossRef CAS PubMed.
- T. Yoshida and H. Minoura, Adv. Mater., 2000, 16, 1219 CrossRef.
- D. Lincot, Thin Solid Films, 2005, 487, 40 CrossRef CAS PubMed.
- M. Dinamani, P. V. Kamath and R. Seshadri, Chem. Mater., 2001, 13, 3981 CrossRef CAS.
- S. J. Limmer, E. A. Kulp and J. A. Switzer, Langmuir, 2006, 22, 10535 CrossRef CAS PubMed.
- D. Raiser and J. P. Deville, J. Electron Spectrosc. Relat. Phenom., 1991, 57, 91 CrossRef CAS.
- X. Li, D. Shen, J. Yang, C. Yao, R. Che, F. Zhang and D. Y. Zhao, Chem. Mater., 2013, 25, 106–112 CrossRef CAS.
- M. Pollnau, D. R. Gamelin, S. R. Lüthi, H. U. Güdel and M. P. Hehlen, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 61, 3337 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |