Phosphorus-doped macroporous carbon spheres for high efficiency selective oxidation of cyclooctene by air†
Chuanxi Li,
Manman Yang,
Ruihua Liu,
Fangfang Zhao,
Hui Huang*,
Yang Liu* and
Zhenhui Kang
Institute of Functional Nano & Soft Materials (FUNSOM), Jiangsu Key Laboratory for Carbon-based Functional Materials and Devices, Collaborative Innovation Center of Suzhou Nano Science and Technology, Soochow University, Suzhou, Jiangsu 215123, China. E-mail: hhuang0618@suda.edu.cn; yangl@suda.edu.cn; Fax: +86 512-65882846; Tel: +86 512-65880957
First published on 29th April 2014
Abstract
The phosphorus-doped macroporous carbon spheres (PMCS) with a high specific surface area (574.68 m2 g−1) were synthesized by a template method. A series of catalytic experiments showed that the PMCS possessed excellent catalytic ability for the selective oxidation of cyclooctene (conversion based on cyclooctene was 50.69% and the selectivity to epoxycyclooctane was 88.47%).
Increasing industrial production demands have focused increased attention on the research for the oxidation of alkenes with high activity, environmental benignity and low cost. In the oxidation process, the selection of catalysts plays a vital role in the types of products, conversion efficiency and selectivity.1–3 Nanocatalysts, as a new generation catalyst, possess excellent catalytic abilities and great potential in practical applications. The design and preparation of nanocatalysts are significant in industry, energy and environmental problems in order to gain relatively high conversion and selectivity. Among the oxidation of alkenes, selective hydrocarbon oxidation is momentous in industrial production, especially for the selective oxidation of cyclooctene. Recently, numerous types of nanocatalysts have been used to improve the catalytic ability for the selective oxidation of cyclooctene, including Au/C,4 Au/graphite,5 mesoporous mixed oxides (Ga, Ga–Nb and Ga–Mo)6 and CuO/mesoporous SiO2 nanocomposites.2 In previous studies, our group has designed several nanocatalysts, such as Au/Cu nanoparticle-modified Si nanowires, Au/SiOx composite thin films, cobalt-based 3D porous framework and carbon quantum dots/SiO2 porous nanocomposites.7–10 These nanocatalysts enriched and elevated the catalytic activity for the selective oxidation of cyclooctene. Despite tremendous efforts being made to design effective nanocatalysts, seeking high activity and low cost nanocatalysts remains a great challenge.
Carbon materials, especially porous ones, have gained widespread acceptance due to their low price, low toxicity, long-term stability, periodic ordered structure and well-developed preparation methods, which are extensively applied in supercapacitors,11 electrocatalysts,12,13 selective methylation14 and gas adsorption.15 It should be noted that these carbon materials with unique porous structure and relatively high specific surface area make the reaction reagents distribute evenly and efficiently and reduce the resistance of diffusion during reactions, which could be a kind of effective nanocatalyst in the selective oxidation of cyclooctene.16 Nowadays, doping different kinds of heteroatoms into the carbon framework has been aggressively studied for developing novel carbon materials because the introduction of heteroatoms changes parts of the carbon framework, improving the electrical, physical and chemical properties of primitive carbon.17 Phosphorus is a conceivable element for the doping of carbon materials as it contains five valence electrons that form strong valence bonds with the ambient carbon atoms.18,19 Due to the high electron-donating ability of phosphorus, it is necessary to explore the distinctive property of phosphorus-doped carbon materials.20 Phosphorus-doped macroporous carbon spheres, as a kind of non-metal nanocatalyst, have become a promising candidate for the catalysis of hydrocarbon selective oxidation on account of their unique porous structure and phosphorus doping.
Herein, we report that phosphorus-doped macroporous carbon spheres (PMCS) with high specific surface area (574.68 m2 g−1) have been synthesized by a typical template method using phytic acid as the precursors of carbon and phosphorus. Owing to the unique porous structure and phosphorus doping, a series of catalytic experiments confirmed that the PMCS showed excellent catalytic ability for the selective oxidation of cyclooctene (conversion based on cyclooctene was 50.69% and the selectivity to epoxycyclooctane was 88.47%) under mild conditions.
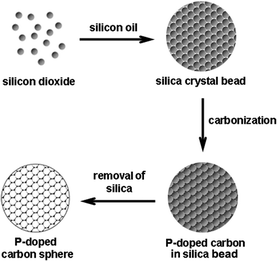
In a typical experiment, the PMCS were prepared by a typical template method. Firstly, the silica beads were synthesized by stirring the colloidal silica crystals. Fig. S1† shows the SEM images of monodispersed silica particles and silica crystal beads. Then, appropriate phytic acid was injected into the pore space of silica templates. Finally, after the carbonization of phytic acid under nitrogen protection, the silica templates were removed by hydrofluoric acid; consequently, the PMCS formed (detailed experiment shown in ESI†). The schematic diagram of the preparation steps for PMCS is shown in Scheme 1.
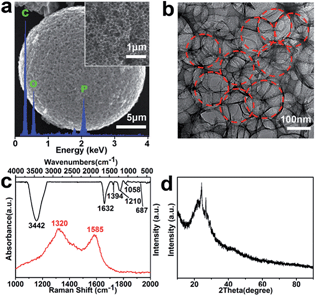
Fig. 1(a) shows the typical scanning electron microscopy (SEM) image of the prepared PMCS, from which the spherical structure is distinctly visible with a diameter of about 20 μm. The inset in the top right corner shows the enlarged SEM image of the PMCS, and the single pore diameter of PMCS is about 150 nm. The pores among the PMCS are uniform in size, nearly spherical and interconnected closely. The energy dispersive X-ray spectroscopy (EDS) spectrum in Fig. 1(a) shows that the PMCS only consist of C, O and P elements. The transmission electron microscopy (TEM) image of the PMCS in Fig. 1(b) indicates that the pores among the PMCS (red dotted circles) are layered upon layers and orderly arraying, which further confirms the macroporous structure of the PMCS. Fig. 1(c) shows the Fourier transform infrared (FT-IR) and Raman spectra of the PMCS. In the FT-IR spectrum (black line), the peaks at 3442 and 1632 cm−1 correspond with the O–H stretching and bending vibrations, respectively. The peaks at 1394 and 1058 cm−1 show the asymmetric and symmetric stretching vibrations of C–O–C bond,21,22 while the peaks at 1210 and 687 cm−1 can be attributed to P–O and P–C stretching vibrations, respectively.23,24 In the Raman spectrum (red line), two characteristic peaks located at 1320 and 1585 cm−1 are observed, which represent the D-band and G-band of carbon, respectively.25 Fig. 1(d) shows the X-ray diffraction (XRD) patterns of the PMCS. The typical peak at 26° indicates that the PMCS are made up of carbon.26
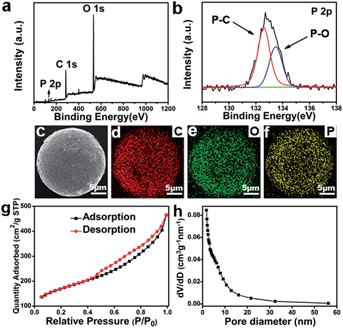
The successful doping of phosphorus into macroporous carbon spheres was further verified by the X-ray photoelectron spectroscopy (XPS) measurements and EDS mapping. The full XPS survey spectrum in Fig. 2(a) shows a peak at 284.6 eV corresponding to C 1s; a peak at 532.4 eV, to O 1s; and a peak at 133.2 eV, to P 2p.19 The high-resolution P 2p XPS spectrum in Fig. 2(b) reveals that phosphorus was doped into the macroporous carbon spheres in two bonding types: P–O bonding at 133.6 eV and P–C bonding at 132.5 eV, respectively.20 The value of 133.6 eV is generally known as the oxidation state of P.27,28 The peak at 132.5 eV has been discussed by other research groups,20,29,30 and the accepted understanding is that the peak is identified as P–C bonding.31,32 The EDS mapping of the PMCS was also carried out, and the results are shown in Fig. 2(c)–(f). The red, green, and yellow hues represent the distribution of C, O and P elements, respectively; evidently, element P is uniformly distributed in PMCS. These results confirm that phosphorus has been successfully doped in PMCS.
To study the macroporous characteristics of PMCS, the nitrogen adsorption–desorption measurements were carried out. The obtained curve in Fig. 2(g) shows the nitrogen adsorption–desorption isotherm of PMCS, which exhibits a typical type-IV isotherm with an H1 hysteresis loop. This adsorption behaviour is generally attributed to the capillary condensation of N2 in the pores.33 The specific surface area of PMCS is 574.68 m2 g−1 obtained from the nitrogen BET (Brunauer–Emmett–Teller) adsorption measurements, indicating that the PMCS have a relatively large specific surface area. This porous characteristic of PMCS may further improve the activity of catalytic reaction. Fig. 2(h) shows the pore size distributions of PMCS, revealing a typical curve of macroporous structure with a characteristic of multiple pore diameters.
The catalytic ability of PMCS over the selective oxidation of cyclooctene was investigated with tert-butyl hydroperoxide (TBHP) as a radical initiator and oxygen in air as the oxidant at 353 K. The conversion of cyclooctene and selectivity to oxidation products were measured by gas chromatography measurements (GC-MS). The detailed conversions of cyclooctene and selectivity to oxidation products by using PMCS as catalyst are shown in Table S1.† Fig. 3 reveals the relationship between the conversion efficiency of cyclooctene and selectivity to oxidation products with reaction time by using PMCS as catalyst. As shown in Fig. 3, the conversion of cyclooctene reached 50.69%, while the selectivities to epoxycyclooctane and 2-cyclooctenone were 88.47% and 8.37%, respectively, after 48 h. The selectivity to epoxycyclooctane increased from 65.57% after 6 h to 88.47% after 48 h while the selectivity to 2-cyclooctenone decreased from 32.60% after 6 h to 8.37% after 48 h. The results indicate that the conversion efficiency of cyclooctene and the selectivity to epoxycyclooctane increased, while the selectivity to 2-cyclooctenone decreased simultaneously. From the experimental results, we can conclude that by controlling the reaction time, we can obtain different kinds of oxidation products.
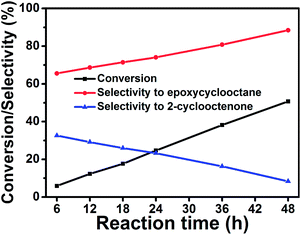 | ||
| Fig. 3 The relationship between the conversion of cyclooctene/selectivity to two oxidation products and reaction time with PMCS as the catalyst. | ||
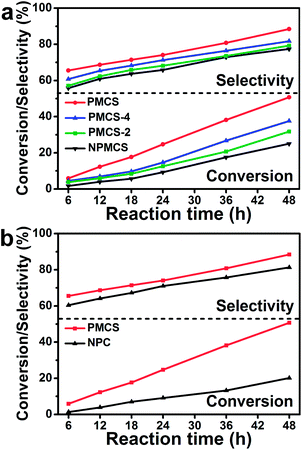
In comparison, the macroporous carbon spheres with different contents of phosphorus and the maximum phosphorus-doped (6%) carbon materials with different structures were also synthesized (detailed synthesis processes shown in ESI†). The different contents of phosphorus were 0%, 2%, 4%, and 6%, which were abbreviated to NPMCS, PMCS-2, PMCS-4 and PMCS, respectively. The maximum phosphorus-doped (6%) carbon material without a macroporous structure was abbreviated to NPC. The elemental analyses of all the samples were calculated by the EDS spectra (Fig. S2 and Table S2†). The FT-IR and Raman spectra of NPMCS, PMCS-4, PMCS-2 and NPC are shown in Fig. S3 and S4,† respectively. Both these spectra indicate that the basic chemical compositions (mainly carbon) of the four contrasts are in accordance with the PMCS. In the following catalytic experiments, the selective oxidation of cyclooctene was also carried out with NPMCS, PMCS-2, PMCS-4 and PMCS as catalysts to verify if the content of phosphorus had an influence on the catalytic activity. The detailed conversion efficiency of cyclooctene and selectivity to two oxidation products are shown in Table S3† and Fig. 4(a). As shown in Fig. 4(a), the conversions of cyclooctene using NPMCS, PMCS-2, PMCS-4 and PMCS as catalysts reached 25.01%, 31.73%, 37.56% and 50.69%, respectively, after 48 h. The highest selectivity to epoxycyclooctane was 88.47% obtained by using PMCS as a catalyst, while the lowest selectivity to epoxycyclooctane was 77.43% by using NPMCS as a catalyst. The macroporous carbon spheres containing lesser amounts of phosphorus (PMCS-2 and PMCS-4) showed an intermediate conversion efficiency and selectivity after 48 h. The conversions of cyclooctene and selectivity to epoxycyclooctane in all of the reactions increased with the reaction time. The results indicated that with an increase in the phosphorus content, both the conversion of cyclooctene and the selectivity to epoxycyclooctane increased gradually. The role of phosphorus should be attributed to the breaking of C–C bond by phosphorus doping and the formation of abundant defect-induced active sites among the macroporous structure,19,20 which accelerate the oxidation of cyclooctene.
To further confirm the influence of the macroporous structure on the catalytic activity, the selective oxidation of cyclooctene was also performed by using NPC and PMCS as the catalysts. The detailed conversion efficiency of cyclooctene and selectivity to two products are shown in Table S4† and Fig. 4(b). As shown in Fig. 4(b), the conversion efficiency of cyclooctene using NPC as the catalyst only reached 20.14% after 48 h, which was much lower than that of PMCS (50.69%). The selectivity to epoxycyclooctane was 81.31% after 48 h, which was lower than the selectivity by using PMCS (88.47%) as well. The porous structure among the carbon spheres is analogous to a kind of microreactor, which can form confinement effect and accelerate the diffusion of the reaction reagents and further enhance the conversion efficiency. The control experiments above revealed that the higher amount of phosphorus in PMCS and the unique porous structure both led to the optimal conversion of cyclooctene and higher selectivity to epoxycyclooctane.
In order to further compare the effects of catalysts above in the selective oxidation of cyclooctene, the detailed information is shown in Table 1. As shown in Table 1, the conversion efficiency of cyclooctene increased with the increase in the contents of phosphorus as well as the specific surface areas of the porous carbon spheres increasing gradually (1–4). The conversion efficiency increased from 25.01% with the specific surface area of 195.60 m2 g−1 to 50.69% with the specific surface area of 574.68 m2 g−1. The increasing amount of P (0 to 6%) resulted in a larger specific surface area of the catalysts (195.60 to 574.68 m2 g−1), which provided many active sites and had positive effects on the selective oxidation of cyclooctene. As shown in Table 1, the conversion of cyclooctene was 20.14% using NPC as the catalyst and the specific surface area was only 33.40 m2 g−1, while the conversion of cyclooctene was 50.69% using PMCS as the catalyst and the specific surface area reached up to 574.68 m2 g−1 (4 and 5). The results showed that the porous structure among the catalysts led to a large specific surface area, which improved the conversion efficiency of cyclooctene tremendously and manifested a confinement effect that may greatly decrease the resistance of the reaction reactants transfer among the porous structure so as to accelerate the catalytic process.16
| No. | Catalysts | COPa (wt%) | MPSb | SSAc (m2 g−1) | Conv. (%) | Σsel C8d |
|---|---|---|---|---|---|---|
| a Content of phosphorus was abbreviated as COP.b Macroporous structure was abbreviated as MPS.c Specific surface area was abbreviated as SSA.d Total selectivity to C8 partial oxidation products. | ||||||
| 1 | NPMCS | 0 | Yes | 195.60 | 25.01 | 93.62 |
| 2 | PMCS-2 | 2 | Yes | 267.45 | 31.73 | 94.03 |
| 3 | PMCS-4 | 4 | Yes | 395.66 | 37.56 | 95.90 |
| 4 | PMCS | 6 | Yes | 574.68 | 50.69 | 96.84 |
| 5 | NPC | 6 | No | 33.40 | 20.14 | 92.80 |
The doping of phosphorus resulted in the high specific surface area of PMCS, which gave numerous active sites including oxygen-containing groups on the surface as well as inside the pores. To further evaluate the effects of oxygen-containing groups on the catalytic reaction, we utilized hydrazine hydrate as the reductant to eliminate the oxygen-containing groups of PMCS in order to verify their catalytic effects. As shown in Table S5,† the conversion of cyclooctene decreased to 18.70% and selectivity to epoxycyclooctane was 80.14%. The results show that the oxygen-containing groups indeed have an effect on the catalytic activity of PMCS, which is consistent with previous reports.34–36
The blank reactions (without catalyst PMCS) did not result in obtaining any epoxide or other products (detailed conversion of cyclooctene and selectivity to oxidation products shown in Table S6†). Moreover, the filtrate solution test (additional 48 h reaction after removing the PMCS from the reaction system) was also performed, which showed that almost no conversion efficiency could be obtained after 48 h. The results indicated that leaching did not play an important role in the reaction system.10 The control experiments above showed that PMCS acted as a valid catalyst in the selective oxidation of cyclooctene.
To confirm the reactive oxygen species in the selective oxidation of cyclooctene, the reactive species trapping experiment was also performed. Here, the p-benzoquinone (BZQ, a radical scavenger) was used to verify that TBHP acted as a radical initiator as well as oxygen in air as the oxidant. In the experiment, the reaction conditions were the same without BZQ before 24 h, yielding a conversion efficiency of about 24% as before. After an appropriate amount of BZQ (0.02 g) was added into the reaction system, the conversion efficiency still stayed at 24%. The result indicated that the reactive oxygen species produced by TBHP was consumed by BZQ and the subsequent reaction using O2 as the oxidant stopped.37
The possible reasons for the enhanced activity and selectivity are as follows: the porous structure among the carbon spheres is analogous to a kind of microreactor, which can form a confinement effect and accelerate the diffusion of the reaction reagents and further enhance the conversion efficiency and selectivity.12–14 In addition, the XPS and FT-IR spectra results show that the P–O and P–C bonds form in PMCS, resulting in defect-induced active sites among PMCS and the excellent activity and selectivity.24,38 Furthermore, phosphorus doping results in high specific surface area of PMCS and gives many active sites including oxygen-containing groups among the pores, leading to the enhanced activity and selectivity. The plausible mechanism is that the tert-butyl hydroperoxide (TBHP) acts as a radical initiator, while the oxygen in air acts as the oxidant. During the reaction, the cyclooctene can diffuse evenly to both the surface as well as inside the porous spheres, which act as the microreactors. Then, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds in cyclooctene are directly oxidized by a peroxide species generated from TBHP and oxygen on the numerous active sites of the carbon spheres and the two products formed.4
C bonds in cyclooctene are directly oxidized by a peroxide species generated from TBHP and oxygen on the numerous active sites of the carbon spheres and the two products formed.4
In general, a good catalyst should be stable for practical applications. The test of the reusability of PMCS was performed for six times using the recycled catalyst. As shown in Fig. 5, PMCS were relatively stable for nearly a constant conversion and selectivity in six cycles during repeated experiments.
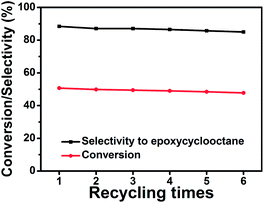 | ||
| Fig. 5 The relationship between the conversion of cyclooctene/selectivity to epoxycyclooctane and recycling times with PMCS as the catalyst. | ||
In our catalysis system, the experimental results and analysis above showed that a higher content of phosphorus contributed toward higher conversion efficiency and selectivity. Due to the phytic acid as the precursors of phosphorus, the highest content of phosphorus in PMCS was 6%. To acquire a higher content of phosphorus, it is necessary to search for a new kind of precursor, which may further elevate the conversion efficiency and selectivity.
Conclusions
We demonstrate a template method for the preparation of PMCS with a pore size of about 150 nm and high specific surface area of 574.68 m2 g−1. The PMCS exhibited high conversion efficiency of cyclooctene and selectivity to epoxycyclooctane with TBHP as a radical initiator and oxygen in air as the oxidant at 353 K. The conversion efficiency of cyclooctene came to 50.69% and the selectivity to epoxycyclooctane reached 88.47% after 48 h. The control experiments above revealed that the higher doped phosphorus and higher specific surface area in PMCS with the unique porous structure led to the optimal conversion of cyclooctene and higher selectivity to epoxycyclooctane. Our present work may be conducive to resolving the problems of selective oxidation of alkenes in industrial production.Acknowledgements
This work was supported by the National Basic Research Program of China (973 Program) (2012CB825800, 2013CB932702), the National Natural Science Foundation of China (51132006), the Specialized Research Fund for the Doctoral Program of Higher Education (20123201110018), a Suzhou Planning Project of Science and Technology (ZXG2012028), and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).Notes and references
- P. Lignier, S. Mangematin, F. Morfin, J. L. Rousset and V. Caps, Catal. Today, 2008, 138, 50 CrossRef CAS PubMed.
- C. Q. Chen, J. Qu, C. Y. Cao, F. Niu and W. G. Song, J. Mater. Chem., 2011, 21, 5774 RSC.
- H. Huang, H. C. Zhang, Z. Ma, Y. Liu, H. Ming, H. T. Li and Z. H. Kang, Nanoscale, 2012, 4, 4964 RSC.
- M. D. Hughes, Y. J. Xu, P. Jenkins, P. McMorn, P. Landon, D. I. Enache, A. F. Carley, G. A. Attard, G. J. Hutchings, F. King, E. H. Stitt, P. Johnston, K. Griffin and C. J. Kiely, Nature, 2005, 437, 1132 CrossRef CAS PubMed.
- S. Bawaked, N. F. Dummer, D. Bethell, D. W. Knight and G. J. Hutchings, Green Chem., 2011, 13, 127 RSC.
- C. A. Deshmane, J. B. Jasinski, P. Ratnasamy and M. A. Carreon, Catal. Commun., 2011, 15, 46 CrossRef CAS PubMed.
- C. H. A. Tsang, Y. Liu, Z. H. Kang, D. D. D. Ma, N. B. Wong and S. T. Lee, Chem. Commun., 2009, 5829 RSC.
- X. Han, H. Huang, H. C. Zhang, X. Zhang, H. T. Li, R. L. Liu, Y. Liu and Z. H. Kang, Mater. Res. Bull., 2013, 48, 3717 CrossRef CAS PubMed.
- X. Han, Y. Z. Han, H. Huang, H. C. Zhang, X. Zhang, R. H. Liu, Y. Liu and Z. H. Kang, Dalton Trans., 2013, 42, 10380 RSC.
- G. J. Zhang, H. Li, F. F. Zhao, H. L. Hu, H. Huang, H. T. Li, X. Han, R. H. Liu, H. Dong, Y. Liu and Z. H. Kang, Dalton Trans., 2013, 42, 9423 RSC.
- X. Y. Chen, C. Chen, Z. J. Zhang and D. H. Xie, J. Mater. Chem. A, 2013, 36, 10903 Search PubMed.
- X. J. Bo and L. P. Guo, Phys. Chem. Chem. Phys., 2013, 15, 2459 RSC.
- R. Silva, D. Voiry, M. Chhowalla and T. Asefa, J. Am. Chem. Soc., 2013, 135, 7823 CrossRef CAS PubMed.
- Y. J. Liu, Y. Q. Jiang, H. B. Yin, W. G. Chen, Y. T. Shen, Y. H. Feng, L. Q. Shen, A. L. Wang, T. S. Jiang and Z. A. Wu, Korean J. Chem. Eng., 2011, 28, 2250 CrossRef CAS PubMed.
- G. P. Hao, Z. Y. Jin, Q. Sun, X. Q. Zhang, J. T. Zhang and A. H. Lu, Energy Environ. Sci., 2013, 6, 3740 CAS.
- J. M. Zhang, Y. X. Zhang, S. Y. Lian, Y. Liu, Z. H. Kang and S. T. Lee, J. Colloid Interface Sci., 2011, 361, 503 CrossRef CAS PubMed.
- X. R. Wang, X. L. Li, L. Zhang, Y. Yoon, P. K. Weber, H. L. Wang, J. Guo and H. J. Dai, Science, 2009, 324, 768 CrossRef CAS PubMed.
- C. H. Choi and S. H. Park, J. Mater. Chem., 2012, 22, 12107 RSC.
- Z. W. Liu, F. Peng, H. J. Wang, H. Yu, W. X. Zheng and J. A. Yang, Angew. Chem., Int. Ed., 2011, 50, 3257 CrossRef PubMed.
- D. S. Yang, D. Bhattacharjya, S. Inamdar, J. Park and J. S. Yu, J. Am. Chem. Soc., 2012, 134, 16127 CrossRef CAS PubMed.
- X. W. Du, S. L. Hu, K. Y. Niu, J. Sun, J. Yang and N. Q. Zhao, J. Mater. Chem., 2009, 19, 484 RSC.
- H. Zhu, X. L. Wang, Y. L. Li, Z. J. Wang, F. Yang and X. R. Yang, Chem. Commun., 2009, 5118 RSC.
- C. Dayanand, G. Bhikshamaiah, V. J. Tyagaraju, M. Salagram and A. S. R. K. Murthy, J. Mater. Sci., 1996, 8, 1945 CrossRef.
- R. Li, Z. D. Wei, X. L. Gou and W. Xu, RSC Adv., 2013, 3, 9978 RSC.
- H. Ming, Z. Ma, Y. Liu, K. M. Pan, H. Yu, F. Wang and Z. H. Kang, Dalton Trans., 2012, 41, 9526 RSC.
- Z. H. Ma, T. Kyotani and A. Tomita, Chem. Commun., 2000, 2365 RSC.
- J. Gorham, J. Torres, G. Wolfe, A. dAgostino and D. H. Fairbrother, J. Phys. Chem. B, 2005, 109, 20379 CrossRef CAS PubMed.
- A. M. Puziy, O. I. Poddubnaya, R. P. Socha, J. Gulgul and M. Wisniewski, Carbon, 2008, 46, 2113 CrossRef CAS PubMed.
- D. S. Yu, Y. H. Xue and L. M. Dai, J. Phys. Chem. Lett., 2012, 3, 2863 CrossRef CAS.
- J. P. Paraknowitsch, Y. J. Zhang, B. Wienert and A. Thomas, Chem. Commun., 2013, 49, 1208 RSC.
- E. C. Silva, F. L. Urias, E. M. Sandoval and B. Sumpter, ACS Nano, 2009, 3, 1913 CrossRef PubMed.
- M. Sevilla and A. B. Fuertes, Carbon, 2006, 44, 468 CrossRef CAS PubMed.
- S. Kang, J. S. Yu, M. Kruk and M. Jaroniec, Chem. Commun., 2002, 1670 RSC.
- J. Zhang, D. S. Su, A. H. Zhang, D. Wang, R. Schlögl and C. Hébert, Angew. Chem., Int. Ed., 2007, 119, 7460 CrossRef.
- M. F. R. Pereira, J. J. Órfão and J. L. Figueiredo, Appl. Catal., A, 1999, 184, 153 CrossRef CAS.
- J. L. Figueiredo and M. F. R. Pereira, Catal. Today, 2010, 150, 2 CrossRef CAS PubMed.
- X. F. Yang, H. Y. Cui, Y. Li, J. L. Qin, R. X. Zhang and H. Tang, ACS Catal., 2013, 3, 363 CrossRef CAS.
- C. Z. Zhang, N. Mahmood, H. Yin, F. Liu and Y. L. Hou, Adv. Mater., 2013, 25, 4932 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Preparation of all catalysts, additional figures, FT-IR spectra and Raman spectra; detailed conversion and selectivity data. See DOI: 10.1039/c4ra01972e |
| This journal is © The Royal Society of Chemistry 2014 |