CVD graphene based immunosensor†
Adeline Huiling Loo,
Adriano Ambrosi,
Alessandra Bonanni* and
Martin Pumera*
Division of Chemistry & Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, 637371, Singapore. E-mail: a.bonanni@ntu.edu.sg; pumera@ntu.edu.sg; pumera.research@outlook.com
First published on 22nd May 2014
Abstract
Graphene synthesis by chemical vapour deposition (CVD) method has been receiving much attention from researchers. This is due to the fact that high quality graphene can be obtained at relatively low production costs. While there has been much advancement in CVD synthesis of graphene, little study has been done on the biosensing applications of CVD graphene. Herein, we aim to draw attention to employing CVD grown graphene as a potential platform for immunosensing of IgG. Using electrochemical impedance spectroscopy (EIS), we obtained a sensitive detection of rabbit IgG in the range of 0.1–100 μg ml−1 with untreated CVD graphene as the electrode interface for direct immobilization of the recognition antibodies. From our report, it can be concluded that CVD grown graphene exhibits great potential to be utilized as a platform for immunosensing applications.
Introduction
Comprising of a single layer of carbon atoms that are densely packed in a honeycomb two-dimensional matrix, graphene has been the key interest of many researchers since its discovery in 2004.1 The enormous attention which graphene is receiving is due to the extraordinary properties it displays. Examples of these properties include superior electron and thermal conductivity, robust mechanical strength and large surface area.2–5 As a result of the above mentioned remarkable properties, graphene has also been dubbed as a “miracle material”.6 However, some of these mentioned outstanding properties can only be achieved by samples exhibiting the highest quality, such as mechanically exfoliated graphene.1 Presently, no graphene prepared via other techniques has demonstrated equivalent characteristics. Nevertheless, these synthetic methods are rapidly improving.The methods for the preparation of graphene can be broadly categorized into two main approaches; bottom-up or top-down. For the instance of top-down approach, the working principle is based on stripping individual sheets of graphene from a graphite source material and this approach encompasses techniques such as oxidation of graphite to graphite oxide with subsequent thermal, chemical or electrochemical reduction, and mechanical or liquid-phase exfoliation of graphite.7–9 On the other hand, for the case of bottom-up approach, the working principle is based on employing small carbon sources to fabricate graphene. The bottom-up approach comprises of epitaxial growth on silicon carbide10 and chemical vapour deposition.11
In recent times, much effort has been channelled into research on graphene synthesis by chemical vapour deposition (CVD) method, which can generate high quality graphene with controlled number of layers and possibly at low mass production costs.12 Although metal catalysts such as iridium, ruthenium, cobalt, platinum and iron have been successfully employed to grow graphene, nickel and copper represent the current most frequently adopted metal catalysts due to their lower cost and ease of controlling the number of graphene layers.13–17 CVD grown graphene has been advantageously adopted for the fabrication of transparent electrodes, touch screens, and electronic devices.18 This is in contrary to thermally/chemically reduced graphenes, which are in the form of powders. However, the use of CVD graphene for biosensing applications has not been fully explored yet. In particular, there have only been a few attempts on the utilization of CVD graphene as an electrochemical biosensing platform and these works include the detection of DNA hybridization and glucose sensing.19–21 Till date, there has been no study conducted with the immunology systems.
Hence, in this work, electrochemical impedance spectroscopy (EIS) was employed to monitor the specific interactions between anti-rabbit IgG probes, which were immobilized on a CVD graphene platform, and rabbit IgG protein targets. A stable and uniform immobilization of the recognition element, anti-rabbit IgG, was achieved without any pre-treatment performed on the CVD graphene. In addition, a sensitive and specific detection of rabbit IgG protein, adopted as the model analyte, was obtained. Hence, this work demonstrates great promises for the future use of CVD grown graphene as a biosensing platform.
Experimental section
Materials
Immunoglobulin G from rabbit serum (rabbit IgG), anti-rabbit immunoglobulin G produced in goat (anti-rabbit IgG), albumin from bovine serum (BSA), avidin, human hemoglobin, hydrochloric acid (conc. 37%), sodium phosphate dibasic, sodium chloride, Tween® 20, potassium hexacyanoferrate(II) trihydrate and potassium hexacyanoferrate(III) were purchased from Sigma-Aldrich (Singapore).Ultrapure water used in this study was obtained from a Milli-Q ion exchange column (Millipore) of resistivity 18.2 MΩ cm.
Buffer solutions used in this study are as follows: PBS (0.01 M phosphate, 0.135 M sodium chloride, pH 7.4), PBS-B (0.01 M phosphate, 0.135 M sodium chloride, 1% BSA, pH 7.4) and PBS-T (0.01 M phosphate, 0.135 M sodium chloride, 0.05% Tween® 20, pH 7.4).
Multilayer graphene (105 nm thick on average) on nickel foil (CVD graphene) was purchased from Graphene Laboratories Inc (Calverton, New York).
Equipment
All electrochemical measurements were conducted with a μAutolab type III electrochemical analyzer (Eco Chemie, Utrecht, The Netherlands) connected to a personal computer. Impedance measurements were controlled by NOVA software version 1.8 and recorded between 0.1 MHz and 0.1 Hz at a sinusoidal voltage perturbation of 10 mV amplitude. The obtained impedance spectra, presented as Nyquist plots in the complex plane, underwent electrochemical circle fitting. All electrochemical measurements were performed at room temperature with 10 mM K4[Fe(CN)6]/K3[Fe(CN)6] (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) in PBS buffer solution as the redox probe, and Ag/AgCl as the reference electrode.
1 molar ratio) in PBS buffer solution as the redox probe, and Ag/AgCl as the reference electrode.
Raman spectra were acquired by using a confocal micro-Raman LabRam HR instrument (Horiba Scientific) in backscattering geometry with a CCD detector. A 514.5 nm Ar laser and a 100× objective lens mounted on a Olympus optical microscope were employed for the focusing of the samples. The initial calibration was conducted at 0 and 520 cm−1 with a silicon wafer as the reference to give a peak position resolution of less than 1 cm−1.
Scanning electron microscope images were attained by utilizing a JSM-7600F Schottky Field Emission Scanning Electron Microscope (JEOL, Japan) at 5 kV accelerating voltage.
Atomic force microscopy was performed with a MultiMode 8 atomic force microscope (Bruker, Singapore) controlled by Nanoscope 8.15 software and using the ScanAsyst mode.
X-ray photoelectron spectroscopy data were obtained with a Phoibos 100 spectrometer and an Mg X-ray radiation source (SPECS, Germany) for the measurement of wide-scan survey spectra and high-resolution N 1s spectra.
Procedures
Commercial CVD graphene was washed gently with ultrapure water before use.Anti-rabbit IgG was immobilized onto the surface of CVD graphene by dry physical adsorption. 50 μl of anti-rabbit IgG in PBS buffer solution at a concentration of 100 μg ml−1 was deposited onto CVD graphene and left to dry under the lamp for 30 minutes. Subsequently, the modified surface underwent gentle washings with PBS-T buffer solution, PBS buffer solution and ultrapure water to remove the excess anti-rabbit IgG that was not well adsorbed on the surface.
50 μl of PBS-B buffer solution was next drop-cast on the anti-rabbit IgG modified CVD graphene surface and placed under the lamp for 10 minutes for drying. After which, gentle washings with PBS-T buffer solution, PBS buffer solution and ultrapure water were performed to remove the excess BSA which was not well adsorbed on the surface.
CVD graphene modified with anti-rabbit IgG and BSA then underwent incubation with rabbit IgG in PBS-T buffer solution. The incubation was performed at 37 °C for 1 hour in the oven. Finally, gentle washings with PBS-T buffer solution, PBS buffer solution and ultrapure water were performed to remove the excess of non-specifically adsorbed species. For selectivity study, negative controls were conducted using BSA, hemoglobin and avidin.
Results & discussions
We investigate here the analytical proof-of-concept of performing immunosensing of IgG on CVD graphene. First of all, in order to gain a better understanding of the material employed as the sensing platform, characterizations by scanning electron microscopy (SEM), atomic force microscopy (AFM) and Raman spectroscopy were conducted with CVD graphene.Characterization of CVD graphene was first performed with SEM to acquire information regarding its surface morphology. The SEM images obtained are shown in Fig. S1 (ESI).† Imaging of CVD graphene was carried out at two different magnifications of 370× (Fig. S1A†) and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000× (Fig. S1B†). From the SEM images, it can be observed that the CVD graphene, which was grown on nickel foil, demonstrates several grain boundaries between continuous islands of graphene. In addition, different colours ranging from white to dark grey, indicating regions with different number of graphene layers, can also be seen. Such observation is a common occurrence in samples grown on thin nickel films.22 The homogeneous colour distribution, however, suggests a homogeneous graphene film thickness. Fig. S1B† clearly shows the edges of the graphene islands grown during the catalytic process. The edges are indicated by white lines which also account for folded graphene. On the other hand, the darker lines seen in Fig. S1B† may indicate structural discontinuity or cracks.
000× (Fig. S1B†). From the SEM images, it can be observed that the CVD graphene, which was grown on nickel foil, demonstrates several grain boundaries between continuous islands of graphene. In addition, different colours ranging from white to dark grey, indicating regions with different number of graphene layers, can also be seen. Such observation is a common occurrence in samples grown on thin nickel films.22 The homogeneous colour distribution, however, suggests a homogeneous graphene film thickness. Fig. S1B† clearly shows the edges of the graphene islands grown during the catalytic process. The edges are indicated by white lines which also account for folded graphene. On the other hand, the darker lines seen in Fig. S1B† may indicate structural discontinuity or cracks.
In order to gain more insights about the surface of the CVD graphene employed, AFM analysis was next conducted. Fig. S2 (see ESI†) presents the AFM characterization of CVD graphene. The two-dimensional and three-dimensional profiles of CVD graphene are depicted in Fig. S2A and B† respectively. From the two figures, it can be confirmed that several of the boundaries visible in SEM as white lines, resemble folded graphene sections which emerge from the film surface at a height of approximately 60 nm (Fig. S2C†).
Lastly, Raman spectroscopy was carried out to attain further structural information such as the presence of defects and the number of layers in CVD graphene. Fig. S3† displays the Raman spectrum obtained in this study and it was noted that there is an absence of the D band (∼1350 cm−1) in the spectrum. This suggests that there is no significant presence of structural defects in the sp2 lattice of CVD graphene. The average ratio of the intensity of G band (∼1560 cm−1) to 2D band (∼2700 cm−1) was calculated to be approximately 2.21. This implies that the CVD graphene is of multilayer structure.23 Moreover, another piece of evidence which indicates the multilayer property is the slight shoulder observed at 2D band as single layer graphene will exhibit a symmetrical 2D band.24
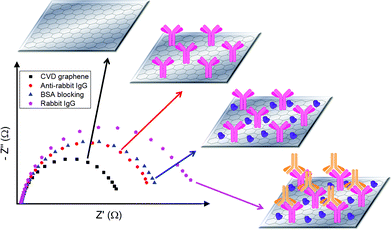
Following the various characterizations, CVD graphene was employed as the transducing platform for the immunosensing of rabbit IgG by using electrochemical impedance spectroscopy (EIS) as the detection technique.25 Scheme 1 illustrates the analytical protocol adopted. In summary, anti-rabbit IgG probes were first immobilized onto the surface of CVD graphene by physical adsorption. The successful attachment of anti-rabbit IgG probes onto CVD graphene was characterized by the appearance of a N 1s peak in X-ray photoelectron spectroscopy (XPS) study after the anti-rabbit IgG immobilization process (see Fig. S4, ESI†). After which, BSA blocking was performed by drop-cast method in order to block off the remaining CVD graphene surface and deter non-specific binding. Lastly, incubation with rabbit IgG target was carried out. For each stage of the analytical procedure, impedance measurement was conducted and the respective Nyquist plots are shown in Scheme 1.
Briefly, bare CVD graphene demonstrated relatively the lowest charge transfer resistance as it was entirely accessible to the redox probe. Subsequently, anti-rabbit IgG probes were immobilized onto the surface of CVD graphene and the magnitude of charge transfer resistance increased owing to the decrease in accessibility of CVD graphene by the redox probe. After which, blocking of the remaining CVD graphene surface with BSA was performed and this further lowered the accessibility by redox probe and charge transfer resistance was further enhanced. Last of all, incubation with rabbit IgG, which binds specifically to anti-rabbit IgG, was carried out and it led to another increase in charge transfer resistance. This can be either attributed to the additional steric hindrance caused by the bulky rabbit IgG protein molecule, or the electrostatic interactions between rabbit IgG and redox probe.26,27 It should be noted that in our previous article, we have compared physical adsorption, chemical linker and biotin/avidin linker for aptasensing on graphenes and we found that the highest sensitivity was exhibited by physical adsorption.28
Selectivity is a fundamental requirement of a functional sensor. Hence, the selectivity performance of the proposed immunosensing platform was investigated by conducting negative control experiments with BSA, hemoglobin and avidin. The conclusions from the experiments are exemplified in Fig. 1.
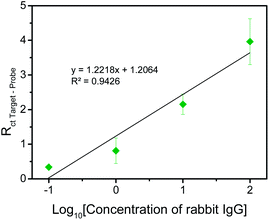
From Fig. 1, it can be noticed that the impedimetric signal (Rct Target–Probe) of rabbit IgG is much greater than that of the negative control proteins, BSA, hemoglobin and avidin. This indicates that the negative control proteins, BSA, hemoglobin and avidin, have negligible interactions with the immobilized anti-rabbit IgG probes. As such, the increase in charge transfer resistance after incubation with the negative control proteins is small, resulting in the low impedimetric signals. Therefore, the proposed immunosensing platform was deduced to be selective for rabbit IgG.
Subsequently, to assess the sensitivity of the proposed sensing system and to evaluate the range of detection, the variation of impedimetric signal with rabbit IgG concentration was examined. As depicted in Fig. 2, the impedimetric signal increases with increasing concentration of rabbit IgG and the linear range of detection was determined to be from 0.1 to 100 μg ml−1 with the limit of detection assessed to be 0.136 μg ml−1. With our acquired range of detection, the immunosensor has the potential to detect IgG in human samples as IgG is present in human serum and plasma samples in the range of 4–16 mg ml−1
With the selectivity and sensitivity aspects of the proposed sensing platform studied, we next move on to the optimization of the analytical protocol employed.
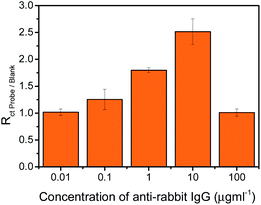
Anti-rabbit IgG optimization was carried out to establish the optimum concentration of the anti-rabbit IgG probe to be deposited onto the surface of CVD graphene in order to achieve maximum surface coverage. Impedance measurements were performed for a series of anti-rabbit IgG concentration and the spectra acquired were analysed and displayed as histograms shown in Fig. 3.
As exhibited in Fig. 3, the impedimetric signal (Rct Probe/Blank) increases progressively as the concentration of anti-rabbit IgG increases from 0.01 to 10 μg ml−1. However, when the concentration of anti-rabbit IgG further increases to 100 μg ml−1, a decrease in the signal can be observed. A higher impedimetric signal correlates to a greater amount of anti-rabbit IgG being successfully immobilized onto the surface of CVD graphene. For that reason, 10 μg ml−1 is determined to be the optimum concentration of anti-rabbit IgG to be deposited onto the surface of CVD graphene as it ensures the greatest coverage of the surface.
CVD graphene offers several advantages. For example, the previous research on chemically modified graphenes exhibited poorer performance, with a detection range of 0.3–7 μg ml−1.29 In addition, here we compare the selectivity with BSA, hemoglobin and avidin while previously the comparison of selectivity was done only with BSA.29 Moreover, CVD graphene is transparent and it can be easily integrated into transparent electronic devices.
In addition, in order to ensure that the physiological pH (7.4) employed for the EIS measurements was suitable for the proposed immunosensing concept, a pH dependent EIS study was conducted (see Fig. S5, ESI†). From the obtained results, it can be confirmed that pH 7.4 is the most appropriate pH to be utilized for this study.
Last of all, the stability of the fabricated immunosensor was assessed by performing a stability study over a time period of seven days (see Fig. S6, ESI†). The results indicate that after seven days, the recorded signal decrease was 48% with an increase in the % RSD value.
Conclusions
In summary, we have demonstrated in this study the proof-of-principle of performing immunosensing of IgG on CVD graphene. The proposed sensing concept was shown to be selective for IgG, with good discrimination from hemoglobin, avidin and BSA. Furthermore, the linear range of detection was determined to be from 0.1 to 100 μg ml−1, thereby conferring reasonable sensitivity to the proposed system. Probe optimization study concluded that 10 μg ml−1 is the optimum concentration of anti-rabbit IgG to be deposited on the surface of CVD graphene. CVD graphene based immunosensors should find their way into biomedical applications in the near future.Acknowledgements
M.P. acknowledges Tier 2 grant (MOE2013-T2-1-056) from Ministry of Education, Singapore.References
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, M. I. Katsnelson, I. V. Grigorieva, S. V. Dubonos and A. A. Firsov, Nature, 2005, 438, 197–200 CrossRef CAS PubMed.
- M. Pumera, Mater. Today, 2011, 14, 308 CrossRef CAS.
- N. A. Kotov, Nature, 2006, 442, 254–255 CrossRef CAS PubMed.
- C. Lee, X. D. Wei, J. W. Kysar and J. Hone, Science, 2008, 321, 385–388 CrossRef CAS PubMed.
- K. S. Novoselov, V. I. Fal'ko, L. Colombo, P. R. Gellert, M. G. Schwab and K. Kim, Nature, 2012, 490, 192–200 CrossRef CAS PubMed.
- P. Blake, P. D. Brimicombe, R. R. Nair, T. J. Booth, D. Jiang, F. Schedin, L. A. Ponomarenko, S. V. Morozov, H. F. Gleeson, E. W. Hill, A. K. Geim and K. S. Novoselov, Nano Lett., 2008, 8, 1704–1708 CrossRef PubMed.
- Y. Hernandez, V. Nicolosi, M. Lotya, F. M. Blighe, Z. Sun, S. De, I. T. McGovern, B. Holland, M. Byrne, Y. K. Gun'Ko, J. J. Boland, P. Niraj, G. Duesberg, S. Krishnamurthy, R. Goodhue, J. Hutchison, V. Scardaci, A. C. Ferrari and J. N. Coleman, Nat. Nanotechnol., 2008, 3, 563–568 CrossRef CAS PubMed.
- D. R. Dreyer, S. Park, C. W. Bielawski and R. S. Ruoff, Chem. Soc. Rev., 2010, 39, 228–240 RSC.
- I. Forbeaux, J. M. Themlin and J. M. Debever, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 58, 16396–16406 CrossRef CAS.
- L. Chen, Y. Hernandez, X. Feng and K. Müllen, Angew. Chem., Int. Ed., 2012, 51, 7640–7654 CrossRef CAS PubMed.
- X. Li, W. Cai, J. An, S. Kim, J. Nah, D. Yang, R. Piner, A. Velamakanni, I. Jung, E. Tutuc, S. K. Banerjee, L. Colombo and R. S. Ruoff, Science, 2009, 324, 1312–1314 CrossRef CAS PubMed.
- P. W. Sutter, J.-I. Flege and E. A. Sutter, Nat. Mater., 2008, 7, 406–411 CrossRef CAS PubMed.
- A. Ambrosi, A. Bonanni, Z. Sofer and M. Pumera, Nanoscale, 2013, 5, 2379 RSC.
- J. Coraux, A. T. N'Diaye, C. Busse and T. Michely, Nano Lett., 2008, 8, 565–570 CrossRef CAS PubMed.
- V. Roumen, M. Alexander, H. P. Roumen, K. Raymond, M. Myrjam, V. Annick and H. Chris Van, Nanotechnology, 2010, 21, 095602 CrossRef PubMed.
- D. Kondo, S. Sato, K. Yagi, N. Harada, M. Sato, M. Nihei and N. Yokoyama, Appl. Phys. Express, 2010, 3, 025102 CrossRef.
- A. Ambrosi and M. Pumera, J. Phys. Chem. C, 2013, 117, 2053 CAS.
- A. Gutés, C. Carraro and R. Maboudian, Biosens. Bioelectron., 2012, 33, 56–59 CrossRef PubMed.
- S.-R. Guo, J. Lin, M. Penchev, E. Yengel, M. Ghazinejad, C. S. Ozkan and M. Ozkan, J. Nanosci. Nanotechnol., 2011, 11, 5258–5263 CrossRef CAS PubMed.
- Y. H. Kwak, D. S. Choi, Y. N. Kim, H. Kim, D. H. Yoon, S.-S. Ahn, J.-W. Yang, W. S. Yang and S. Seo, Biosens. Bioelectron., 2012, 37, 82–87 CrossRef CAS PubMed.
- K. S. Kim, Y. Zhao, H. Jang, S. Y. Lee, J. M. Kim, K. S. Kim, J.-H. Ahn, P. Kim, J.-Y. Choi and B. H. Hong, Nature, 2009, 457, 706–710 CrossRef CAS PubMed.
- A. C. Ferrari, Solid State Commun., 2007, 143, 47–57 CrossRef CAS PubMed.
- A. C. Ferrari, J. C. Meyer, V. Scardaci, C. Casiraghi, M. Lazzeri, F. Mauri, S. Piscanec, D. Jiang, K. S. Novoselov, S. Roth and A. K. Geim, Phys. Rev. Lett., 2006, 97, 187401 CrossRef CAS.
- A. Bonanni, A. H. Loo and M. Pumera, TrAC, Trends Anal. Chem., 2012, 37, 12–21 CrossRef CAS PubMed.
- J. S. Daniels and N. Pourmand, Electroanalysis, 2007, 19, 1239–1257 CrossRef CAS PubMed.
- E. Katz and I. Willner, Electroanalysis, 2003, 15, 913–947 CrossRef CAS.
- A. H. Loo, A. Bonanni and M. Pumera, Chem.–Asian J., 2013, 8, 198–203 CrossRef CAS PubMed.
- A. H. Loo, A. Bonanni, A. Ambrosi, H. L. Poh and M. Pumera, Nanoscale, 2012, 4, 921 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra03506b. |
| This journal is © The Royal Society of Chemistry 2014 |