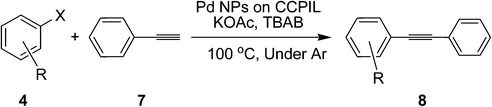
Design and synthesis of a new phosphinite-functionalized clay composite for the stabilization of palladium nanoparticles. Application as a recoverable catalyst for C–C bond formation reactions†
Habib Firouzabadi*a,
Nasser Iranpoor*a,
Arash Ghaderiab,
Mohammad Gholinejadc,
Sajjad Rahimia and
Safura Jokara
aDepartment of Chemistry, College of Sciences, Shiraz University, Shiraz 71454, Iran. E-mail: firouzabadi@chem.susc.ac.ir; iranpoor@chem.susc.ac.ir; Fax: +98 711228 0926; Tel: +98 711 228 4822
bDepartment of Chemistry, College of Sciences, Hormozgan University, Bandar Abbas, 71961, Iran. Fax: +98 761 766 0032; Tel: +98 761 766 0042
cDepartment of Chemistry, Institute for Advanced Studies in Basic Sciences (IASBS), Gava zang, Zanjan 45137-6731, Iran. Fax: +98-241-4214949
First published on 21st May 2014
Abstract
A new clay composite carrying phosphinite-functionalized ionic liquid moieties (CCPIL) has been designed and synthesized. Palladium nanoparticles were uniformly ligated by CCPIL. After characterization, this composite has been applied as a heterogeneous catalyst for the Suzuki–Miyaura reaction of aryl halides with phenylboronic acid on water in the presence of NaOH as the base. These nanoparticles have also been applied for copper-free Sonogashira–Hagihara reaction in TBAB and Mizoroki–Heck reaction under solvent-free conditions. The catalyst was also found to be stable towards moisture and air and its catalytic activity remained almost unchanged after exposure to air for three months.
Introduction
In today's economic and industrial environment, the use of less toxic reaction solvents such as water and ionic liquids are gaining more importance in view of green chemistry. In addition, removal of metal wastes from reaction media and the recovery of expensive metal catalysts are two important issues to be examined. Along this line, chemists are interested in researching for using ionic liquids (ILs) as novel and green solvents, catalysts and reagents.1 ILs have desirable properties, such as non-volatility, non-flammability and a wide temperature range over the liquid phase.2 They have polarities similar to that of light alcohols, in which polar organic compounds, gases and biocatalysts are dissolved, thus reactions can be performed under homogeneous conditions.3 By considering these facts, replacing organic solvents with ionic liquids in organic reactions would be of great interest. However, ionic liquids are expensive, and their use as a solvent is not desirable economically. Nevertheless, by immobilization of ILs onto the surface of a solid support, organic reactions can proceed on the thin IL layer on the surface of the support and minimize the amount of ILs used. In addition, supporting ionic liquids on inorganic materials facilitates their purification processes, recycling and isolation of products from the reaction mixtures.4–6 Incorporation of ionic liquids may be achieved by two approaches; the first approach is their immobilization by covalent binding to solid support4,5 and the second is the adsorption of ILs on the surface of a solid support.6Clay minerals occur widely in nature and their high surface area and ion exchange properties make them suitable for use as solid supports.7 A class of clays is the Mg-organosilicates containing pendant organic groups. The composition of these organo-functionalized clays has been reported as R8Si8Mg6O16(OH)4, where R is an organic group.8
Transition metals have come to play an important role in metal-catalyzed reactions in creating C–C bonds.9 One of the most booming protocols for C–C bond formation between two sp2 carbons is the Suzuki–Miyaura reaction in which biaryl compounds are produced.10 Various palladium nanoparticles have been used as catalysts for the promotion of this reaction11 and numerous reviews are available in the literature in which the use of palladium nanoparticles in the Suzuki–Miyaura reaction have been discussed.10a,12 Sonogashira–Hagihara reaction which is one of the most successful methods to form substituted alkynes, has been widely applied in the synthesis of natural products and biologically active molecules and different materials.13 Another palladium-catalyzed reaction which also proceeds between two sp2 carbons is called the Mizoroki–Heck reaction. It produces functionalized alkenes that are applied in the synthesis of many organic compounds.14 Very recently, palladium nanoparticles supported on amine- and phenyl-functionalized clay has been reported to catalyze carbon–carbon bond formation reactions.15
In recent years, we have reported the synthesis and application of functionalized ILs,16a,b and heterogeneous palladium catalysts for different palladium catalyzed reactions.16c–f In this study, we combined the advantages of homogeneous IL phase, phosphorylated ligands and a new heterogeneous clay support for Pd-catalyzed C–C bond formation in green media.
Results and discussion
Initially, the epoxy-functionalized clay (1) was prepared by the method reported in the literature.17 In order to build up the ionic moiety on the surface of the clay, the epoxy-functionalized clay was dispersed in CH2Cl2 by sonication. 1-Methylimidazole was added to the reaction vessel followed by diphenyl phosphine chloride and the reaction was allowed to stir at 50 °C for 24 h under argon atmosphere. The resulting clay composite carrying phosphinite-functionalized ionic liquid moieties (CCPIL, 2) was separated by simple filtration and washed with CH2Cl2 to give the purified organic-inorganic hybrid material (Scheme 1).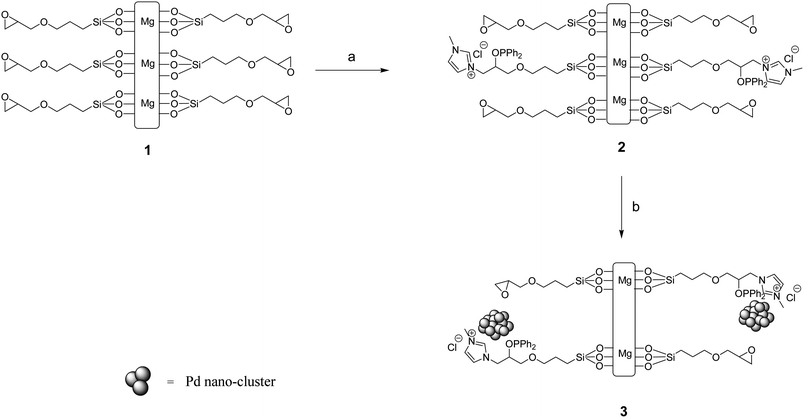 | ||
| Scheme 1 Synthesis of CCPIL and deposition of palladium nanoparticles on it. Reaction conditions: (a) 1-methylimidazole, Ph2PCl, CH2Cl2, 50 °C, under Ar, 24 h; (b) PdCl2 (aqueous solution), rt, 24 h. | ||
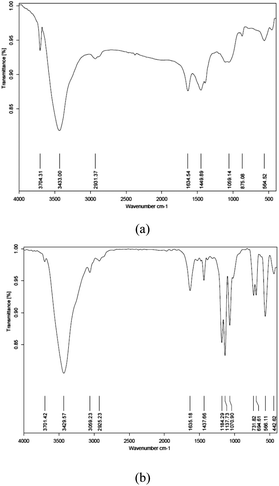
FT-IR spectrum of 1 showed bands at 3433 cm−1 (Si–OH group) and 565 cm−1 (Mg–O absorption) (Fig. 1a). FT-IR spectrum of 2 also showed the same bands plus absorption bands for P–O (1071 cm−1), C–O (1138 cm−1) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching of aromatic rings (1438 cm−1), confirming that phosphinite-functionalized ionic liquid moieties are covalently bonded to the clay producing an organoclay composite (Fig. 1b).
C stretching of aromatic rings (1438 cm−1), confirming that phosphinite-functionalized ionic liquid moieties are covalently bonded to the clay producing an organoclay composite (Fig. 1b).
According to the %C of 1 in CHN analysis, there are 26 mmol organic residues per 1 g of the organoclay composite. Based on the %N of 2, which originates from the imidazolium group, we concluded that 4 mmol of the epoxy group has reacted with 1-methylimmidazole to give CCPIL 2.
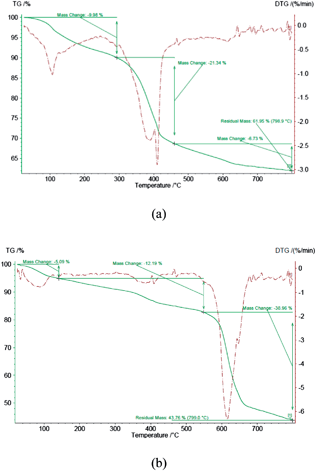
Thermogravimetric analysis (TGA) of 1 confirms the presence of organic groups in the material with a decomposition temperature range of 200–450 °C affording 62% residual mass (Fig. 2a). However, TGA analysis of 2 shows the decomposition of organic groups at 200–700 °C giving 39% residual mass (Fig. 2b). In comparison with 1, the decrease of the residual mass from 62% to 39% in CCPIL 2, verifies the increasing presence of organic groups which is related to the reaction of epoxide moiety with N-methylimidazole and PPh2Cl.
The complexation of palladium particles with the synthesized organoclay was achieved by stirring the organoclay in an aqueous solution of PdCl2 at room temperature for 24 h. Subsequent washing of filtrate with water in a Soxhlet apparatus (to remove the adsorbed PdCl2) and drying by evacuation at 50 °C for 24 h resulted in our target material 3 (Scheme 1). The amount of loading of palladium on the surface of modified clay was determined by ICP analysis as 0.072 mmol of palladium per 1 g of the composite 3, showing the capability of the support to absorb about 72% palladium from the solution. It is very likely that palladium nanoparticles were mostly ligated by the phosphorus group rather than C-2 position of the imidazole.16a
Solid state UV-vis spectrum of palladium chloride shows a peak at 280 nm corresponding to Pd(II).18 The reduction of Pd(II) to Pd(0) was confirmed by the disappearance of this peak. Moreover, increasing the absorption bands in the higher regions indicates the formation of Pd(0) nanoparticles19 (Fig. 3).
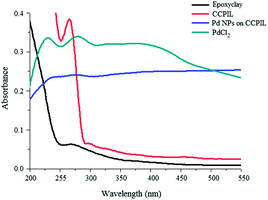 | ||
| Fig. 3 Solid state UV-vis spectra of PdCl2 (green line); epoxyclay (black line); CCPIL (red line) and PdNPs supported on CCPIL (blue line). | ||
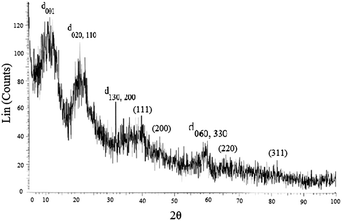
The X-ray diffraction (XRD) pattern of the PdNPs supported on CCPIL shows a low-angle reflection (d001) corresponding to the bilayer arrangement of the organic-modified clay.18 The broad in-plane reflections at higher angles (d020,110, d130,200) and the characteristic reflection (060) in smectites confirm the formation of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 trioctahedral Mg-organosilicate clay with talc-like structure.17 The characteristic peaks for Pd(0) can also be observed at (111), (200), (220) and (311) crystallographic planes (Fig. 4).16d
1 trioctahedral Mg-organosilicate clay with talc-like structure.17 The characteristic peaks for Pd(0) can also be observed at (111), (200), (220) and (311) crystallographic planes (Fig. 4).16d
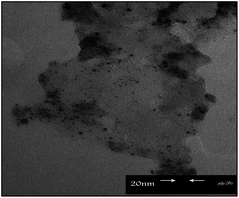
The TEM picture of the supported palladium nanoparticles on the surface of the organoclay composite showed the average size of the palladium particles of around 3–5 nm, although smaller particles to the size of 1 nm or smaller can also be observed (Fig. 5).
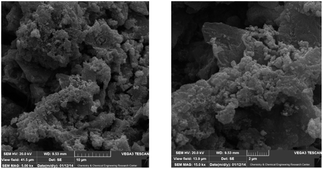
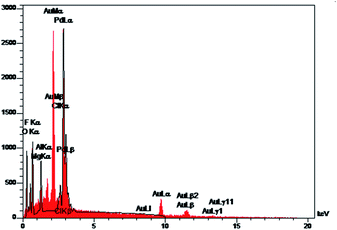
The morphology of the surface of the composite 3 has been examined by collecting two SEM images at two different magnifications (Fig. 6). The SEM-EDX pattern indicates the presence of palladium nanoparticles in the structure of the clay composite (Fig. 7).
The nitrogen adsorption–desorption isotherm for the final catalyst shows a type-IV curve with H3 hysteresis loop which is typical for slit-shaped pores (Fig. S1†).20 BET calculations show a specific surface area of 84.8 m2 g−1 and a total pore volume of 0.09 cm3 g−1.
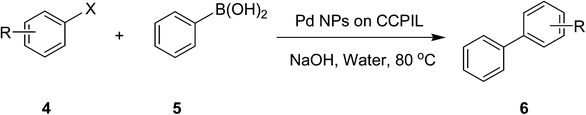
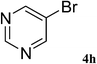
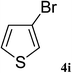
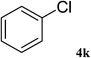
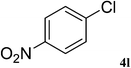
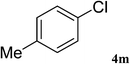
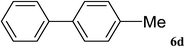
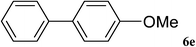
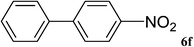
After characterization, this composite was used as a catalyst for the Suzuki–Miyaura reaction. It was found that the use of a combination of an aryl halide (1 mmol), phenylboronic acid (1.2 mmol), palladium nanoparticles deposited on CCPIL (0.01 g containing 7.2 × 10−4 mmol Pd) and NaOH (1.5 mmol), followed by heating in water (3 mL) at 80 °C under air resulted the desired biaryls in excellent yields (Table 1). Since the reactants are insoluble in water, this reaction is an “on water” reaction.21 As shown in Table 1, a range of aryl iodides, bromides and chlorides reacted with phenylboronic acid to give the desired products in high yields. The reaction of iodobenzene (4a) was faster in comparison with the aryl iodides bearing an electron donating group (4b) or having steric hindrance (4c) (Table 1, entries 1–3). This reaction could be easily conducted at room temperature although for a longer reaction time (Table 1, entry 1). In the case of 1-iodo-4-aminobenzene (4b), the Suzuki–Miyaura reaction was performed exclusively upon the carbon halide bond and the N-arylation product was not observed (Table 1, entry 2). The coupling reaction of phenylboronic acid with both electron-releasing and electron-withdrawing aryl bromides afforded products in high yields (Table 1, entries 4–10). Heterocyclic bromides such as 5-bromo pyrimidine (4h), 3-bromo thiophene (4i) and 3-bromo pyridine (4j), led to the corresponding heterobiaryls in high yields (Table 1, entries 8–10). The catalytic system effect was also manifested in the reaction of aryl chlorides with phenylboronic acid. In these cases, prolonged reaction times were required to give the expected products in high yields (Table 1, entries 11–13).
| Entry | Substrate | Product | Time (h) | Yield (%) |
|---|---|---|---|---|
| a Reaction conditions: aryl halide (1 mmol), phenylboronic acid (1.2 mmol), Pd nanoparticles supported on CCPIL (0.01 g), NaOH (1.5 mmol) on water (3 mL) at 80 °C. The data shown in parenthesis refers to the reaction conducted at room temperature. | ||||
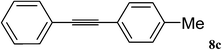
| 1 | 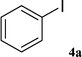 |
 |
40 min (12) | 95 (84) |
| 2 | 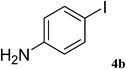 |
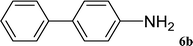 |
4 | 91 |
| 3 | 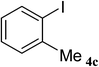 |
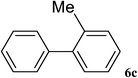 |
3.5 | 95 |
| 4 | 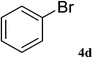 |
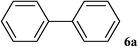 |
9 | 87 |
| 5 | 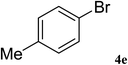 |
 |
10.5 | 93 |
| 6 | 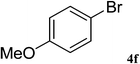 |
 |
16 | 90 |
| 7 | 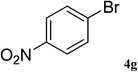 |
 |
2 | 90 |
| 8 | 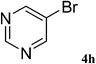 |
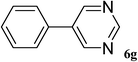 |
8 | 96 |
| 9 | 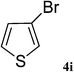 |
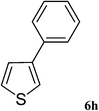 |
18 | 89 |
| 10 | 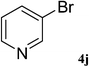 |
 |
10 | 85 |
| 11 |  |
 |
24 | 88 |
| 12 |  |
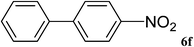 |
16 | 85 |
| 13 | 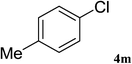 |
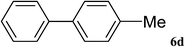 |
30 | 94 |
In comparison with our previously reported system using palladium nanoparticles supported on aminoclay,16d the current system is more effective for the activation of C–Cl bonds in the Suzuki–Miyaura reaction. However, this heterogeneous catalyst is much less reactive in the Suzuki–Miyaura reaction than the reported water-soluble NHC–Pd polymer with triethylene glycol legs which acts homogeneously.22
The reusability of the catalyst was tested upon the reaction of 1-bromo-4-nitro benzene with phenylboronic acid employing 0.01 g of the catalyst in the presence of NaOH at 80 °C. In the first run, the reaction was completed within 2 h to give the product in 90% isolated yield. Similarly, the reactions for the repeated runs were conducted after separation of the catalyst. After completion of the reaction in the first run, the aqueous solution was removed by a syringe. Ethyl acetate (5 mL) was added to the reaction mixture to extract the organic compounds.
The ethyl acetate solution was removed by a syringe and the residual catalyst was dried under a nitrogen flow. After complete drying, the catalyst was charged again into the vessel containing the reacting substrates, and the reaction was performed under similar conditions. This recycling was repeated for five consecutive runs. As shown in Table 2, no significant changes on the reaction times were observed during the five runs conducted. This observation indicates that the amount of Pd leached into the reaction mixture should be low. This has been quantitatively approved by ICP analysis, which showed that after recycling the catalyst for five times, only 7% of Pd leached from the support into the reaction mixture.
We have also shown that the catalyst is stable towards air. For this aim, we prepared the catalyst and left it in air for three months. Then, the catalyst was applied for the reaction of 1-bromo-4-nitrobenzene with phenylboronic acid under the above-mentioned conditions. The desired final product was obtained in 88% isolated yield within 2.25 h.
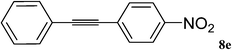
This composite has also been used for Sonogashira–Hagihara reaction. Inspired by the successful experience from our previous work,16c we initiated the optimization study under the conditions reported therein, namely, using KOAc as the base and molten TBAB as the solvent at 100 °C. Employing these conditions, the reaction of 1-bromo-4-nitrobenzene with phenylacetylene went to completion within 40 min (Table 3, entry 1). Switching the solvent to water gave the desired product in 92% conversion after 6 h (Table 3, entry 2). Addition of TBAB to the reaction mixture made a positive impact on the reaction (Table 3, entry 3). Different solvents as well as bases were tested for this reaction (Table 3). These studies, like our previous study, led us to choose TBAB as the solvent and KOAc as the base at 100 °C as the optimized conditions.
| Entry | Solvent | Base | Time (h) | Conversion (%) |
|---|---|---|---|---|
| a TBAB (1 mmol) was added to the reaction mixture. | ||||
| 1 | TBAB | KOAc | 40 min | 100 |
| 2 | H2O | KOAc | 6 | 92 |
| 3a | H2O/TBAB | KOAc | 2.5 | 100 |
| 4 | DMSO | KOAc | 3 | 100 |
| 5 | PEG400 | KOAc | 3 | 100 |
| 6 | TBAB | NaOAc | 1 | 100 |
| 7 | TBAB | None | 24 | 23 |
| 8 | TBAB | Cs2CO3 | 12 | 100 |
| 9 | TBAB | NaOH | 12 | Trace |
| 10 | TBAB | Et3N | 12 | Trace |
| 11 | TBAB | Morpholine | 12 | Trace |
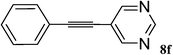
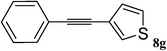
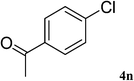
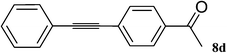
Utilizing the optimized conditions, a wide range of aryl halides including aryl chlorides reacted with phenylacetylene to give the desired products in high to excellent yields (Table 4). For example the reaction of p-chloroacetophenone with phenylacetylene gave the desired product in 80% isolated yield within 3.75 h (Table 4, entry 12). The acetyl group remained intact in this reaction and no condensation reaction was observed.
The catalyst was also recovered for five runs without any significant decrease in its reactivity (Table 5). ICP analysis showed only 3% leaching of the catalyst after the 5th run.
| Run | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Time for completion of the reaction (min) | 40 | 40 | 45 | 45 | 45 |
In order to show the wide range of application of the catalyst, we have also applied this nanocatalyst in the Mizoroki–Heck reaction. For this purpose, the reaction between bromobenzene and n-butyl acrylate using nPr3N as the base at 130 °C as the model reaction was studied. In the result presented in Scheme 2, the reaction proceeded smoothly to completion after 12 h in the absence of solvent at 130 °C to give the desired product in 77% isolated yield.
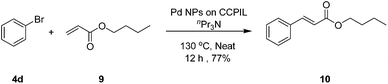 | ||
| Scheme 2 Mizoroki–Heck reaction of bromobenzene with n-butyl acrylate in the presence of Pd(0) nanoparticles under neat conditions. | ||
Conclusions
In conclusion, we have designed and synthesized a new class of ionic liquid supported on the surface of organofunctionalized clay. To this support, a phosphinite group was anchored to act as a suitable ligand for transition metals. Palladium nanoparticles were deposited on the surface of this support. This composite has been applied as a recyclable catalyst for the Suzuki–Miyaura reaction of aryl halides (I, Br and Cl) with phenylboronic acid on water under air atmosphere with small amount of Pd leaching. The composite was stable towards air and moisture. In order to show the merit of the compound as a catalyst, we performed the Sonogashira–Hagihara reaction in molten TBAB and also the Mizoroki–Heck reaction under solvent-free conditions.Acknowledgements
The authors are thankful for the support of this work by National Elite Foundation of Iran by grant number BN048, Iran National Science Foundation (INSF-Grant number 92027195), Shiraz University Research Council and Institute for Advanced Studies in Basic Sciences (IASBS) Research Council. A.G. and M.G. are also thankful to Professor Babak Karimi and Dr S. Jafar Hoseini for their useful suggestions and discussions.Notes and references
- (a) L. A. Blanchard, D. Hancu, E. J. Beckman and J. F. Brennecke, Nature, 1999, 398, 28 CrossRef PubMed; (b) T. Welton, Chem. Rev., 1999, 99, 2071 CrossRef CAS PubMed; (c) P. Wasserscheid and W. Keim, Angew. Chem., Int. Ed., 2000, 39, 3773 CrossRef; (d) N. Iranpoor, H. Firouzabadi and R. Azadi, Tetrahedron Lett., 2006, 47, 5531 CrossRef CAS PubMed.
- T. Prinz, W. Keim and B. Driessen-Hollscher, Angew. Chem., Int. Ed., 1996, 35, 1708 CrossRef CAS.
- P. J. Dyson and T. J. Geldbach in Metal Catalyzed Reactions in Ionic Liquids, Springer, 2005, vol. 29, ch. 1 Search PubMed.
- (a) M. H. Valkenberg, C. deCastro and W. F. Holderich, Green Chem., 2002, 4, 88 RSC; (b) T. Sasaki, C. Zhong, M. Tada and Y. Iwasawa, Chem. Commun., 2005, 2506 RSC.
- (a) B. Karimi and D. Enders, Org. Lett., 2006, 8, 1237 CrossRef CAS PubMed; (b) C. P. Mehnert, R. A. Cook, N. C. Dispenziere and M. Afework, J. Am. Chem. Soc., 2002, 124, 12932 CrossRef CAS PubMed.
- C. P. Mehnert, E. J. Mozeleski and R. A. Cook, Chem. Commun., 2002, 3010 RSC.
- (a) R. S. Varma, Tetrahedron, 2002, 58, 1235 CrossRef CAS; (b) V. Kannana and K. Sreekumar, J. Mol. Catal. A: Chem., 2013, 376, 34 CrossRef PubMed; (c) A. V. Martinez, J. A. Mayoral and J. I. Garcia, Appl. Catal., A, 2014, 472, 21 CrossRef CAS PubMed; (d) C. B. A. H. Pizarro, J. A. Casas and J. J. Rodriguez, Appl. Catal., B, 2014, 148–149, 330 Search PubMed; (e) S. Mohammed, A. K. Padala, B. A. Dar, B. Singh, B. Sreedhar, R. A. Vishwakarma and S. B. Bharate, Tetrahedron, 2012, 68, 8156 CrossRef CAS PubMed; (f) A. Alvarez, S. Moreno, R. Molina, S. Ivanova, M. A. Centeno and J. A. Odriozol, Appl. Clay Sci., 2012, 69, 22 CrossRef CAS PubMed; (g) T. Subramanian and K. Pitchumani, Catal. Commun., 2012, 29, 109 CrossRef CAS PubMed; (h) M. R. Castillo, L. Fousse, J. M. Fraile, J. I. Garcia and J. A. Mayoral, Chem.–Eur. J., 2007, 13, 287 CrossRef CAS PubMed.
- E. Muthusamy, D. Walsh and S. Mann, Adv. Mater., 2002, 14, 969 CrossRef CAS.
- (a) X. Shang and Z. Q. Liu, Chem. Soc. Rev., 2013, 42, 3253 RSC; (b) N. Kambe, T. Iwasaki and J. Terao, Chem. Soc. Rev., 2011, 40, 4937 RSC.
- (a) F. Alonso, I. P. Beletskaya and M. Yus, Tetrahedron, 2008, 64, 3047 CrossRef CAS PubMed; (b) R. Martin and S. L. Buchwald, Acc. Chem. Res., 2008, 41, 1461 CrossRef CAS PubMed.
- (a) Y. Zheng, P. D. Stevens and Y. Gao, J. Org. Chem., 2006, 71, 537 CrossRef CAS PubMed; (b) N. Gurbuz, I. Ozdemir, B. Cetinkaya and T. Seckin, Appl. Organomet. Chem., 2003, 17, 776 CrossRef CAS; (c) C. Yang, H. Wustefeld, M. Kalwei and F. Schueth, Stud. Surf. Sci. Catal., 2004, 154, 2574 CrossRef; (d) R. B. Bedford, U. Singh, R. I. Walton, R. T. Williams and S. A. Davis, Chem. Mater., 2005, 17, 701 CrossRef CAS.
- (a) M. Sasaki and H. Fuwa, Synlett, 2004, 1851 CrossRef CAS PubMed; (b) N. T. S. Phan, M. Van Der Sluys and C. W. Jones, Adv. Synth. Catal., 2006, 348, 609 CrossRef CAS.
- R. Chinchilla and C. Najera, Chem. Soc. Rev., 2011, 40, 5084 RSC.
- (a) Q. Du, W. Zhang, H. Ma, J. Zheng, B. Zhou and Y. Li, Tetrahedron, 2012, 68, 3577 CrossRef CAS PubMed; (b) P. Sun, Y. Zhu, H. Yang, H. Yan, L. Lu, X. Zhang and J. Mao, Org. Biomol. Chem., 2012, 10, 4512 RSC; (c) A. V. Martinez, J. A. Mayoral and J. I. Garcia, Appl. Catal., A, 2014, 472, 21 CrossRef CAS PubMed.
- J. B. B. Varadwaj, S. Rana and K. Parida, J. Phys. Chem. C, 2014, 118, 1640 Search PubMed.
- (a) N. Iranpoor, H. Firouzabadi and R. Azadi, Eur. J. Org. Chem., 2007, 2197 CrossRef CAS; (b) A. Safavi, N. Maleki, N. Iranpoor, H. Firouzabadi, A. R. Banazadeh, R. Azadi and F. Sedaghati, Chem. Commun., 2008, 6155 RSC; (c) H. Firouzabadi, N. Iranpoor and A. Ghaderi, Org. Biomol. Chem., 2011, 9, 865 RSC; (d) H. Firouzabadi, N. Iranpoor, A. Ghaderi, M. Ghavami and S. J. Hoseini, Bull. Chem. Soc. Jpn., 2011, 84, 100 CrossRef CAS; (e) H. Firouzabadi, N. Iranpoor and M. Gholinejad, J. Mol. Catal. A: Chem., 2010, 321, 110 CrossRef CAS PubMed; (f) H. Firouzabadi, N. Iranpoor and M. Gholinejad, Tetrahedron, 2009, 65, 7079 CrossRef CAS PubMed.
- N. T. Whilton, S. L. Burkrtt and S. Mann, J. Mater. Chem., 1998, 8, 1927 RSC.
- K. K. R. Datta, M. Eswaramoorthy and C. N. R. Rao, J. Mater. Chem., 2007, 17, 613 RSC.
- B. M. Choudary, S. Madhi, N. S. Chowdari, M. L. Kantam and B. Sreedhar, J. Am. Chem. Soc., 2002, 124, 14127 CrossRef CAS PubMed.
- F. Rouquerol, J. Rouquerol and K. Sing, Adsorption by powders and porous solids, Academic Press, London, 1999 Search PubMed.
- A. Chanda and V. V. Fokin, Chem. Rev., 2009, 109, 725 CrossRef CAS PubMed.
- B. Karimi and P. Fadavi Akhavan, Chem. Commun., 2011, 47, 7686 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section and spectral data for all compounds. See DOI: 10.1039/c4ra03645j |
| This journal is © The Royal Society of Chemistry 2014 |