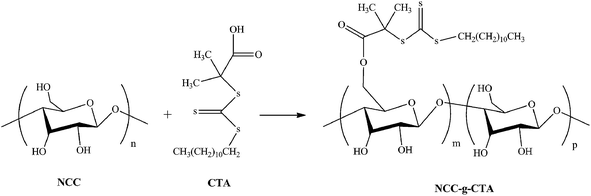
Nanocrystalline cellulose grafted random copolymers of N-isopropylacrylamide and acrylic acid synthesized by RAFT polymerization: effect of different acrylic acid contents on LCST behavior
Elnaz Zeinalia,
Vahid Haddadi-Asl*a and
Hossein Roghani-Mamaqani*b
aDepartment of Polymer Engineering and Color Technology, Amirkabir University of Technology, P.O. Box 15875-4413, Tehran, Iran. E-mail: haddadi@aut.ac.ir; Fax: +98 21 64542403; Tel: +98 21 64542403
bDepartment of Polymer Engineering, Sahand University of Technology, P.O. Box 51335-1996, Tabriz, Iran. E-mail: r.mamaghani@sut.ac.ir; Fax: +98 411 3459089; Tel: +98 411 3459089
First published on 3rd July 2014
Abstract
Free and nanocrystalline cellulose (NCC) attached homopolymers of N-isopropylacrylamide and acrylic acid (AA) as temperature- and pH-sensitive materials and also their dual-sensitive copolymers with different contents of AA were synthesized by RAFT polymerization. NCCs were obtained from microcrystalline cellulose by an acid hydrolysis process. Then, the surface of NCC was chemically modified by 2-(dodecylthiocarbonothioylthio)-2-methylpropionic acid (DDMAT) as chain transfer agent. In situ synthesis of polymers in the presence of 1 wt% of NCC was carried out at 70 °C via the R-group approach. Successful attachment of DDMAT and polymer chains on the backbone of NCC was studied by X-ray photoelectron (XPS), Fourier transform infrared, proton nuclear magnetic resonance, and Raman spectroscopies. Elemental analysis, thermogravimetric analysis, and XPS were also used to evaluate the grafting of DDMAT and polymers. Thermal behaviour of the NCC-attached polymers was also studied by differential scanning calorimetry. The lower critical solution temperatures (LCST) of polymers as phase separation temperatures were measured by the cloud point method using dynamic light scattering. Addition of NCC, AA content, and pH at higher pH values results in increase of LCST. At low pH values, increase of LCST occurred by an increase of NCC and also pH value. The morphology and crystalline structure of polymer-grafted NCCs were examined by transmission electron microscopy and X-ray diffraction respectively.
1. Introduction
Copolymers based on poly(acrylic acid) (PAA) and poly(N-isopropylacrylamide) (PNIPAAm) attracted a great deal of interest because of their dual responsive behaviors. PAA is a pH-sensitive polymer which precipitates at pH < 4 in aqueous solutions due to the protonation of its carboxyl groups. PNIPAAm shows lower critical solution temperature (LCST) in aqueous solutions because its phase transition is at about 32 °C.1,2 Although thermoresponsive polymers are soluble in water at low temperatures, they separate upon heating because of the delicate balance between the hydrophobic and hydrophilic interactions.3 This spontaneous process is endothermic and is driven by increasing entropy accompanied with the release of hydrophobically bound water molecules.4 The LCST of these temperature sensitive polymers can be tuned by copolymerization with other monomers. A more hydrophilic comonomer raises the LCST and a more hydrophobic comonomer lowers the LCST.5 Sensitive comonomers to pH in copolymerization with N-isopropylacrylamide (NIPAAm) can increase LCST as the phase separation temperatures to 37 °C.5,6 Random copolymers of NIPAAm with carboxylic acid monomers of acrylic acid and methacrylic acid have been carried out using free radical polymerization techniques.6,7 Random copolymers of NIPAAm and AA respond to the combined external stimuli of temperature and pH. Addition of acrylic acid comonomer content results in an increase of LCST value at all pH ranges, which is mainly on account of the higher hydrophilicity of acrylic acid compared with NIPAAm. Also, because of the low value of pKa for the acrylic acid polymers, the phase transition of the resultant copolymers are usually falls below pH 5.6–9 Consequently, tailoring NIPAAm copolymers which respond to the physiologically relevant pHs between 5.0 and 7.4 can be a challenge.10 Surfing the literature reveals that composition, molecular architecture, molecular weight, and solution concentration affect the micellar properties.11–14Design of nanocomposites based on renewable natural resources which are biodegradable and nontoxic is very interested. Nanocrystalline cellulose (NCC) with a very high modulus of elasticity, high aspect ratio, specific surface area in the range of 150–300 m2 g−1, low density, easy production by acid hydrolysis of extremely abundant cellulose resources, nonabrasive nature, and recyclability have attracted considerable attention for exploring new applications in the production of biobased nanocomposites.15–17 Besides these, NCC is economically interested since it can be prepared from a wide variety of natural sources and used to prepare new ecologically friendly biocomposites. NCC because of its renewability, high mechanical properties and its formability into different structures can be a good support for hydrogels.18 It is an ideal carrier for many functional polymers, like electro-conductive polymers.19 Poly(4-vinylpyridine) coated NCC can also have reversible flocculation and sedimentation behavior with changes in pH which may offer new applications for biomedical devices, as clarifying agents, and in industrial separation processes.20 Enzyme immobilization, drug delivery, biosensor, and many other applications can be given from the NNC composites.21–24
Chemical nature of cellulose causes that NCC surface bears numerous hydroxyl functions which can be used for grafting reactions. Commonly, there are three methods for the covalent attachment of polymers to the surface of a substrate, which are “grafting from”, “grafting through”, and “grafting to” methods. The “grafting from” method, which has commonly been used for synthesis of graft polymer chains, relies on the surface functionalization of substrates by a polymer precursor and subsequent propagation of polymer chains from the surface-attached initiators by in situ polymerization.
Controlled radical polymerization (CRP) which results in polymers with narrow molecular weight distribution and well-defined topology has been studied numerously in the preparation of graft polymers.25,26 Atom transfer radical polymerization (ATRP) and reversible addition fragmentation chain transfer (RAFT) are the two common methods of CRP to prepare functional polymers. Functionality in polymers plays a key role in synthesis of graft polymer chains on various substrates. Therefore, in situ CRP has attracted a great deal of interest in synthesis of various graft polymers in the recent years.27–31
Cellulose and its nanocrystalline parts have commonly been used for synthesis of biodegradable based nanocomposites by in situ CRP methods with grafting from approach.32–43 Glaied et al. reported synthesis of cellulose fibers densely grafted with poly[2-(meth-acryloyloxy)ethyl]-trimethylammonium chloride through aqueous ATRP.32 They exploit the hydroxyl groups present on the cellulose surface to attach the ATRP initiator. Morandi et al. reported a similar way to synthesize polystyrene-grafted NCCs by surface initiated ATRP.33 By a similar procedure, synthesis of temperature sensitive copolymers of poly(N,N-dimethylaminoethyl methacrylate)- and polystyrene-grafted NCCs were carried out by Yi et al.34,35 Considering RAFT polymerization, Stenzel et al. were the first that report preparation of trithiocarbonate-based chain transfer agent (CTA) on cellulose and also hydroxyisopropyl cellulose to obtain polystyrene comb polymers via the Z-group approach.36,37 Roy et al. synthesized polystyrene-grafted cellulose.38 They converted hydroxyl groups of the cellulose fiber into thiocarbonylthio-based CTA, and further used to mediate the RAFT polymerization of styrene. Roy et al. also polymerized 2-(dimethylamino)ethyl methacrylate from cellulosic filter paper via RAFT polymerization.39 Hiltunen et al., Fleet et al., and Semsarilar et al. reported similar studies using hydroxypropyl, methyl, and ethyl hydroxyethyl cellulose.40–42 Also, Barsbay et al. grafted sodium 4-styrenesulfonate from cellulose by RAFT polymerization via 4-cyanopentanoic acid dithiobenzoate mediation.43
Grafting of responsive polymers on substrates can also affect the characteristics of the grafted chains, their conformation in solution, and therefore their phase transition behavior. In this study, we report our investigation of the grafting NIPAAm and AA random copolymers on the surface of NCC by RAFT polymerization. According to our knowledge, there is not a comprehensive report on graft reaction of dual responsive polymers on NCC by RAFT polymerization. NCC with its hydrophilic OH groups is used as the bio-based nanofiller which can result in increasing of LCST. Therefore, synthesis of NIPAAm and AA homopolymers and also their random copolymers by RAFT polymerization, in situ grafting of the polymers on NCC via R-approach RAFT polymerization, investigation of the effect of NCC on LCST and response of the copolymers, study the effect of various contents of AA feed content on LCST and response of the copolymers, and finally on the thermal behavior of the products are evaluated in this study.
2. Experimental section
Materials
N-Isopropylacrylamide (NIPAAm, Aldrich, 97%) was recrystallized twice from hexane and dried under vacuum prior to use. Acrylic acid (AA, Aldrich, 99%) was distilled and stored at −40 °C. Azobisisobutyronitrile (AIBN, Aldrich, 98%) was recrystallized twice from methanol. Tricaprylylmethylammonium chloride (Aldrich), 1-dodecanetiol (Riedel-de Haen, 99%), carbon disulfide (Sigma-Aldrich, >99%), sodium hydroxide (NaOH, Merck), acetone (Merck), chloroform (Merck), n-hexane (sigma-Aldrich, >95%), 2-propanol (Merck) were used without any purification for the synthesis of DDMAT. Microcrystalline cellulose (MCC, Aldrich), sulfuric acid (H2SO4, Merck), N,N′-dicyclohexylcarbodiimide (DCC, Aldrich, 99%), 4-dimethylaminopyridine (DMAP, Aldrich, 99%), methanol (Merck, 99%) were used in the preparation of functional NCC. Also, tetrahydrofuran (THF, Merck) was used as solvent in polymer separation.Characterization
Particle size and its distribution was analyzed using a dynamic light scattering instrument (DLS, Malvern Nano Zetasizer ZS 90, United Kingdom) with a scattering angle of 176.1. The measurement was done after the samples were diluted by diethyl ketone. X-ray photoelectron spectroscopy (XPS) was carried out on a Gammadata-Scienta Esca 200 hemispherical analyzer equipped with an Al Kα (1486.6 eV) X-ray source. Elemental analysis was carried out with an Elementar Vario max CHNOS Analyser (Hanau, German). Total carbon, hydrogen, nitrogen, and oxygen were determined by dry combustion method. Raman spectra were collected in the range from 3500 to 400 cm−1 using Bruker Dispersive Raman Spectrometer fitted with a 785 nm laser source, a CCD detector, and a confocal depth resolution of 2 μm. The laser beam was focused on the sample using an optical microscope. Fourier transform infrared (FTIR) spectra were recorded on a Bomem FTIR spectrophotometer within a range of 500–4000 cm−1 using a resolution of 4 cm−1. An average of 32 scans has been reported for each sample. The cell pathlength was kept constant during all the experiments. Samples were prepared on a KBr pellet in vacuum desiccators under a pressure of 0.01 Torr. 1H NMR (300 MHz) spectra were recorded on a Bruker Avance 300 spectrometer using CDCl3 as the solvent and tetramethylsilane as the internal standard. A pulse delay of 1 s was used to ensure complete relaxation of spins. Thermal gravimetric analyses were carried out with a PL thermo-gravimetric analyzer (Polymer Laboratories, TGA 1000, UK). The thermograms were obtained from ambient temperature to 700 °C at a heating rate of 10 °C min−1. A sample weight of about 10 mg was used for all the measurements, and nitrogen was used as the purging gas at a flow rate of 50 mL min−1. Thermal analysis was carried out using a differential scanning calorimetry (DSC) instrument (NETZSCH DSC 200 F3, Netzsch Co, Selb/Bavaria, Germany). Nitrogen at a rate of 50 mL min−1 was used as the purging gas. Aluminum pans containing 2–3 mg of the samples were sealed using the DSC sample press. The samples were heated from ambient temperature to 250 °C at a heating rate of 10 °C min−1. Tg was obtained as the inflection point of the heat capacity jump. X-ray diffraction (XRD) patterns were collected on an X-ray diffraction instrument (Siemens D5000) with a Cu target (λ = 0.1540 nm) at room temperature. The system consists of a rotating anode generator, and operated at 35 kV and a current of 20 mA. The samples were scanned from 2 to 40° at the step scan mode, and the diffraction pattern was recorded using a scintillation counter detector. The basal spacing of the samples was calculated using the Bragg's equation (λ = 2d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ). A Vega Tescan SEM was used to evaluate the morphology of NCCs which were gold-coated using a sputtering coater. A transmission electron microscope, Philips EM 208, with an accelerating voltage of 120 kV was used to study the morphology of the NCC and NCC-grafted polymers.
θ). A Vega Tescan SEM was used to evaluate the morphology of NCCs which were gold-coated using a sputtering coater. A transmission electron microscope, Philips EM 208, with an accelerating voltage of 120 kV was used to study the morphology of the NCC and NCC-grafted polymers.
Synthesis of 2-(dodecylthiocarbonothioylthio)-2-methylpropionic acid (DDMAT)
DDMAT was synthesized by a similar way to the previously reported procedure.44 1-Dodecanethiol (20.2 g), acetone (58.0 g), and tricaprylylmethylammonium chloride (1.0 g) were cooled to 0 °C under nitrogen atmosphere and subsequently aqueous sodium hydroxide (50%) (8.4 g) was added over 10 min. After the mixture was stirred for an additional 20 min, carbon disulfide (7.6 g) in acetone (10.0 g) was added over 30 min. After addition of chloroform (17.8 g), aqueous sodium hydroxide (50%, 40 g) was added dropwise and stirring was continued overnight. 200 mL of water and 80 mL of concentrated HCL were added to acidify the aqueous solution. After removing acetone, the solid was collected, and then stirred in 300 mL of 2-propanol. The resulting solid was recrystallized from hexane to afford 22.0 g of yellow crystalline solid (mp ≈ 60 °C).1H NMR (in CDCL3): δ 1.0 (t, 3H), 1.30–1.5 (m, 18H), 1.6–1.8 (m, 8H), 3.2 (t, 2H), 11.0 (s, 1H).
FTIR: 2849 cm−1 (CH), 1716 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1069 cm−1 (C
O), 1069 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S), 814 cm−1 (C–S).
S), 814 cm−1 (C–S).
Preparation of NCC from the source of MCC
NCC was prepared by acid hydrolysis of MCC similar to the literature.45,46 MCC powder (10.0 g) was hydrolyzed by sulphuric acid (87.5 mL, 64% w/v) for 5 h at 45 °C. The hydrolysis was quenched by adding a large amount of water (500 mL). The resulting mixture was cooled to room temperature and centrifuged. Water (500 mL) was added to the precipitate and the mixture was then sonicated. Then, 1.0% NaOH solution was added into the mixture and stirring was applied for 48 h. After addition of 500 mL water, centrifugation and sonication process was continued for three times. Then, the suspension was filtered using an Amicon stirred cell until the pH of suspension reached a constant value.Synthesis of DDMAT-grafted NCC
NCC was modified with DDMAT similar to the literature.42 A 50 mL round-bottom flask was charged with NCC (0.1 g) dissolved in chloroform (10 mL), DDMAT (1.0 g, 4.2 mmol), DCC (0.4 g, 1.94 mmol) of, and DMAP (0.26 g, 2.13 mmol). The reaction was sealed and placed in a preheated oil bath at 40 °C. The reaction was left to run for 6 days. The reaction mixture was then precipitated in methanol, dissolved in chloroform, and precipitated in methanol again. This cycle was repeated three times to obtain a pure product of NCC-g-CTA.Synthesis of polymers and nanocomposites
Batch polymerizations were performed in glass vials which were placed in an oil bath thermostated at 70 °C. At first, monomer (14.38 g), DDMAT (0.68 g), AIBN (0.022 g), and dioxane (35.1 mL) was added into a 250 mL lab reactor and mixed for 2 h. Then, the reactor's contents were transferred into the glass vials by means of a syringe and was then degassed under the nitrogen atmosphere, sealed and placed in an oil bath. Polymerizations were run during 4 h for AA and 25 h for NIPAAm monomers. The polymerizations were stopped by cooling the reaction mixture in an ice bath after distinct time periods. Also, cool methanol was added into the vials for precipitating polymer. Synthesis of random copolymers was accomplished similarly. However, NIPAAm (11.825 g), AA, AIBN (0.015 g), DDMAT (0.165 g) and 12.5 mL dioxane was used and polymerizations were run during 17 h. Various molar fraction of AA was 5, 10, 15, 20, and 30 molar percent. Samples are designated as the general name of PNIPAAm-co-PAAX, in which X denotes the AA molar ratio in the feed. In the case of NCC-grafted polymers, the same procedure was run other than DDMAT was added half of the amount used for polymers. Also, NCC-g-CTA (1 wt% relative to monomer) was added at the first stage of reactant addition. Samples are designated as the general name of NCC-g-PNIPAAm-co-PAAX, in which X denotes the AA molar ratio in the feed.Separation of polymer chains from particles
The prepared nanocomposites were dissolved in THF and the polymer chains were separated from NCC by high-speed ultracentrifugation (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm). The solution was passed through a 0.2 micrometer PTFE filter and then poured into methanol (500 mL) to precipitate polymer chains. After filtration, polymer was dried overnight in a vacuum oven at temperature 50 °C. The remained solid on the filter was the NCC-grafted polymers. Grafted polymers were also separated from NCCs by acid hydrolysis. By a typical procedure, 0.2 g of NCC-grafted polymers with 15 mL of HCl solution and 30 mL of THF were added into a flask and stirred at 85 °C for 5 days. After that the reaction mixture was filtered and the filtrate was dried at 65 °C in a vacuum oven to give separated graft polymers.
000 rpm). The solution was passed through a 0.2 micrometer PTFE filter and then poured into methanol (500 mL) to precipitate polymer chains. After filtration, polymer was dried overnight in a vacuum oven at temperature 50 °C. The remained solid on the filter was the NCC-grafted polymers. Grafted polymers were also separated from NCCs by acid hydrolysis. By a typical procedure, 0.2 g of NCC-grafted polymers with 15 mL of HCl solution and 30 mL of THF were added into a flask and stirred at 85 °C for 5 days. After that the reaction mixture was filtered and the filtrate was dried at 65 °C in a vacuum oven to give separated graft polymers.
3. Results and discussion
NCC was prepared by acid hydrolysis of MCC. During this process, small crystalline blocks of cellulose are separated from the amorphous parts. These naonocrystals are in the form of rods with an average length of below 100 nm. Fig. 1 shows the SEM and TEM images for the MCC and NCC. Cellulose microcrystals with dimension of 10–50 μm in length and 10–25 μm in diameter are clearly shown in the Fig. 1(A). These MCC granules with large sizes are composed of microfibrils with strong hydrogen bonding and nanocrystals can obtained after acid hydrolysis.47 Rod like shapes of NCC is also displayed in SEM and TEM images (Fig. 1(B) and (C)). NCC with length of 100–200 nm and diameter of 10–20 nm are an order of magnitude smaller than the microcrystals. Because of the nature of NCCs, they formed laterally aggregated morphology. High specific area and strong hydrogen bonds in addition to the surface ionic charge caused by acid treatment can also results in this arrangement.48 Similar size ranges were also reported by Bai et al. for the NCCs prepared from MCC source.45 TEM image also shows the separated nanocrystals which were obtained by dispersion of NCCs in water (0.1 wt%). DLS from 0.1 wt% solution of nanocrystals in water was used to evaluate the size of nanocrystals and its distribution and the corresponding result is displayed in Fig. 1(D). A narrow monomodal peak at the vicinity of about 107 nm clearly shows that NCCs are not accumulated and uniformly distributed in the water. Calculation of hydrodynamic radius of the nanoparticles by their diffusion coefficient in a medium from the Stokes–Einstein equation in DLS shows that the value is given by this technique is the radius of a sphere having the same diffusion coefficient as the rodlike NCCs. The size of 107 nm is close to the mean length of 130 nm reported by Azzam et al.49 and Elazzouzi-Hafraoui et al.50 for NCCs prepared from the source of cotton (Fig. 2). | ||
| Fig. 1 SEM images of (A) MCC and (B) NCC, (C) TEM image of NCC, and (D) intensity distribution of the NCCs in water measured by DLS. | ||
DDMAT was grafted on NCC from its R group by an esterification reaction. This insures that grafting polymers on NCC will proceed by R-approach (Fig. 3). Elemental analysis and XPS were employed to investigate the grafting percent of DDMAT on NCC. Table 1 shows the content of carbon, oxygen, nitrogen, and sulfur atoms for MCC, NCC, and NCC-g-CTA. Composition of oxygen in NCC can be used for calculation of hydroxyl groups composition. Repeating units of NCC is composed of two glucose groups with the formula of C6H10O5. Therefore, the amount of oxygen atoms in the form of hydroxyl group is equal to 6 in every repeating unit. Therefore, 47.9% of oxygen in NCC shows that 28.7% of NCC consists of hydroxyl groups. According to the results of elemental analysis, total amount of sulphur atom which originates from the attached CTA is about 2.3 percents which gives C/S value of 17.74.
 | ||
| Fig. 3 Wide scan XPS for (A) NCC and (B) NCC-g-CTA, and C 1s core-level spectrum for the (C) NCC and (D) NCC-g-CTA. | ||
| Sample | N | C | H | O | S | C/O | C/S |
|---|---|---|---|---|---|---|---|
| MCC | <0.1 | 45.3 | 6.1 | 47.2 | <0.1 | 0.96 | — |
| NCC | <0.1 | 44.4 | 5.9 | 47.9 | <0.1 | 0.93 | — |
| NCC-g-CTA | <0.1 | 40.8 | 4.9 | 42.1 | 2.3 | 0.97 | 17.74 |
XPS was used to investigate the surface composition of NCC and NCC-g-CTA (Fig. 3 and Table 2). Fig. 3 shows the survey data and also the higher resolution data of the S 2p, O 1s, and C 1s areas. Survey-scan spectra of both the neat and modified NCCs show that carbon and oxygen are their main components. Survey-scan spectrum of NCC varies from the NCC-g-CTA considerably at the binding energy of 175.9 eV, which relates to sulfur. Appearance of S 2p and S 2s band spectra at the binding energies of 175.86 and 238 eV clearly shows that DDMAT has been anchored on the surface of NCC.43 The inset of Fig. 3(B) shows the band of S 2p at 175.9 eV assigned to C–S groups. C 1s band spectrum at the binding energy of 286–294 eV is used to evaluate the proportion of various functional groups in NCC and NCC-g-CTA, which reflects the local environments of the carbon atoms (C–C and C–H, C–O and C–S, O–C–O, O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). The relative amounts of each carbon bond were determined before and after surface modification. The deconvoluted peak related to C–S bond which is observed at the binding energy of 290 eV implies the successful attachment of DDMAT on the NCC surface.51 The results of Fig. 3(C) and (D) are presented in Table 2 and show that appearance of sulfur results in increased values of C–O/C–S and O–C
O). The relative amounts of each carbon bond were determined before and after surface modification. The deconvoluted peak related to C–S bond which is observed at the binding energy of 290 eV implies the successful attachment of DDMAT on the NCC surface.51 The results of Fig. 3(C) and (D) are presented in Table 2 and show that appearance of sulfur results in increased values of C–O/C–S and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peak area. In addition, appearance of O–C
O peak area. In addition, appearance of O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peak in the spectrum of NCCs shows the presence of residual cell wall polysaccharides with carboxylic groups that remain intimately linked to MCC. It may also results from the oxidation of the reducing end groups of MCC.51,52 The ratio of O–C–O/C–O is 0.28 which is very close to the theoretical value of 0.2 calculated from the formula of pure cellulose (C6H9O5)n. This ratio decreases to 0.23 because of the appearance of the C–S group. Sulfur content of 6.39% according to the data obtained from survey scan spectra of NCC-g-CTA shows that the graft percent of DDMAT on NCC is about 17.14% which is close the value resulted from elemental analysis.
O peak in the spectrum of NCCs shows the presence of residual cell wall polysaccharides with carboxylic groups that remain intimately linked to MCC. It may also results from the oxidation of the reducing end groups of MCC.51,52 The ratio of O–C–O/C–O is 0.28 which is very close to the theoretical value of 0.2 calculated from the formula of pure cellulose (C6H9O5)n. This ratio decreases to 0.23 because of the appearance of the C–S group. Sulfur content of 6.39% according to the data obtained from survey scan spectra of NCC-g-CTA shows that the graft percent of DDMAT on NCC is about 17.14% which is close the value resulted from elemental analysis.
| Sample | Composition (%) | Composition of C in groups | ||||||
|---|---|---|---|---|---|---|---|---|
| C | O | S | C/O | (C–C/C–H) | (C–O/C–S) | (O–C–O) | (O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) O) |
|
| NCC | 34.9 | 65.09 | — | 53.6 | 42.44 | 41.07 | 11.54 | 4.94 |
| NCC-g-CTA | 31.48 | 62.13 | 6.39 | 50.6 | 33.82 | 48.47 | 11.23 | 6.48 |
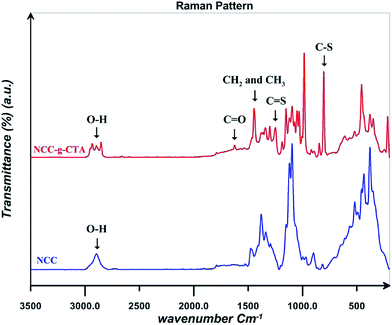
Raman spectra for NCC and NCC-g-CTA are displayed in Fig. 4. Results shows that C–S and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S peaks appear at the wave numbers of 804 and 1249 cm−1 respectively in the spectrum of NCC-g-CTA, which relates to DDMAT and confirms its successful attachment on NCC.38 The peak assigned to the CH2 and CH3 groups of DDMAT is also observed at the wave number of 1444 cm−1. In addition, C
S peaks appear at the wave numbers of 804 and 1249 cm−1 respectively in the spectrum of NCC-g-CTA, which relates to DDMAT and confirms its successful attachment on NCC.38 The peak assigned to the CH2 and CH3 groups of DDMAT is also observed at the wave number of 1444 cm−1. In addition, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and O–H groups are observed at the wave numbers of 1624 and 2894 cm−1 respectively.43
O and O–H groups are observed at the wave numbers of 1624 and 2894 cm−1 respectively.43
FTIR is used to confirm the successful surface modification of NCC by DDMAT and polymers. In addition, effect of the addition of NCC on the spectra of PNIPAAm, PAA, and their random copolymers with various AA contents is studied. FTIR spectra for PNIPAAm, PAA, and their random copolymers are shown in Fig. 5(A). In the spectrum of PNIPAAm, the bands at 1538 and 1641 cm−1 is related to the amide groups (type I and II).53,54 In addition, the peaks at 1362 and 1380 cm−1 are attributed to the isopropyl groups. The peaks at 1450 and 2850–2980 cm−1 are assigned to CH and CH2 units. Also, stretching vibration of N–H group is seen at 3293 cm−1. PAA spectra show two peaks at 1730 and 3419 cm−1 which are assigned to the stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and O–H groups respectively.8 FTIR spectra confirm the formation of copolymers by the appearance of the characterization bands of PAA and PNIPAAm. By increasing AA content, C
O and O–H groups respectively.8 FTIR spectra confirm the formation of copolymers by the appearance of the characterization bands of PAA and PNIPAAm. By increasing AA content, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O band area increases in the random copolymers spectra. In addition, electropositive N–H group of NIPAAm causes a shift of carbonyl wave number to lower values.
O band area increases in the random copolymers spectra. In addition, electropositive N–H group of NIPAAm causes a shift of carbonyl wave number to lower values.
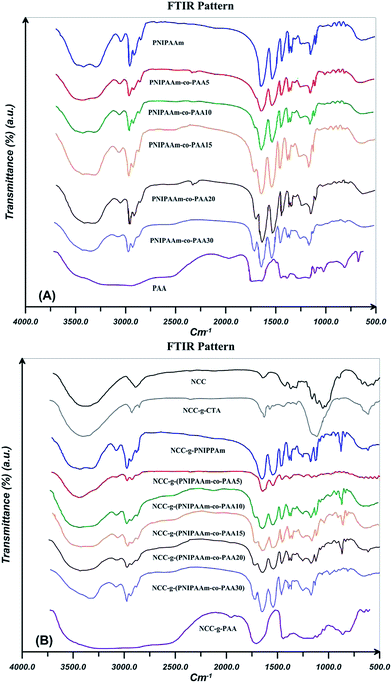 | ||
| Fig. 5 FTIR spectra for PNIPAAm, PAA, and their random copolymers by varying AA content (A) without and (B) in the presence of 0.1 wt% NCC. | ||
FTIR spectra for NCC, NCC-g-CTA, and polymer grafted NCCs are presented in Fig. 5(B). Bending and stretching vibrations of C–H in NCC are appeared at 1370 and 2893 cm−1 respectively. Water absorption of NCC causes a peak at 897 cm−1 which assigned to the O–H stretching vibration. The characteristic peaks at 1410 and 1640 cm−1 relate to the symmetric and asymmetric stretching carboxyl groups.55 The OH stretch for NCC and NCC-g-CTA does not show a remarkable difference. This shows that DDMAT is attached to hydroxyl groups at the surface (with low percentage) and interior hydroxyl groups of the crystallite (with high percentage) remained unreacted.56 The peak at 1635 cm−1 arises from the carbonyl groups of DDMAT and is a proven for successful surface modification of NCC with DDMAT. Appearance of the characteristic bands of PNIPAAm and AA in the spectra of the modified NCCs shows that grafting reactions have appropriate been carried out. The increased strength of C–H stretching vibrations at 2850–2980 cm−1 indicates higher amount of methylene groups in the polymer modified NCCs than in the neat NCC an NCC-g-CTA. This demonstrates the presence of polymer chains in the samples after the extraction of free chains and this is an evidence for the successful grafting reactions.52 Polymer grafting on the surface of nanocellulose results in a decreased value of hydroxyl vibration peak area which arises from the hydrogen bonding at 3030–3680 cm−1. This shows that the content of hydroxyl groups decreases during the grafting process.57
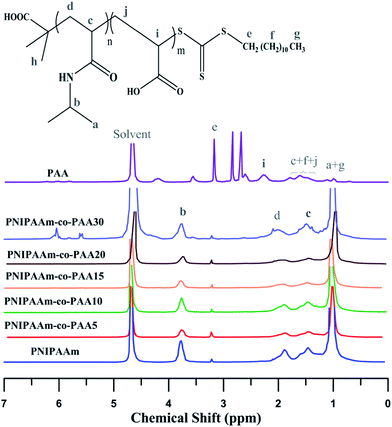
1H NMR spectra of PNIPAAm, PAA, and their random copolymers by varying AA content are exhibited in Fig. 6. Feature signals of NIPAAm (CH–CH3: δ = 1, CH–CH3: δ = 3.7, CH–CH2: δ = 1.4, CH–CH2: δ = 1.8) and DDMAT (S–CH2: δ = 3.2, CH2–(CH2)10: δ = 1.2) in addition to PAA (CH2–CH: δ = 1.49–1.79, CH2–CH: δ = 2.26) and DDMAT ((CH2)10–CH3: δ = 1, CH2–(CH2)10: δ = 1.12, S–CH2: δ = 3.56) can be observed in the spectra. The peak at δ = 4.68 is related to the solvent.8,38 In random copolymers, the signal at δ = 1.2–1.6 which arises from the hydrogen atoms (CH2–CH in NIPAAm and CH2–CH in PAA) increases with addition of AA content.
Molecular weights of the synthesized polymers calculated from 1H NMR data are presented in Table 3. The theoretical ratio of AA in the random copolymers (FAA) is necessary for the molecular weight estimation which can be obtained from the eqn (1) (Mayo and Lewis):58
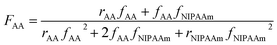 | (1) |
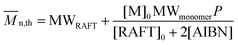 | (2) |
 | (3) |
| Polymer | AA% | Mn (g mol−1) | ||||
|---|---|---|---|---|---|---|
| Feed | Polymer (eqn (1)) | Polymer (1H NMR) | ||||
| Free | NCC-grafted | Free | NCC-grafted | |||
| PNIPAAm | 0 | 0 | 0 | 0 | 7455 | 7834 |
| PNIPAAm-co-pAA5 | 5 | 8.5 | 7.3 | 8.1 | 14763 | 15140 |
| PNIPAAm-co-pAA10 | 10 | 12.6 | 13.2 | 13.3 | 13691 | 14229 |
| PNIPAAm-co-pAA15 | 15 | 18.4 | 16.8 | 18.1 | 11323 | 11892 |
| PNIPAAm-co-pAA20 | 20 | 26.2 | 24.3 | 27.4 | 9212 | 10058 |
| PNIPAAm-co-pAA30 | 30 | 35.6 | 33.8 | 38.3 | 7205 | 7932 |
| PAA | 100 | 100 | 100 | 100 | 5882 | 6302 |
LCST behavior for PNIPAAm and its random copolymers in solution was studied by their cloud point variations. Contribution of PAA as pH-sensitive and PNIPAAm as temperature responsive polymers results in copolymers with dual sensitive characteristics. Therefore, DLS results in two pH ranges of 3–4 and 7–8 were used to study the cloud point and LCST behavior. LCST of thermoresponsive polymers is mainly attributed to a change in the hydrophilic and hydrophobic balance of the polymers which originates from the interactions between polymer chains and water molecules and most importantly hydrogen bonding. At low temperatures, strong interactions between the polar groups and water molecules results in an appropriate solubility of the polymers. Lower entropy of water molecules surrounding apolar groups compared with the free molecules results in an entropic penalty. Increasing apolar surface area of polymer results in an increase of entropic penalty and also decrease of LCST.1
Hydrodynamic diameter for the 0.2 wt% solutions of PNIPAAm and NCC-g-PNIPAAm at different temperatures in addition to their DLS diagrams near the LCST points are shown in Fig. 7. Spatial conformation and solubility of PNIPAAm vary with temperature. At temperatures lower than LCST, strong hydrogen bonding between the polymer chains and water molecules results in the polymer solubility. However, polymer chains collapse at temperatures higher than LCST because of hydrogen bonding between N–H and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O.59 PNIPAAm chains become poorly soluble and collapsed together when temperature is increased, and there is a sharp transition at 34 °C, which corresponds to the phase transition temperature of PNIPAAm. By increasing temperature, also particle size of NCC-g-PNIPAAm varies suddenly at 34 and 40 °C, which is on account of the insolubility of free and graft PNIPAAm chains at higher temperatures. PNIPAAm particle size increases from 12 to 230 nm; however, this variation occurs in two steps for nanocomposites. The first step at temperature range of 33–35 °C originates from the PNIPAAm particles which expand from 76.9 to 106 nm in diameter. The second step relates to the variation of NCC-g-PNIPAAm particle size from 116 to 222 nm. NCC particles increase water solubility of PNIPAAm because of a large number of hydroxyl groups. Therefore, an increase of about 6 °C is observed for LCST by the addition of NCC. Also, PDI values of the particles increases in the presence of NCC.
O.59 PNIPAAm chains become poorly soluble and collapsed together when temperature is increased, and there is a sharp transition at 34 °C, which corresponds to the phase transition temperature of PNIPAAm. By increasing temperature, also particle size of NCC-g-PNIPAAm varies suddenly at 34 and 40 °C, which is on account of the insolubility of free and graft PNIPAAm chains at higher temperatures. PNIPAAm particle size increases from 12 to 230 nm; however, this variation occurs in two steps for nanocomposites. The first step at temperature range of 33–35 °C originates from the PNIPAAm particles which expand from 76.9 to 106 nm in diameter. The second step relates to the variation of NCC-g-PNIPAAm particle size from 116 to 222 nm. NCC particles increase water solubility of PNIPAAm because of a large number of hydroxyl groups. Therefore, an increase of about 6 °C is observed for LCST by the addition of NCC. Also, PDI values of the particles increases in the presence of NCC.
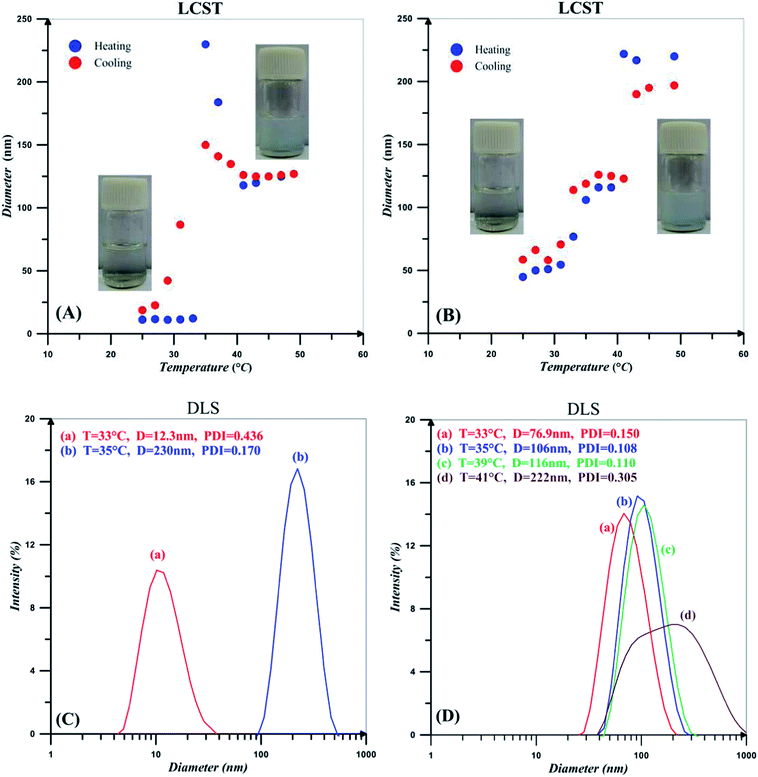 | ||
| Fig. 7 Hydrodynamic diameter for the 0.2 wt% solutions of (A) PNIPAAm and (B) NCC-g-PNIPAAm in addition to the DLS intensity diameters near the LCST points for (C) PNIPAAm and (D) NCC-g-PNIPAAm. | ||
Effect of pH on the LCST behaviour of free and NCC-attached PNIPAAm-co-PAA15 is studied in two pH ranges of 3–4 and 7–8 and the results are shown in Fig. 8. Also, LCST diagram of the 0.2 wt% solutions of NIPAAm and AA random copolymers with various AA content and also NCC-grafted copolymers in different pH values is displayed in Fig. 9. Addition of AA in random copolymers results in increase of LCST point at pH = 7–8; however, a decrease in LCST is observed with increasing AA content at pH = 3–4. Hydrophobic behavior of AA in protic media is the main reason for decrease of LCST. In addition, hydrogen bonding between carboxylic group of AA and amide functional group of NIPAAm in protic media is reasonable. This effect was seen in random copolymers of AA and NIPAAm and also their graft copolymers.1,60 More ionization of the copolymers at higher pH values results in higher LCST values. Also, copolymer chains expand because of electrostatic repulsion, and therefore interaction between polymer chains decreases at higher pH values.1 According to the inlet of Fig. 8, it is clear that phase transition of copolymer chains is not sharp at higher pH values. Since more of the AA units of the copolymer chains are ionized at higher pH values, their hydrophilic tendencies interfere with the LCST behavior of the PNIPAAm segments. Therefore, aggregation of PNIPAAm chain segments to form collapsed particles is weakened. In addition, particles with lower diameters were obtained at higher pH values after phase separation. Collapse of polymer chains into large aggregates is decreased because of the stabilization of the smaller particles in the solution by ionized PAA segments. The plateau at higher pH values for the LCST variation by AA content is also reasonable because of the ionization of AA which prevents from the formation of large aggregates. Finally, by increasing pH values, ionized PAA segments reduce the effect of hydrophobically bound water and it may reach to a point that PNIPAAm segments are unable to form aggregates.1 At both pH ranges, NCC-grafted polymers show larger diameters upon the aggregation which is mainly on account of the presence of NCC particles with the average diameter of 107 nm. Steric confinement of the NCC attached polymers results in their sharp phase transition upon heating.
 | ||
| Fig. 9 LCST diagram for the 0.2 wt% solutions of NIPAAm and AA random copolymers with various AA content in different values of pH. | ||
It is clear that LCST can be tuned by three factors of NCC addition, AA content, and pH. Addition of NCC, AA content and pH at higher pH values results in increase of LCST. At low pH values, increase of LCST is occurred by increase of NCC and also pH value. Therefore, LCST can be tuned to desired values at a particular pH by changing the PAA content and also NCC of the copolymer. This shows that grafting density of copolymers and polymerization conversion is also key factors in tailoring the LCST. This dual sensitive NIPAAm-co-PAA copolymers and its biocomposites is tailored to switch from a hydrophobe to an ionized state at predetermined pH values. In addition, its PNIPAAm segments can switch from a soluble to collapsed parts at predetermined temperatures. Also, NCC addition hinders this behavior thermally. This dual behavior of products is useful in a variety of biomedical applications. In drug delivery, the combined sensitivity could be used to enhance drug delivery where pH gradients are shown in body. In addition, in a desired location releasing of drugs temperature in combination of pH may act as a good controlling key.
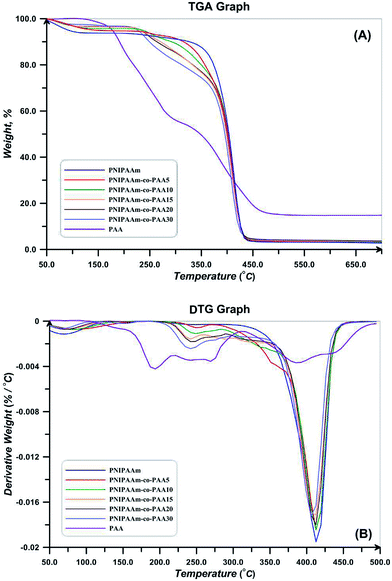
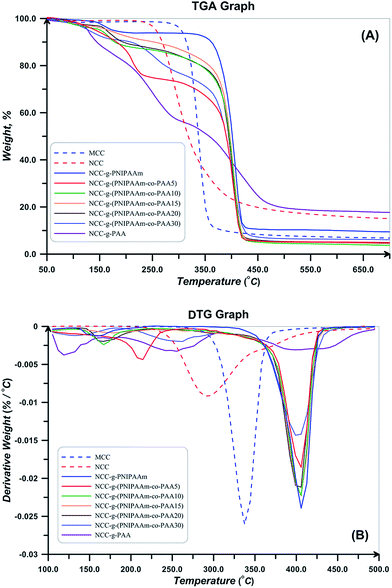
TGA and DTG thermograms for PNIPAAm, PAA, and also NIPAAm and AA random copolymers are presented in Fig. 10. All the samples show a weight loss at temperatures lower than 120 °C which is attributed to the water evaporation. Degradation of PAA is carried out in three steps of hydrogen abstraction, carboxyl abstraction, and PAA chains degradation. The first step takes place by intra- and intermolecular reactions of carboxyl groups. Intramolecular reactions result in hexagonal anhydride rings (Fig. 11(A)). At the second step which is accomplished at temperatures higher than 200 °C, CO2 released upon the degradation of anhydride groups (Fig. 11(B)). At temperatures higher than 300 °C, carboxyl abstraction process is continued. PAA repeating units remains between anhydride rings at the backbone during these processes. Broken short chains which consist of two or more repeating units are formed during the third step at temperatures higher than 350 °C (Fig. 11(C)). Also, CO is released at higher temperatures.61 Degradation temperature of random copolymers decreases with increasing AA content which results from the lower degradation temperature of PAA. In addition, their degradation process consists of some distinguished stages because of multi-stage degradation pattern of PAA. Short chains of PAA after degradation results in high values of char content for random copolymers (Table 4).
| Polymer | 1st step | 2nd step | 3rd step | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Td,s | Td,m | D | Td,s | Td,m | D | Td,s | Td,m | D | |
| PNIPAAm | 285 | 411 | 87.4 | — | — | — | — | — | — |
| NCC-g-PNIPAAm | 140 | 160 | 4.5 | 340 | 405 | 81.3 | — | — | — |
| PNIPAAm-co-pAA5 | 233 | 412 | 90.5 | — | — | — | — | — | — |
| NCC-g-(PNIPAAm-co-pAA5) | 115 | 212 | 21.8 | 287 | 404 | 67.6 | — | — | — |
| PNIPAAm-co-pAA10 | 219 | 248 | 4.9 | 284 | 411 | 86.6 | — | — | — |
| NCC-g-(PNIPAAm-co-pAA10) | 135 | 167 | 8.2 | 300 | 406 | 78.4 | — | — | — |
| PNIPAAm-co-pAA15 | 222 | 240 | 4.5 | 359 | 410 | 69.9 | — | — | — |
| NCC-g-(PNIPAAm-co-pAA15) | 135 | 176 | 5.5 | 290 | 406 | 82.1 | — | — | — |
| PNIPAAm-co-pAA20 | 210 | 241 | 10.3 | 351 | 411 | 72.1 | — | — | — |
| NCC-g-(PNIPAAm-co-pAA20) | 145 | 165 | 7.9 | 276 | 411 | 80.2 | — | — | — |
| PNIPAAm-co-pAA30 | 202 | 241 | 14.7 | 343 | 406 | 70.8 | — | — | — |
| NCC-g-(PNIPAAm-co-pAA30) | 101 | 134 | 6.8 | 191 | 253 | 13.9 | 317 | 403 | 68.1 |
| PAA | 108 | 193 | 19.9 | 219 | 269 | 24.2 | 312 | 387 | 39.2 |
| NCC-gPAA | 100 | 119 | 15.2 | 194 | 252 | 26.1 | 318 | 501 | 36.1 |
TGA and DTG thermograms for MCC, NCC, and polymer grafted NCCs are also shown in Fig. 12. Thermal degradation behavior of NCC differs from MCC at temperatures higher than 150 °C, the decomposition of CNC started at lower temperatures (230 with respect to the 290 °C), continues in higher temperature range, and also its char value is higher. This distinction is a result of the difference in their particle size. CNC because of its small particle size and high specific surface area has higher number of free end chains in the surface which decompose at lower temperatures. The decomposition of cellulose at low temperature usually facilitated the formation of char residue (18.03 with respect to the 7.79% at 500 °C). Amorphous chains on the surface of NCC degrade at lower temperatures and results in lower thermal stability of NCC.55 Considering DTG thermogram of NCC, its degradation takes place at one step and there is not any degradation step at temperatures in the range of 150–200 °C. It is reported that sulfate-containing NCCs show a distinguished degradation step at this region because of the presence of sulfate groups.55 Therefore, repeated centrifugation and ultrasonic processes in combination with base treatment with NaOH results in sulfate free NCC product. Additionally, DTG peak point which is commonly known as the degradation temperature is lower for NCC (291 °C with respect to 337 °C). Moreover, deconvolution of DTG curves of NCC results in two peaks, the first one originates from the degradation of cellulose macromolecule (66.8%) and the second one is assigned to the degradation of the remained solid (33.2%).
As shown in Fig. 12, the polymer-grafted NCCs displays a two-stage decomposition profile. The first degradation step is observed in temperature range of 150–220 °C. The second degradation step starts at around 410 °C, which is slightly higher than the pure NCC decomposition temperature and it is lower than that of the free polymers. The thermal degradation profile of the NCC-grafted polymers is an intermediate of the thermal profile of each component. This completely shows the grafting of polymers on NCC. Also, char value of the NCC-grafted polymers is higher than the neat polymers as a result of the remained nanocrystals. Degradation temperature of NCC-g-PAA is lower than PAA and also takes place at broad temperature ranges. Random copolymers show two degradation steps in their thermal profiles. Degradation onset is 300 and 317 °C for both of the synthesized copolymers which are higher than the degradation start point of NCC (238 °C) and shows that thermal stability of the NCC increased. The entire polymer grafted NCCs show a two step degradation pattern; the first one relates to the decomposition of NCC and occurs at temperatures in the range of 150–250 °C and the other originates from the copolymers at temperature span of 275–400 °C.38,62
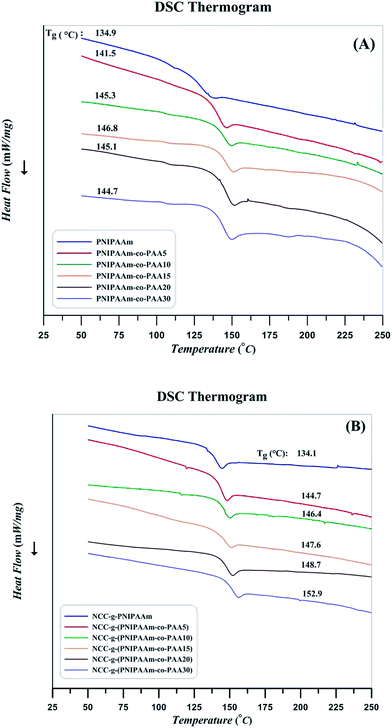
Relaxation behavior of copolymers by the addition of AA content and also effect of attachment on the chain confinement were evaluated by DSC at the temperature range of 25–250 °C. The curves were obtained after removing thermal history as follows: after a heating procedure, the samples were cooled to room temperature to distinguish the phase conversion and other irreversible thermal behaviors. Subsequently, glass transition as a macroscopic indication of the relaxation behavior was obtained through the second heating cycle. The strength and nature of the interface can result in various Tg values. Graft polymer chains relax in a different manner in comparison with the free chains; this difference indicates interactions between the substrate and chains with covalent bonding.63 Substrate confinement commonly increases Tg value. DSC results of PNIPAAm, random copolymers, and their nanocomposites are presented in Fig. 13. According to the results, glass transition temperature of random copolymers increases by the addition of AA content. Similarly, polymer grafting on NCC results in higher Tg values as a results of confinement of NCC on the relaxation of polymer chains.43 Heat flow differences near the Tg which can be a rough estimation of heat capacity variation (ΔCp) are somewhat different in the case of the polymers and its nanocomposites. According to the ΔCp values derived from DSC results and presented in Table 5, it is concluded that NCC not only confines the segmental motions of the polymers but also it reduces the heat capacity variation at glass transition temperature. Also, by increasing AA content of the copolymers, ΔCp values decrease. Decrease of molecular weight by the addition of AA content in combination with the increase of a PAA which does not response to the increase of temperature at this temperature region may be the reasons. Decrease of PNIPAAm content in the copolymers by the addition of AA is also concluded by the ratio of ΔCp values for the free and attached polymers.64
| Sample | Tg (°C) | ΔCp (mW °C−1) | %PNIPAAm | ||
|---|---|---|---|---|---|
| Free | Attached | Free | Attached | ||
| PNIPAAm | 134.9 | 134.1 | 64.52 | 55.06 | 85.3 |
| PNIPAAm-co-pAA5 | 141.5 | 144.7 | 95.64 | 72.30 | 75.6 |
| PNIPAAm-co-pAA10 | 145.3 | 146.4 | 87.74 | 66.49 | 75.8 |
| PNIPAAm-co-pAA15 | 146.8 | 147.6 | 82.05 | 61.61 | 75.0 |
| PNIPAAm-co-pAA20 | 145.1 | 148.7 | 78.72 | 57.60 | 73.1 |
| PNIPAAm-co-pAA30 | 144.7 | 152.9 | 79.50 | 57.49 | 72.0 |
XRD results for the MCC, NCC, and also free and NCC-attached polymers are displayed in Fig. 14. According to the results, the pattern for MCC and NCC are similar which shows that the crystalline structure of cellulose I was not destroyed upon the hydrolysis with acid. It is clear that the crystalline peak area increases after acid hydrolysis because the amorphous parts of MCC are hydrolysed by the acid. MCC and NCC display four diffraction peaks at 14.8, 16.1, 22.5, and 34.5°, which are typical of cellulose.65,66 Two peaks at 14.8 and 16.1° corresponds to the cellulose I polymorph structure and the peak at 22.5° is assigned to the crystalline parts. Degree of crystallinity for MCC and NCC is equals to 69.3 and 68.5% respectively which are in typical crystallinity value range of 55–80%.67 Decrease of crystallinity degree for NCC is ascribed to its higher amorphous surfaces which is a result of its high aspect ratio.55 The characteristic peaks of NCC at the diffraction angles of 16.2 and 22.5° are not present in the patterns of NCC-grafted polymers which is a result of its low content in the nanocomposites and also lower diffraction intensity of MCC compared with the polymers. Two peaks at diffraction angles of 8.5 and 20.5° for all the free polymers show their crystalline structure. NCC-grafted polymers, however, show weak peaks at these diffraction angles which is a result of NCC particles.57
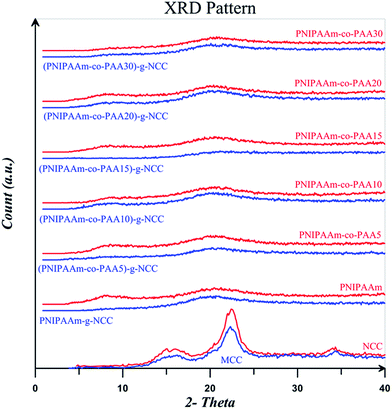 | ||
| Fig. 14 XRD patterns for MCC, NCC, and free and NCC-attached PNIPAAm, PNIPAAm-co-PAA15, and PNIPAAm-co-PAA30. | ||
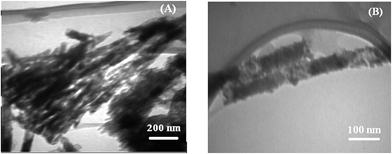
TEM images of the polymer-attached NCC is provided in Fig. 15. Because of intra- and intermolecular hydrogen bonding, NCC particles are mainly in the form of clusters as shown in Fig. 1 (C). The overall morphology of the nanocrystals has not been changed upon the grafting reaction. However, after polymer grafting, NCC particles observed in the separated forms. Polymer-grafted NCCs show polymer covering of nanocrystals as a layer on them.68
4. Conclusions
Dual-sensitive free and NCC-grafted random copolymers of NIPAAm and AA with different AA contents were synthesized by RAFT polymerization. Grafting reaction was carried out via R-group approach using DDMAT-grafted NCCs. DLS results show that NCC particles with 107 nm in length and narrow size distribution are formed. Rod like shapes of NCC is also displayed in SEM and TEM images. Sulfur content of 6.39% from XPS shows that the graft percent of DDMAT on NCC is about 17.14%. Appearance of the C–S and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S peaks at the wave numbers of 804 and 1249 cm−1 in Raman spectra and also the peak at 1635 cm−1 arising from the carbonyl groups of DDMAT are evidences for the successful surface modification of NCC with CTA. 1H NMR shows that molecular weight of copolymers reduces by the addition of AA content. Addition of NCC, AA content, and pH at higher pH values results in increase of LCST. At low pH values, increase of LCST is occurred by increase of NCC and also pH value. Polymer-grafted NCCs are thermally more stable than NCC. Glass transition temperature of random copolymers increases by the addition of AA content and also NCC. XRD results indicates that by grafting polymers on NCC, their crystalline peaks area diminished. Polymer covering on the NCCs was shown by TEM.
S peaks at the wave numbers of 804 and 1249 cm−1 in Raman spectra and also the peak at 1635 cm−1 arising from the carbonyl groups of DDMAT are evidences for the successful surface modification of NCC with CTA. 1H NMR shows that molecular weight of copolymers reduces by the addition of AA content. Addition of NCC, AA content, and pH at higher pH values results in increase of LCST. At low pH values, increase of LCST is occurred by increase of NCC and also pH value. Polymer-grafted NCCs are thermally more stable than NCC. Glass transition temperature of random copolymers increases by the addition of AA content and also NCC. XRD results indicates that by grafting polymers on NCC, their crystalline peaks area diminished. Polymer covering on the NCCs was shown by TEM.
Acknowledgements
Iran's Research Institute of Petroleum Industry is greatly appreciated for its financial support.References
- X. Yin, A. S. Hoffman and P. S. Stayton, Biomacromolecules, 2006, 7, 1381–1385 CrossRef CAS PubMed.
- C. M. Schilli, M. Zhang, E. Rizzardo, S. H. Thang, Y. K. Chong, K. Edwards, G. Karlsson and A. H. E. Müller, Macromolecules, 2004, 37, 7861–7866 CrossRef CAS.
- C. Vasilea, G. G. Bumbua, R. P. Dumitriua and G. Staikos, Eur. Polym. J., 2004, 40, 1209–1215 CrossRef PubMed.
- H. G. Schild, Prog. Polym. Sci., 1992, 17, 163–249 CrossRef CAS.
- H. Feil, Y. H. Bae, J. Feijen and S. W. Kim, Macromolecules, 1993, 26, 2496–2500 CrossRef CAS.
- G. Chen and A. S. Hoffman, Nature, 1995, 373, 49–52 CrossRef CAS PubMed.
- V. Bulmus, Z. Ding, C. J. Long, P. S. Stayton and A. S. Hoffman, Bioconjugate Chem., 2000, 11, 78–83 CrossRef CAS PubMed.
- X. Zhao, W. Liu, D. Chen, X. Lin and W. W. Lu, Macromol. Chem. Phys., 2007, 208, 1773–1781 CrossRef CAS.
- K. S. Soppimath, D. C. W. Tan and Y. Y. Yang, Adv. Mater., 2005, 17, 318–323 CrossRef CAS.
- A. F. Olea and J. K. Thomas, Macromolecules, 1989, 22, 1165–1169 CrossRef CAS.
- B. Liu and S. Perrier, J. Polym. Sci., Part A: Polym. Chem., 2005, 43, 3643–3654 CrossRef CAS.
- P. Bilalis, G. Zorba, M. Pitsikalis and N. Hadjichristidis, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 5719–5728 CrossRef CAS.
- K. Nakashima and P. Bahadur, Adv. Colloid Interface Sci., 2006, 123, 75–96 CrossRef PubMed.
- Č. Koňak, D. Oupický, V. Chytrý and K. Ulbrich, Macromolecules, 2000, 33, 5318–5320 CrossRef.
- D. Klemm, F. Kramer, S. Moritz, T. Lindström, M. Ankerfors, D. Gray and A. Dorris, Angew. Chem., Int. Ed., 2011, 50, 2–31 CrossRef PubMed.
- P. Lu and Y. L. Hsieh, Carbohydr. Polym., 2010, 82, 329–336 CrossRef CAS PubMed.
- Y. Habibi, L. A. Lucia and O. J. Rojas, Chem. Rev., 2010, 110, 3479–3500 CrossRef CAS PubMed.
- R. Rodriguez, C. Alvarez-Lorenzo and A. Concheiro, J. Controlled Release, 2003, 86, 253–265 CrossRef CAS.
- C. Ding, X. Qian, J. Shen and X. An, Bioresearch, 2010, 5, 303–315 CAS.
- K. H. M. Kan, J. Li, K. Wijesekera and E. D. Cranston, Biomacromolecules, 2013, 14, 3130–3139 CrossRef CAS PubMed.
- K. A. Mahmoud, K. B. Male, S. Hrapovic and J. H. Luong, ACS Appl. Mater. Interfaces, 2009, 1, 1383–1386 CAS.
- H. Wang and M. Roman, Biomacromolecules, 2011, 12, 1585–1593 CrossRef CAS PubMed.
- J. V. Edwards, N. Prevost, A. French, M. Concha, A. DeLucca and Q. Wu, Engineering, 2013, 5, 20–28 CrossRef.
- E. Lam, K. B. Male, J. H. Chong, A. C. W. Leung and J. H. T. Luong, Trends Biotechnol., 2012, 30, 283–290 CrossRef CAS PubMed.
- H. Roghani-Mamaqani, V. Haddadi-Asl and M. Salami-Kalajahi, Polym. Rev., 2012, 52, 142–188 CrossRef CAS.
- W. A. Braunecker and K. Matyjaszewski, Prog. Polym. Sci., 2007, 32, 93–146 CrossRef CAS PubMed.
- H. Roghani-Mamaqani, V. Haddadi-Asl, M. Najafi and M. Salami-Kalajahi, AIChE J., 2011, 57, 1873–1881 CrossRef CAS.
- H. Roghani-Mamaqani, V. Haddadi-Asl, M. Najafi and M. Salami-Kalajahi, J. Appl. Polym. Sci., 2012, 123, 409–417 CrossRef CAS.
- S. Rahimi-Razin, M. Salami-Kalajahi, V. Haddadi-Asl and H. Roghani-Mamaqani, J. Polym. Res., 2012, 19, 9954 CrossRef PubMed.
- L. Ahmadian-Alam, V. Haddadi-Asl, H. Roghani-Mamaqani, L. Hatami and M. Salami-Kalajahi, J. Polym. Res., 2012, 19, 9773 CrossRef.
- S. Rahimi-Razin, V. Haddadi-Asl, M. Salami-Kalajahi, F. Behboodi-Sadabad and H. Roghani-Mamaqani, J. Iran. Chem. Soc., 2012, 9, 877–887 CrossRef CAS PubMed.
- O. Glaied, M. Dubé, B. Chabot and C. Daneault, J. Colloid Interface Sci., 2009, 333, 145–151 CrossRef CAS PubMed.
- G. Morandi, L. Heath and W. Thielemans, Langmuir, 2009, 25, 8280–8286 CrossRef CAS PubMed.
- J. Yi, Q. Xu, X. Zhang and H. Zhang, Cellulose, 2009, 16, 989–997 CrossRef CAS.
- J. Yi, Q. Xu, X. Zhang and H. Zhang, Polymer, 2008, 49, 4406–4412 CrossRef CAS PubMed.
- M. H. Stenzel, T. P. Davis and A. G. Fane, J. Mater. Chem., 2003, 13, 2090–2097 RSC.
- M. Hernández-Guerrero, T. P. Davis, C. Barner-Kowollik and M. H. Stenzel, Eur. Polym. J., 2005, 41, 2264–2277 CrossRef PubMed.
- D. Roy, J. T. Guthrie and S. Perrier, Macromolecules, 2005, 38, 10363–10372 CrossRef CAS.
- D. Roy, J. S. Knapp, J. T. Guthrie and S. Perrier, Biomacromolecules, 2008, 9, 91–99 CrossRef CAS PubMed.
- M. Hiltunen, S. Riihela and S. L. Maunu, J. Polym. Sci., Part B: Polym. Phys., 2009, 47, 1869–1879 CrossRef CAS.
- R. Fleet, J. B. McLeary, V. Grumel, W. G. Weber, H. Matahwa and R. D. Sanderson, Eur. Polym. J., 2008, 44, 2899–2911 CrossRef CAS PubMed.
- M. Semsarilar, V. Ladmiral and S. Perrier, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 4361–4365 CrossRef CAS.
- M. Barsbay, O. Guven, T. P. Davis, C. Barner-Kowollik and L. Barner, Polymer, 2009, 50, 973–982 CrossRef CAS PubMed.
- J. T. Lai, D. Filla and R. Shea, Macromolecules, 2002, 35, 6754–6756 CrossRef CAS.
- W. Bai, J. Holbey and K. Li, Cellulose, 2009, 16, 455–465 CrossRef CAS PubMed.
- J. Loiseau, N. Doerr, J. M. Suau, J. B. Egraz, M. F. Llaura and C. Ladaviere, Macromolecules, 2003, 36, 3066–3077 CrossRef CAS.
- S. J. Eichhorn and R. J. Young, Cellulose, 2001, 8, 197–207 CrossRef CAS.
- S. Elazzouzi-Hafraoui, Y. Nishiyama, J. L. Putaux, L. Heux, F. Dubreuil and C. Rochas, Biomacromolecules, 2008, 9, 57–65 CrossRef CAS PubMed.
- F. Azzam, L. Heux, J. L. Putaux and B. Jean, Biomacromolecules, 2010, 11, 3652–3659 CrossRef CAS PubMed.
- S. Elazzouzi-Hafraoui, Y. Nishiyama, J. L. Putaux, L. Heux, F. Dubreuil and C. Rochas, Biomacromolecules, 2008, 9, 57–65 CrossRef CAS PubMed.
- Y. Habibi, A. L. Goffin, N. Schiltz, E. Duquesne, P. Dubois and A. Dufresne, J. Mater. Chem., 2008, 18, 5002–5010 RSC.
- X. Cao, Y. Habibi and L. A. Lucia, J. Mater. Chem., 2009, 19, 7137–7145 RSC.
- Z. Q. Cao, W. G. Liu, G. X. Ye, X. L. Zhao, X. Z. Lin, P. Gao and K. D. Yao, Macromol. Chem. Phys., 2006, 207, 2329 CrossRef CAS.
- G. Morandi, L. Heath and W. Thielemans, Langmuir, 2009, 25, 8280–8286 CrossRef CAS PubMed.
- R. Cha, Z. He and Y. Ni, Carbohydr. Polym., 2012, 88, 713–718 CrossRef CAS PubMed.
- G. Chen, A. Dufresne, J. Huang and P. R. Chang, Macromol. Mater. Eng., 2009, 294, 59–67 CrossRef CAS.
- M. Salami-kalajahi, V. Haddadi-Asl, F. Behboodi-Sadabad, S. Rahimi-Rezin and H. Roghani-Mamaqani, J. Polym. Eng., 2012, 32, 13–22 CAS.
- C. Y. Hong and C. Y. Pan, Macromolecules, 2006, 39, 3517–3524 CrossRef CAS.
- G. Chen and A. S. Hoffman, Nature, 1995, 373, 49–52 CrossRef CAS PubMed.
- I. C. McNeil and S. M. T. Sadeghi, Polym. Degrad. Stab., 1990, 29, 233–246 CrossRef.
- N. Wang, E. Ding and R. Cheng, Polymer, 2007, 48, 3486–3493 CrossRef CAS PubMed.
- H. Roghani-Mamaqani, V. Haddadi-Asl, K. Khezri, E. Zeinali and M. Salami-Kalajahi, J. Polym. Res., 2014, 21, 333 CrossRef.
- M. Yu, R. Yang, L. Huang, X. Cao, F. Yang and D. Liu, BioResources, 2012, 7, 1802–1812 Search PubMed.
- J. O. Zoppe, Y. Habibi, O. J. Rojas, R. A. Venditti, L. S. Johansson, K. Efimenko, M. Osterberg and J. Laine, Biomacromolecules, 2010, 11, 2683–2691 CrossRef CAS PubMed.
- Y. Nishiyama, P. Langan and H. Chanzy, J. Am. Chem. Soc., 2002, 124, 9074–9082 CrossRef CAS PubMed.
- J. Sugiyama, R. Vuong and H. Chanzy, Macromolecules, 1991, 24, 4168–4175 CrossRef CAS.
- S. Wei, V. Kumar and G. S. Banker, Int. J. Pharm., 1996, 142, 175–181 CrossRef CAS.
- F. R. Mayo and F. M. Lewis, J. Am. Chem. Soc., 1944, 66, 1594–1601 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |